Abstract
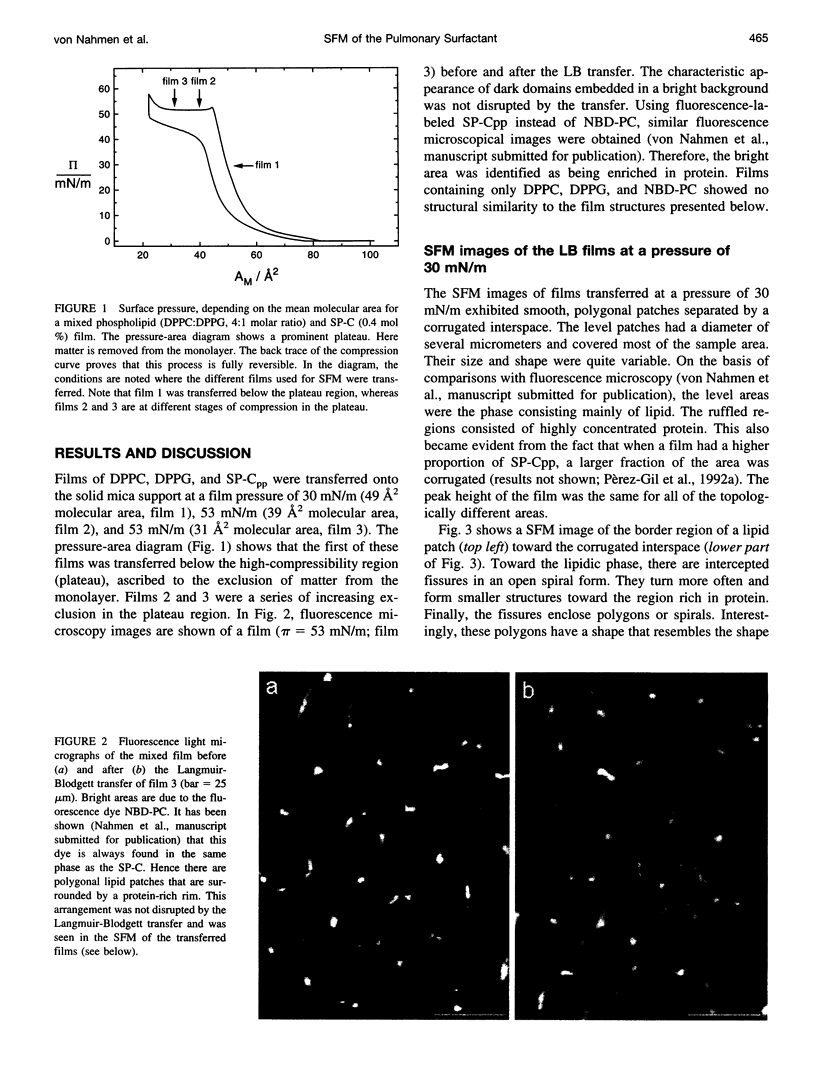
The structures formed by a pulmonary surfactant model system of dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylglycerol (DPPG), and recombinant surfactant-associated protein C (SP-C) were studied using scanning force microscopy (SFM) on Langmuir-Blodgett films. The films appeared to be phase separated, in agreement with earlier investigations by fluorescence light microscopy. There were smooth polygonal patches of mostly lipid, surrounded by a corrugated rim rich in SP-C. When the films were compressed beyond the equilibrium surface pressure, the protein-rich phase mediated the formation of layered protrusions. The height of these multilamellar structures embodied equidistant steps slightly higher than a DPPC double layer in the gel phase. At the air-water interface too, a high compressibility at low surface tension was indicative of the exclusion of matter. The exclusion process proved to be fully reversible. The present study demonstrates that some of the matter of the model pulmonary surfactant can move in and out of the active monolayer. The SFM images revealed a lipid-protein complex that was responsible for the reversible exclusion of double-layer structures. This mechanism may be important in the natural system too, to keep the surface tension of the alveolar air/water interface constantly low over the range of area encountered upon breathing.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN E. S. ISOLATION AND ASSAY OF DIPALMITYL LECITHIN IN LUNG EXTRACTS. Am J Physiol. 1964 Aug;207:402–406. doi: 10.1152/ajplegacy.1964.207.2.402. [DOI] [PubMed] [Google Scholar]
- Bangham A. D., Morley C. J., Phillips M. C. The physical properties of an effective lung surfactant. Biochim Biophys Acta. 1979 Jun 21;573(3):552–556. doi: 10.1016/0005-2760(79)90229-7. [DOI] [PubMed] [Google Scholar]
- CLEMENTS J. A., BROWN E. S., JOHNSON R. P. Pulmonary surface tension and the mucus lining of the lungs: some theoretical considerations. J Appl Physiol. 1958 Mar;12(2):262–268. doi: 10.1152/jappl.1958.12.2.262. [DOI] [PubMed] [Google Scholar]
- Creuwels L. A., Demel R. A., van Golde L. M., Benson B. J., Haagsman H. P. Effect of acylation on structure and function of surfactant protein C at the air-liquid interface. J Biol Chem. 1993 Dec 15;268(35):26752–26758. [PubMed] [Google Scholar]
- Curstedt T., Johansson J., Persson P., Eklund A., Robertson B., Löwenadler B., Jörnvall H. Hydrophobic surfactant-associated polypeptides: SP-C is a lipopeptide with two palmitoylated cysteine residues, whereas SP-B lacks covalently linked fatty acyl groups. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2985–2989. doi: 10.1073/pnas.87.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enhorning G. Pulsating bubble technique for evaluating pulmonary surfactant. J Appl Physiol Respir Environ Exerc Physiol. 1977 Aug;43(2):198–203. doi: 10.1152/jappl.1977.43.2.198. [DOI] [PubMed] [Google Scholar]
- Harwood J. L. Lung surfactant. Prog Lipid Res. 1987;26(3):211–256. doi: 10.1016/0163-7827(87)90004-x. [DOI] [PubMed] [Google Scholar]
- Johansson J., Curstedt T., Robertson B. The proteins of the surfactant system. Eur Respir J. 1994 Feb;7(2):372–391. doi: 10.1183/09031936.94.07020372. [DOI] [PubMed] [Google Scholar]
- Johansson J., Szyperski T., Curstedt T., Wüthrich K. The NMR structure of the pulmonary surfactant-associated polypeptide SP-C in an apolar solvent contains a valyl-rich alpha-helix. Biochemistry. 1994 May 17;33(19):6015–6023. doi: 10.1021/bi00185a042. [DOI] [PubMed] [Google Scholar]
- Notter R. H., Tabak S. A., Mavis R. D. Surface properties of binary mixtures of some pulmonary surfactant components. J Lipid Res. 1980 Jan;21(1):10–22. [PubMed] [Google Scholar]
- Oosterlaken-Dijksterhuis M. A., Haagsman H. P., van Golde L. M., Demel R. A. Characterization of lipid insertion into monomolecular layers mediated by lung surfactant proteins SP-B and SP-C. Biochemistry. 1991 Nov 12;30(45):10965–10971. doi: 10.1021/bi00109a022. [DOI] [PubMed] [Google Scholar]
- Oosterlaken-Dijksterhuis M. A., Haagsman H. P., van Golde L. M., Demel R. A. Interaction of lipid vesicles with monomolecular layers containing lung surfactant proteins SP-B or SP-C. Biochemistry. 1991 Aug 20;30(33):8276–8281. doi: 10.1021/bi00247a024. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. Properties, function, and origin of the alveolar lining layer. Proc R Soc Lond B Biol Sci. 1958 Feb 18;148(931):217–240. doi: 10.1098/rspb.1958.0015. [DOI] [PubMed] [Google Scholar]
- Possmayer F. A proposed nomenclature for pulmonary surfactant-associated proteins. Am Rev Respir Dis. 1988 Oct;138(4):990–998. doi: 10.1164/ajrccm/138.4.990. [DOI] [PubMed] [Google Scholar]
- Post A., Nahmen A. V., Schmitt M., Ruths J., Riegler H., Sieber M., Galla H. J. Pulmonary surfactant protein C containing lipid films at the air-water interface as a model for the surface of lung alveoli. Mol Membr Biol. 1995 Jan-Mar;12(1):93–99. doi: 10.3109/09687689509038502. [DOI] [PubMed] [Google Scholar]
- Pérez-Gil J., Nag K., Taneva S., Keough K. M. Pulmonary surfactant protein SP-C causes packing rearrangements of dipalmitoylphosphatidylcholine in spread monolayers. Biophys J. 1992 Jul;63(1):197–204. doi: 10.1016/S0006-3495(92)81582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Gil J., Tucker J., Simatos G., Keough K. M. Interfacial adsorption of simple lipid mixtures combined with hydrophobic surfactant protein from pig lung. Biochem Cell Biol. 1992 May;70(5):332–338. doi: 10.1139/o92-051. [DOI] [PubMed] [Google Scholar]
- Qanbar R., Possmayer F. On the surface activity of surfactant-associated protein C (SP-C): effects of palmitoylation and pH. Biochim Biophys Acta. 1995 Apr 6;1255(3):251–259. doi: 10.1016/0005-2760(94)00224-m. [DOI] [PubMed] [Google Scholar]
- Schürch S., Qanbar R., Bachofen H., Possmayer F. The surface-associated surfactant reservoir in the alveolar lining. Biol Neonate. 1995;67 (Suppl 1):61–76. doi: 10.1159/000244207. [DOI] [PubMed] [Google Scholar]
- Schürch S. Surface tension at low lung volumes: dependence on time and alveolar size. Respir Physiol. 1982 Jun;48(3):339–355. doi: 10.1016/0034-5687(82)90038-x. [DOI] [PubMed] [Google Scholar]
- Shelley S. A., Balis J. U., Paciga J. E., Espinoza C. G., Richman A. V. Biochemical composition of adult human lung surfactant. Lung. 1982;160(4):195–206. doi: 10.1007/BF02719293. [DOI] [PubMed] [Google Scholar]
- Taneva S. G., Keough K. M. Dynamic surface properties of pulmonary surfactant proteins SP-B and SP-C and their mixtures with dipalmitoylphosphatidylcholine. Biochemistry. 1994 Dec 13;33(49):14660–14670. doi: 10.1021/bi00253a003. [DOI] [PubMed] [Google Scholar]
- Taneva S., Keough K. M. Pulmonary surfactant proteins SP-B and SP-C in spread monolayers at the air-water interface: I. Monolayers of pulmonary surfactant protein SP-B and phospholipids. Biophys J. 1994 Apr;66(4):1137–1148. doi: 10.1016/S0006-3495(94)80895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneva S., Keough K. M. Pulmonary surfactant proteins SP-B and SP-C in spread monolayers at the air-water interface: II. Monolayers of pulmonary surfactant protein SP-C and phospholipids. Biophys J. 1994 Apr;66(4):1149–1157. doi: 10.1016/S0006-3495(94)80896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoreloff P., Gulik A., Denizot B., Proust J. E., Puisieux F. A structural study of interfacial phospholipid and lung surfactant layers by transmission electron microscopy after Blodgett sampling: influence of surface pressure and temperature. Chem Phys Lipids. 1991 Sep;59(2):151–165. doi: 10.1016/0009-3084(91)90004-u. [DOI] [PubMed] [Google Scholar]
- Wang Z., Hall S. B., Notter R. H. Dynamic surface activity of films of lung surfactant phospholipids, hydrophobic proteins, and neutral lipids. J Lipid Res. 1995 Jun;36(6):1283–1293. [PubMed] [Google Scholar]
- Weaver T. E. Pulmonary surfactant-associated proteins. Gen Pharmacol. 1988;19(3):361–368. doi: 10.1016/0306-3623(88)90029-8. [DOI] [PubMed] [Google Scholar]