Abstract
Monte Carlo simulations of fluorescence recovery after photobleaching (FRAP) experiments on two-component lipid bilayers systems in the solid-fluid phase coexistence region were carried out to study the geometry and size of fluid domains in these bilayers. The gel phase was simulated by superposable elliptical domains, which were either of predetermined dimensions, increasing in number with increasing gel phase fraction, or of predetermined number, increasing in dimensions with increasing gel phase fraction. The simulations were done from two perspectives: 1) a time-independent analysis of fractional fluorescence recovery as a function of fractional fluid phase in the system; and 2) a time-dependent analysis of fractional fluorescence recovery as a function of time at a given fraction of fluid phase in the system. The time-dependent simulations result in recovery curves that are directly comparable to experimental FRAP curves and provide topological and geometrical models for the coexisting phases that are consistent with the experimental result.
Full text
PDF



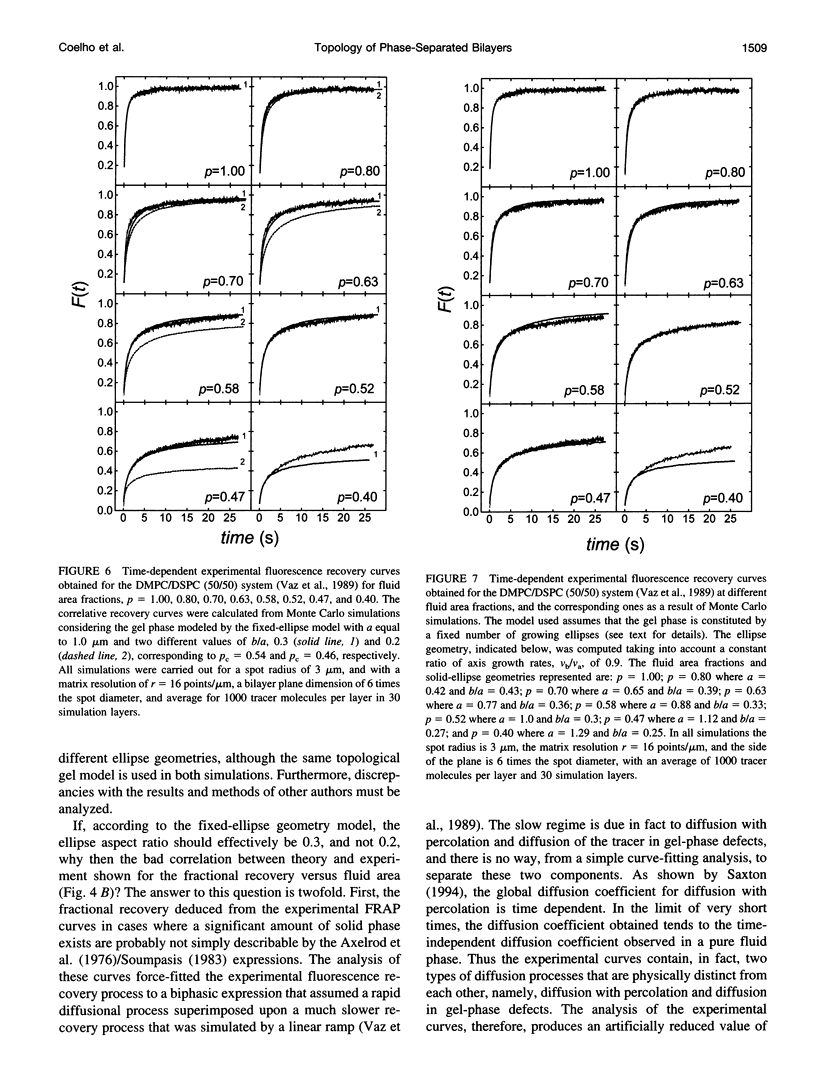
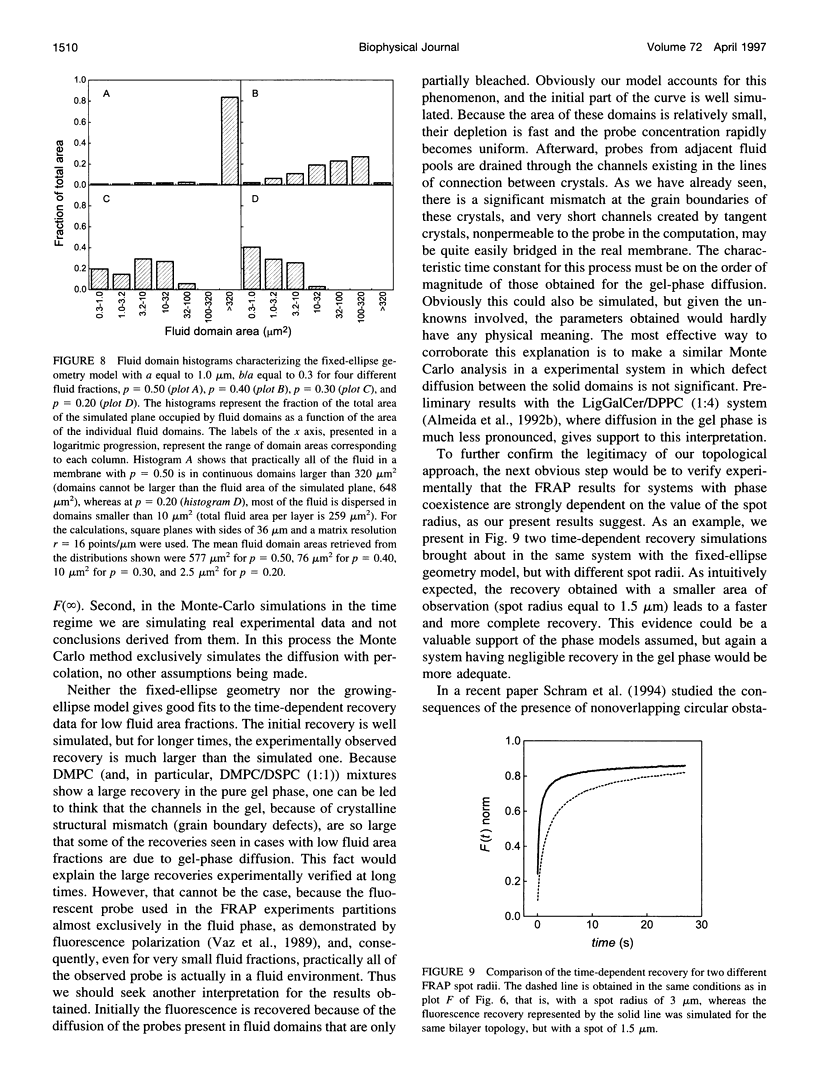

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion and percolation in two-phase, two-component lipid bilayers. Topology of the solid-phase domains in-plane and across the lipid bilayer. Biochemistry. 1992 Aug 11;31(31):7198–7210. doi: 10.1021/bi00146a024. [DOI] [PubMed] [Google Scholar]
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry. 1992 Jul 28;31(29):6739–6747. doi: 10.1021/bi00144a013. [DOI] [PubMed] [Google Scholar]
- Almeida P. F., Vaz W. L., Thompson T. E. Percolation and diffusion in three-component lipid bilayers: effect of cholesterol on an equimolar mixture of two phosphatidylcholines. Biophys J. 1993 Feb;64(2):399–412. doi: 10.1016/S0006-3495(93)81381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultmann T., Vaz W. L., Melo E. C., Sisk R. B., Thompson T. E. Fluid-phase connectivity and translational diffusion in a eutectic, two-component, two-phase phosphatidylcholine bilayer. Biochemistry. 1991 Jun 4;30(22):5573–5579. doi: 10.1021/bi00236a033. [DOI] [PubMed] [Google Scholar]
- Derzko Z., Jacobson K. Comparative lateral diffusion of fluorescent lipid analogues in phospholipid multibilayers. Biochemistry. 1980 Dec 23;19(26):6050–6057. doi: 10.1021/bi00567a016. [DOI] [PubMed] [Google Scholar]
- Hong-wei S., McConnell H. Phase separations in phospholipd membranes. Biochemistry. 1975 Feb 25;14(4):847–854. doi: 10.1021/bi00675a032. [DOI] [PubMed] [Google Scholar]
- Hui S. W. Geometry of phase-separated domains in phospholipid bilayers by diffraction-contrast electron microscopy. Biophys J. 1981 Jun;34(3):383–395. doi: 10.1016/S0006-3495(81)84857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Tamm L. K., Böhm, Ramalingam T. S., Betzig E., Edidin M. Nanoscale complexity of phospholipid monolayers investigated by near-field scanning optical microscopy. Science. 1995 Oct 27;270(5236):610–614. doi: 10.1126/science.270.5236.610. [DOI] [PubMed] [Google Scholar]
- Knoll W., Ibel K., Sackmann E. Small-angle neutron scattering study of lipid phase diagrams by the contrast variation method. Biochemistry. 1981 Oct 27;20(22):6379–6383. doi: 10.1021/bi00525a015. [DOI] [PubMed] [Google Scholar]
- Luna E. J., McConnell H. M. Multiple phase equilibria in binary mixtures of phospholipids. Biochim Biophys Acta. 1978 Jun 2;509(3):462–473. doi: 10.1016/0005-2736(78)90240-7. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo E. C., Lourtie I. M., Sankaram M. B., Thompson T. E., Vaz W. L. Effects of domain connection and disconnection on the yields of in-plane bimolecular reactions in membranes. Biophys J. 1992 Dec;63(6):1506–1512. doi: 10.1016/S0006-3495(92)81735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn R., Liang G. L., Strauss H. L., Snyder R. G. IR spectroscopic determination of gel state miscibility in long-chain phosphatidylcholine mixtures. Biophys J. 1995 Nov;69(5):1987–1998. doi: 10.1016/S0006-3495(95)80069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen O. G., Jørgensen K. Dynamical order and disorder in lipid bilayers. Chem Phys Lipids. 1994 Sep 6;73(1-2):3–25. doi: 10.1016/0009-3084(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Radmacher M., Tillamnn R. W., Fritz M., Gaub H. E. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 1992 Sep 25;257(5078):1900–1905. doi: 10.1126/science.1411505. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Marsh D., Thompson T. E. Determination of fluid and gel domain sizes in two-component, two-phase lipid bilayers. An electron spin resonance spin label study. Biophys J. 1992 Aug;63(2):340–349. doi: 10.1016/S0006-3495(92)81619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Anomalous diffusion due to obstacles: a Monte Carlo study. Biophys J. 1994 Feb;66(2 Pt 1):394–401. doi: 10.1016/s0006-3495(94)80789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Distance dependence of the diffusion coefficient. Biophys J. 1989 Sep;56(3):615–622. doi: 10.1016/S0006-3495(89)82708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. The effect of mobile obstacles. Biophys J. 1987 Dec;52(6):989–997. doi: 10.1016/S0006-3495(87)83291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram V., Tocanne J. F., Lopez A. Influence of obstacles on lipid lateral diffusion: computer simulation of FRAP experiments and application to proteoliposomes and biomembranes. Eur Biophys J. 1994;23(5):337–348. doi: 10.1007/BF00188657. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Soumpasis D. M. Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys J. 1983 Jan;41(1):95–97. doi: 10.1016/S0006-3495(83)84410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Fluid phase connectivity in an isomorphous, two-component, two-phase phosphatidylcholine bilayer. Biophys J. 1990 Jul;58(1):273–275. doi: 10.1016/S0006-3495(90)82373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Translational diffusion and fluid domain connectivity in a two-component, two-phase phospholipid bilayer. Biophys J. 1989 Nov;56(5):869–876. doi: 10.1016/S0006-3495(89)82733-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., Suter R. M., Nagle J. F. Structure of the fully hydrated gel phase of dipalmitoylphosphatidylcholine. Biophys J. 1989 Feb;55(2):315–325. doi: 10.1016/S0006-3495(89)82807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Thorpe MF. Percolation properties of random ellipses. Phys Rev A Gen Phys. 1988 Sep 1;38(5):2650–2656. doi: 10.1103/physreva.38.2650. [DOI] [PubMed] [Google Scholar]



