Abstract
Neutrophil emigration into inflamed tissue is mediated by beta 2-integrin and L-selectin adhesion receptors. Homotypic neutrophil aggregation is also dependent on these molecules, and it provides a model system in which to study adhesion dynamics. In the current study we formulated a mathematical model for cellular aggregation in a linear shear field based on Smoluchowski's two-body collision theory. Neutrophil suspensions activated with chemotactic stimulus and sheared in a cone-plate viscometer rapidly aggregate. Over a range of shear rates (400-800 s-1), approximately 90% of the single cells were recruited into aggregates ranging from doublets to groupings larger than sextuplets. The adhesion efficiency fit to these kinetics reached maximum levels of > 70%. Formed aggregates remained intact and resistant to shear up to 120 s, at which time they spontaneously dissociated back to singlets. The rate of cell disaggregation was linearly proportional to the applied shear rate, and it was approximately 60% lower for doublets as compared to larger aggregates. By accounting for the time-dependent changes in adhesion efficiency, disaggregation rate, and the effects of aggregate geometry, we succeeded in predicting the reversible kinetics of aggregation over a wide range of shear rates and cell concentrations. The combination of viscometry with flow cytometry and mathematical analysis as presented here represents a novel approach to differentiating between the effects of hydrodynamics and the intrinsic biological processes that control cell adhesion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alon R., Hammer D. A., Springer T. A. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995 Apr 6;374(6522):539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- Bargatze R. F., Kurk S., Butcher E. C., Jutila M. A. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994 Nov 1;180(5):1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T. A., Schammel C. M., Lynam E. B., Guyer D. A., Mellors A., Edwards B., Rogelj S., Sklar L. A. Evidence for a third component in neutrophil aggregation: potential roles of O-linked glycoproteins as L-selectin counter-structures. J Leukoc Biol. 1995 Nov;58(5):510–518. doi: 10.1002/jlb.58.5.510. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L. Origins of DNA replication in metazoan chromosomes. J Biol Chem. 1993 Jan 5;268(1):1–4. [PubMed] [Google Scholar]
- Feehan C., Darlak K., Kahn J., Walcheck B., Spatola A. F., Kishimoto T. K. Shedding of the lymphocyte L-selectin adhesion molecule is inhibited by a hydroxamic acid-based protease inhibitor. Identification with an L-selectin-alkaline phosphatase reporter. J Biol Chem. 1996 Mar 22;271(12):7019–7024. doi: 10.1074/jbc.271.12.7019. [DOI] [PubMed] [Google Scholar]
- Finger E. B., Puri K. D., Alon R., Lawrence M. B., von Andrian U. H., Springer T. A. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996 Jan 18;379(6562):266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
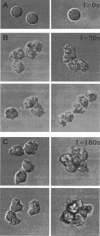
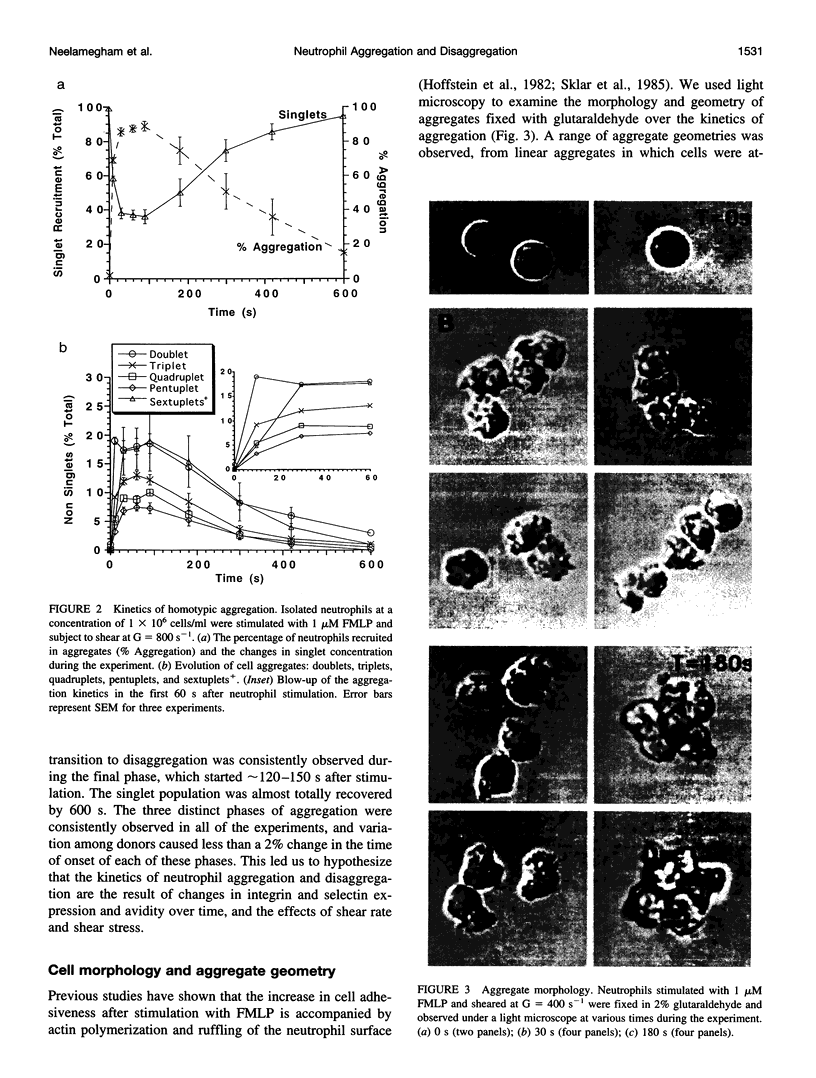
- Hoffstein S. T., Friedman R. S., Weissmann G. Degranulation, membrane addition, and shape change during chemotactic factor-induced aggregation of human neutrophils. J Cell Biol. 1982 Oct;95(1):234–241. doi: 10.1083/jcb.95.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. Y., Hellums J. D. Aggregation and disaggregation kinetics of human blood platelets: Part I. Development and validation of a population balance method. Biophys J. 1993 Jul;65(1):334–343. doi: 10.1016/S0006-3495(93)81078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. J., Hollers J. C., Crockett-Torabi E., Smith C. W. Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. J Clin Invest. 1992 Nov;90(5):1687–1696. doi: 10.1172/JCI116041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., Abbassi O., McIntire L. V., McEver R. P., Smith C. W. P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys J. 1993 Oct;65(4):1560–1569. doi: 10.1016/S0006-3495(93)81195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Rochon Y. P., Frojmovic M. M. Dynamics of human neutrophil aggregation evaluated by flow cytometry. J Leukoc Biol. 1991 Nov;50(5):434–443. doi: 10.1002/jlb.50.5.434. [DOI] [PubMed] [Google Scholar]
- Rochon Y. P., Frojmovic M. M. Regulation of human neutrophil aggregation: comparable latent times, activator sensitivities, and exponential decay in aggregability for FMLP, platelet-activating factor, and leukotriene B4. Blood. 1993 Dec 1;82(11):3460–3468. [PubMed] [Google Scholar]
- Simon S. I., Burns A. R., Taylor A. D., Gopalan P. K., Lynam E. B., Sklar L. A., Smith C. W. L-selectin (CD62L) cross-linking signals neutrophil adhesive functions via the Mac-1 (CD11b/CD18) beta 2-integrin. J Immunol. 1995 Aug 1;155(3):1502–1514. [PubMed] [Google Scholar]
- Simon S. I., Chambers J. D., Butcher E., Sklar L. A. Neutrophil aggregation is beta 2-integrin- and L-selectin-dependent in blood and isolated cells. J Immunol. 1992 Oct 15;149(8):2765–2771. [PubMed] [Google Scholar]
- Simon S. I., Chambers J. D., Sklar L. A. Flow cytometric analysis and modeling of cell-cell adhesive interactions: the neutrophil as a model. J Cell Biol. 1990 Dec;111(6 Pt 1):2747–2756. doi: 10.1083/jcb.111.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. I., Rochon Y. P., Lynam E. B., Smith C. W., Anderson D. C., Sklar L. A. Beta 2-integrin and L-selectin are obligatory receptors in neutrophil aggregation. Blood. 1993 Aug 15;82(4):1097–1106. [PubMed] [Google Scholar]
- Simon S. I., Schmid-Schönbein G. W. Biophysical aspects of microsphere engulfment by human neutrophils. Biophys J. 1988 Feb;53(2):163–173. doi: 10.1016/S0006-3495(88)83078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Omann G. M., Painter R. G. Relationship of actin polymerization and depolymerization to light scattering in human neutrophils: dependence on receptor occupancy and intracellular Ca++. J Cell Biol. 1985 Sep;101(3):1161–1166. doi: 10.1083/jcb.101.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- Taylor A. D., Neelamegham S., Hellums J. D., Smith C. W., Simon S. I. Molecular dynamics of the transition from L-selectin- to beta 2-integrin-dependent neutrophil adhesion under defined hydrodynamic shear. Biophys J. 1996 Dec;71(6):3488–3500. doi: 10.1016/S0006-3495(96)79544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tees D. F., Coenen O., Goldsmith H. L. Interaction forces between red cells agglutinated by antibody. IV. Time and force dependence of break-up. Biophys J. 1993 Sep;65(3):1318–1334. doi: 10.1016/S0006-3495(93)81180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tha S. P., Goldsmith H. L. Interaction forces between red cells agglutinated by antibody. I. Theoretical. Biophys J. 1986 Dec;50(6):1109–1116. doi: 10.1016/S0006-3495(86)83555-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcheck B., Moore K. L., McEver R. P., Kishimoto T. K. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation of P-selectin in vitro. J Clin Invest. 1996 Sep 1;98(5):1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]