Abstract

Colorectal cancer (CRC) is one of the most treatable cancers, yet it ranks second in mortality worldwide. Early detection significantly impacts treatment outcomes, but early stage CRC often presents no symptoms or nonspecific symptoms. The current screening methods are invasive and lacks specificity, hindering widespread CRC screening efforts. This underscores the urgent need for improved CRC screening tools. In this study, a label-free impedimetric immunosensor for detecting colon cancer-secreted protein-2 (CCSP-2), which exhibits a mean 78-fold increase in primary colon cancers compared to normal mucosa, was developed. Our cost-effective and noninvasive electrochemical immunosensor for CCSP-2 biomarker detection aims to facilitate early diagnosis and monitoring of CRC. The designed immunosensor features a functionalized gold electrode (Au) modified with cysteine-modified recombinant protein G (RPGCys) to immobilize the CCSP-2 antibody (Ab), and bovine serum albumin (BSA) used to prevent sensor surface fouling. Electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) were employed to analyze the electrochemical response to the binding of CCSP-2 antigen (Ag) and Ab. The changes in relative charge transfer resistance (ΔRct/Rcti) with varying concentrations of Ag were plotted and a calibration curve was established between ΔRct/Rcti and logarithm of Ag concentration to assess sensor’s sensitivity. The sensor demonstrated a linear response (R2 = 0.95) within the range of 10–100 ng/μL, plateauing after 100 ng/μL, with a detection limit of 0.71 ng/μL. Statistical analysis of specificity and selectivity studies showed significant differences in Ag detection compared to blank and nonspecific protein BSA, both with and without cell extracts. This immunosensor effectively detects the CRC biomarker CCSP-2 with high sensitivity and specificity. Integrating this sensor with other sensors for serum CRC biomarkers present a promising approach for developing diagnostic and prognostic tools for CRC.
Keywords: immunosensor, CCSP-2, colorectal cancer, electrochemical impedance spectroscopy, label-free
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide in terms of incidence and the second most common in terms of mortality, despite being one of the most treatable cancers.1,2 Early detection is crucial, as the 5-year survival rate for CRC patients is approximately 90% when diagnosed at an early stage, but it drops to 12.5% at advanced stages when the cancer has metastasized.3,4 Regular screening is essential to prevent late-stage deaths; however, mass screening is challenging due to the asymptomatic nature of early stage CRC or the presence of nonspecific symptoms (e.g., changes in bowel habits, abdominal discomfort, unexplained weight loss, and fatigue). Additionally, current CRC screening methods are either invasive, expensive, uncomfortable, or have poor specificity, leading to low patient compliance and screening failure.5−7
According to the American Cancer Society (ACS), CRC screening tests are categorized into visual/structural tests (colonoscopy, CT colonography, flexible sigmoidoscopy) and stool-based tests (fecal immunochemical test [FIT], high-sensitivity guaiac-based fecal occult blood test [gFOBT], and multitarget stool DNA test [MT-sDNA]).8 Despite their effectiveness, these tests have significant limitations. Colonoscopy, for instance, is invasive, carries risks of complications, requires bowel preparation, is resource-intensive, and is costly. FIT, the most common noninvasive test, has limitations such as false positives, lack of specificity, and discomfort.9
Blood-based biomarker tests are emerging as a promising alternative due to their noninvasive nature and higher public acceptance. Adler et al.10 reported that 97% of people who refuse colonoscopy accept a noninvasive test, and 83% prefer a blood test. A notable blood biomarker for CRC is the colon cancer secreted protein (CCSP-2), also known as VWA2 or AMACO. CCSP-2 is significantly upregulated in colon adenomas and cancers, with an average 78-fold increase in stages II, III, and IV CRC, and is absent in normal colon and other body tissues. CCSP-2 is a secreted extracellular matrix protein detectable in blood, making it a valuable tumor marker.11−13
Various detection tools, such as polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), immunohistochemistry (IHC), and flow cytometry, have been developed to detect and measure cancer biomarkers. PCR, though highly sensitive in identifying genetic mutations associated with cancer, is expensive, time-consuming, and requires invasive sample collection.14 ELISA is widely used for protein biomarker detection;15 however, its sensitivity may be insufficient for early stage CRC screening. RIA offers excellent sensitivity but involves radioactive materials, limiting its practicality for widespread use.16 While IHC provides valuable information on tumor biomarker expression, it necessitates invasive biopsies and is confined to large laboratories.17 Similarly, flow cytometry, with its capability to analyze multiple biomarkers, is highly complex and cost-prohibitive for routine screening.18 There is a pressing need for a more accessible, noninvasive, cost-effective tool to increase compliance with CRC screening. Biosensors, particularly electrochemical biosensors, offer potential as alternative tools for early detection due to their low cost, simplicity, and suitability for point-of-care (POC) applications. Electrochemical biosensors can be miniaturized and mass-fabricated, and they can be used at home or in a doctor’s office.19,20 Detection methods in electrochemical biosensors can be labeled or label-free, with label-free systems offering advantages such as shorter analysis times and simplicity.21 However, label free electrochemical biosensors for CRC diagnosis are not yet widely available,22 leaving room for further development and broader use in large-scale screening.
In this study, a simple label-free electrochemical immunosensor for detecting CCSP-2 biomarker was designed. This immunosensor utilizes the specific binding between an antigen and an antibody immobilized on a transducer surface, providing high selectivity and sensitivity.23−25 Gold electrodes were chosen as substrates due to their ability to be readily modified with thiolated molecules, facilitating antibody immobilization.26 RPGCys was used as a linker between the gold surface and Ab, forming a self-assembled monolayer (SAM) on the gold surface. Protein G binds specifically to the Fc fragments of antibodies, leaving the Fab regions available for antigen binding, thus ensuring higher orientation and simplicity in immobilization compared to other methods.27−29
To prevent nonspecific binding, BSA was used as a blocking agent. CV and EIS were employed for the characterization of the fabricated immunosensor and Ag detection, with EIS chosen as the primary detection method due to its enhanced sensitivity. The proposed design of this label-free impedimetric immunosensor (BSA/Ab/RPGCys/Au) for Ag detection holds promise as a simple, inexpensive, rapid, portable, and reliable sensing platform. This CCSP-2 immunosensor can potentially be integrated with other CRC biomarker immunosensors in future, significantly impacting mass screening and early detection of CRC.
Experimental Section
Materials and Equipment
Recombinant protein G cys (N-term) protein (RPGCys) was purchased from Novus Biologicals, CO. Anti-VWA2 antibody produced in rabbit (Ab) and PrEST antigen VWA2 (Ag), bovine serum albumin (BSA), potassium hexacyanoferrate(II) trihydrate (>99.95% trace metals basis), and potassium hexacyanoferrate(III) (99.98% trace metals basis) were purchased from Millipore Sigma. Additionally, potassium chloride, potassium phosphate monobasic, and sodium chloride were obtained from Millipore Sigma, while sodium phosphate dibasic anhydrous was purchased from Fisher Scientific. These chemicals were used to prepare phosphate-buffered saline (PBS) with a pH adjusted to approximately 7.4. Sulfuric acid (Optima grade), used for electrochemical cleaning of working electrodes, was also purchased from Fisher Scientific. The commercial Caco-2 cell line isolated from human colon tissue was obtained from American Type Culture Collection (ATCC).
For electrochemical experiments, a digital potentiostat/galvanostat (Metrohm Autolab) controlled with NOVA 2.1 software was employed. All electrochemical analyses were conducted in a three-electrode system comprising of a platinum counter electrode, a hydrogen reference electrode (RHE) (Gaskatel, Kassel, Deutschland), and gold working electrodes (Au) with a 3.0 mm diameter and 99.95% purity, purchased from BASi, West Lafayette, IN. For atomic force microscopy (AFM), MAXTEK quartz crystal polycrystalline gold crystal sensors, with a diameter of 14 mm, were procured from INFICON.
Cleaning and Characterization of Electrodes for Immunosensor
Prior to the immunosensor design, gold electrodes (Au) were mechanically polished on a microcloth (Buehler) using alumina slurries of 1, 3, and 0.05 μm (Buehler), each for 5 min. This was followed by washing and sonication in an ultrasonic bath with ultrapure water (>18.2 MΩ cm, Millipore Milli-Q) for 2 min to remove any alumina residues. The Au electrodes were then immersed in a solution of 50 mM KOH and 25% H2O2 for 10 min and thoroughly rinsed with Milli-Q water. Electrochemical cleaning was performed by cycling the potential between 0 to 1.7 V vs RHE in 0.5 M H2SO4 at scan rates of 300, 200, and 100 mV/s until a reproducible voltammogram was obtained shown in Supporting Information (Figure S2). Although the Ag/AgCl reference electrode is commonly used in many electrochemical experiments, an additional reduction peak always appeared in the CV of the Au electrode in 0.5 M H2SO4, likely due to chloride or silver ion leakage from the Ag/AgCl reference electrode.30 Switching to a reference electrode RHE resolved this issue, enabling stable and consistent results in the Au electrode voltammogram. The cleaned bare Au electrodes were characterized using CV and EIS, to ensure reproducible and minimal potential difference (ΔEp) in CV and charge transfer resistance (Rct) in EIS. CV and EIS data were recorded in an electrolyte consisting of 2 mM K3Fe(CN)6 and K4Fe(CN)6 in PBS buffer, with the CV potential cycled between 0.5 and 1.2 V starting at open circuit potential at a scan rate of 10 mV/s, and EIS conducted in the frequency range of 0.5 MHz to 0.1 Hz. The ferri/ferrocyanide ([(Fe(CN)6)3–/4–]) redox probe was used for surface characterization due to its high sensitivity to the blocking effects caused by any heterogeneous reaction on the working electrode surface.31
Immunosensor Design and Characterization
The clean bare Au electrodes were modified by drop-casting 15 μL of RPGCys (1 mg/mL), followed by incubation at 4 °C for 2 h to form a self-assembled monolayer (SAM) of thiolated recombinant protein G on the gold surface. This SAM served as a linker between the gold working electrode (WE) and the Ab. The RPGCys/Au surfaces were rinsed with PBS solution to remove any unbound thiolated protein G and dried with N2 gas. Subsequently, 15 μL of Ab (10 ng/μL) was drop-cast on RPGCys/Au and incubated for 1 h at 4 °C. The Ab/RPGCys/Au was rinsed with ultrapure water and dried with N2 to remove any unbound Ab. To prevent undesired adsorption on the immunosensor surface, the Ab/RPGCys/Au was blocked by drop-casting 0.1% bovine serum albumin (BSA) and incubated at 4 °C for 1 h. The prepared immunosensor (BSA/Ab/RPGCys/Au) was then washed multiple times with PBS buffer to remove any unbound residues, dried with N2, and stored in a dry condition at 4 °C.
Each modification step, especially the formation of the SAM of RPGCys and antibody immobilization was verified using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) in an electrolyte consisting of 2 mM K3Fe(CN)6 and K4Fe(CN)6 in PBS buffer, employing the same CV and EIS parameters used for characterizing the bare Au electrodes. The topography of the gold surface (Maxtek Au crystal) before and after immunosensor preparation was studied using atomic force microscopy (AFM) in contact mode with a NanoScope scanning probe microscope system (Digital Instruments, Inc.).
Analyte Preparation and Tests
The stock Ag (4800 ng/μL) was aliquoted and diluted to concentrations of 10, 30, 50, 100, 150, and 200 ng/μL with PBS buffer and stored at −20 °C until use. The growth and subculture of the caco-2 cell line followed the manufacturer’s protocol, detailed in the Supporting Information (Figure S1). Extract preparation from the Caco-2 cell line followed protocols from previous projects in our lab.32 The Caco-2 cell extracts were diluted 1:100 and used to prepare positive control (100 ng/μL Ag in cell extracts), negative control (100 ng/μL BSA in cell extracts), and blank (PBS solution in cell extracts).
The immunosensor (BSA/Ab/RPGCys/Au) was incubated with various concentrations of Ag and with samples of blank, positive, and negative controls at 4 °C for 1 h to evaluate the sensitivity, specificity, and selectivity of the sensor. Subsequently, all electrodes were washed with PBS to remove any loosely bound and unbound analytes from the immunosensor surface, dried with N2, and analyzed using EIS and CV. All measurements were performed with three WEs, and EIS data were collected three times for each WE to ensure reproducibility and to confirm the detection limit and conduct statistical error analysis. The Nyquist plots from the EIS experiments were fitted using the extended Randles equivalent circuit in NOVA software presented in Supporting Information (Figure S3), which consists of a double-layer capacitance (Cdl), solution resistance (Rs), Warburg impedance (W), and charge transfer resistance (Rct).33 The change in relative charge transfer resistance (ΔRct/Rcti) was calculated using eq 1
| 1 |
Apart from electrochemical measurements, the topography of the immunosensor surface (BSA/Ab/RPGCys/Au) after binding with Ag was also examined using AFM.
Results and Discussion
Design and Characterization Confirm the Successful Formation of Biosensing Probe
The schematic representation of the immunosensor design is shown in Figure 1a, illustrating each step of the functionalization of the Au working electrode (WE) to provide a clear depiction of the biosensing layers on the gold surface. CV data for each modification step of the WE are presented in Figure 1b, demonstrating the quality of the probe fabrication. There is a significant decrease in current density with each functionalization step of the WE, confirming the formation of the SAM of RPGCys on the Au surface, the immobilization of Ab, and the blocking with BSA. The increased thickness of the Au surface after each modification layer induced an insulating effect, which reduced the electron charge transfer permittivity of the redox couple [(Fe(CN)6)3–/4–] toward the WE surface and increased the separation between the oxidation and reduction peaks in CV.
Figure 1.
(a) Schematic illustration of the step-by-step immunosensor design. (b) Cyclic voltammetry (CV) of bare Au, RPGCys/Au, Ab/RPGCys/Au, and BSA/Ab/RPGCys/Au. (c) Electrochemical impedance spectroscopy (EIS) of bare Au, RPGCys/Au, Ab/RPGCys/Au and BSA/Ab/RPGCys/Au. (d) Randles equivalent circuit diagram.
The Nyquist plots from the EIS experiments, shown in Figure 1c, further validate the formation of the SAM, the immobilization of antibodies, and the surface blocking step. The Nyquist plot, which represents the real impedance (Z′) versus imaginary impedance (Z″), demonstrates the kinetically controlled high-frequency region determining the charge transfer resistance (Rct) and the diffusion-controlled low-frequency region determining the Warburg impedance (W).33,34 An increase in the charge transfer resistance, Rct (equivalent to diameter of the semicircles) are observed in each step of surface modification, as evidenced in the Nyquist plots in Figure 1c, confirming the successful formation and characterization of the biosensing probe. The EIS data, presented as a Nyquist plot, were fitted using a Randles equivalent circuit, as depicted in Figure 1d. CV and EIS data were recorded for each fabrication step of the immunosensor which showed consistent results in both experimental methods.
Analysis of Ag Detection Indicates the Successful Functionalization of the Bioreceptors
The immunosensor probe was tested for detecting Ag at varying concentrations, with both CV and EIS data collected to examine parameter changes during the interaction of Ag with the Ab-modified biosensor. While both CV and EIS results were consistent, EIS was chosen as the detection method, due to its higher sensitivity.
When the immunosensor probe was exposed to lower concentrations of Ag (10 ng/μL), the diameter of the semicircles (indicative of the charge transfer resistance, Rct) in the Nyquist plot of the EIS data was larger than that of the immunosensor probe, indicating a “signal-on” behavior (Figure 2b). Conversely, at higher concentrations (200 ng/μL) of Ag, the Nyquist plot of EIS showed a reduced semicircle diameter after the binding of Ag with the Ab on the immunosensor, indicating a “signal-off” behavior (Figure 2c).
Figure 2.
(a) Schematic illustration of the interaction between Ag and the immunosensor probe. (b, c) Nyquist plot of the EIS data for bare Au, BSA/Ab/RPGCys/Au, and the immunosensor probe with added (b) Ag (10 ng/μL) and (c) Ag (200 ng/μL).
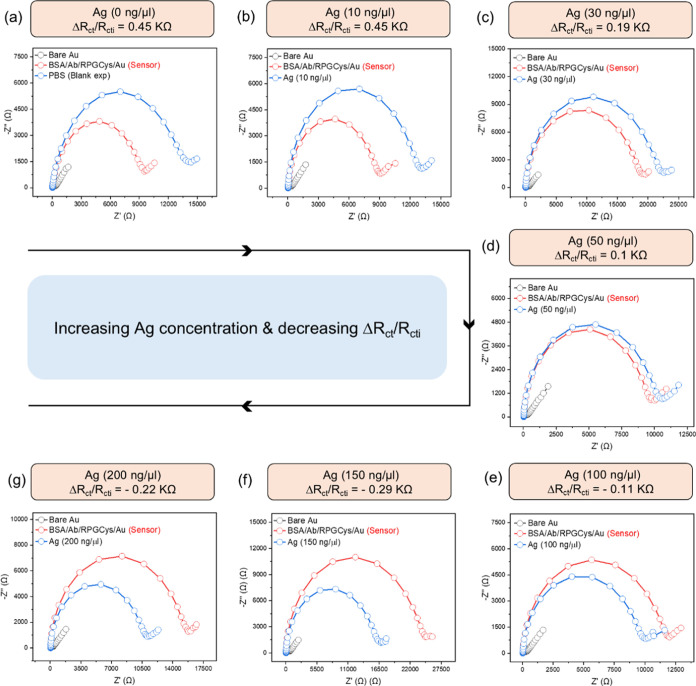
There is a decreasing trend in the diameter of the semicircle in the Nyquist plot as the concentrations of Ag are increased from 0 to 200 ng/μL (Figure 3). In the lower concentration range of Ag (10–50 ng/μL), there was significant hindrance to the charge transfer of the [(Fe(CN)6)3–/4–] redox couple on the surface of the immunosensor probe, which increased the Rct value after antigen–antibody interaction compared to the Rct value of the immunosensor probe. However, in the higher concentration range of Ag (100–200 ng/μL), the opposite behavior was observed, where the Rct value decreased after antibody–antigen interaction, due to an increased electron charge transfer rate of the redox couple used.
Figure 3.
(a–g) Nyquist plot of the EIS data for bare Au, BSA/Ab/RPGCys/Au, and the immunosensor probe in the presence of increasing concentrations of Ag in PBS (a) PBS (blank exp.), (b) Ag (10 ng/μL), (c) Ag (30 ng/μL), (d) Ag (50 ng/μL), (e) Ag (100 ng/μL), (f) Ag (150 ng/μL), (g) Ag (200 ng/μL). The corresponding relative charge transfer resistance ΔRct/Rcti is also shown.
This behavior suggests that as more antigens bind to the antibodies in the immunosensor, forming antibody–antigen complexes, the orientation of the assembled layers on the immunosensor changes, facilitating increased electron transfer rates of the redox couple [(Fe(CN)6)3–/4–]. Additionally, in the concentration range of 100 to 200 ng/μL, the changes in Rct values were not as significant as those observed in the lower concentration ranges (0 to 50 ng/μL), which may be attributed to sensor saturation.
In addition to EIS data, AFM images were taken for the bare Au surface (MAXTEK Au crystal), the immunosensor surface (BSA/Ab/RPGCys/Au), and for Ag (100 ng/μL) on the immunosensor probe which was shown in Supporting Information (Figure S4).
Calibration Curve Assessed the Reproducibility and Limit of Detection (LoD)
Figure 4a shows the plot of relative charge transfer resistance (ΔRct/Rcti) against Ag concentration. A linear response is observed between 0 and 100 ng/μL, beyond which the signal appears to plateau in the range of 100–200 ng/μL. A calibration curve was constructed in the linear range of 0–100 ng/μL by plotting ΔRct/Rcti against the logarithm of Ag concentration (Figure 4b). A strong linear correlation was found in this range, with an R2 value of 0.95, and the linear regression analysis yielded the equation y = −0.53395x + 0.97452, where y represents the changes in relative charge transfer resistance (ΔRct/Rcti) and x represents the logarithm of Ag concentration.
Figure 4.
(a) Plot of relative charge transfer resistance (ΔRct/Rcti) versus Ag concentrations, showing linearity from 0 to 100 ng/μL, followed by a plateau from 100 to 200 ng/μL. (b) Calibration curve of ΔRct/Rcti against logarithmic Ag concentrations, demonstrating a strong linear correlation (R2 = 0.95).
The lowest detection limit of the immunosensor was calculated using the standard methods for determining the Limit of Blank (LoB) and the Limit of Detection (LoD) as published by the Clinical and Laboratory Standards Institute (CLSI)35
| 2 |
| 3 |
For this immunosensor probe, the LoB defines the highest apparent analyte (Ag) concentration expected to be found when replicates of a sample containing no analyte (PBS buffer) are tested and was calculated using eq 2. The LoD was calculated using eq 3, where the LoD defines the lowest analyte (Ag) concentration likely to be reliably distinguished from the LoB and at which detection is feasible. The immunosensor for detecting Ag was found to have a LoB value of 0.48 ng/μL and an LoD value of 0.71 ng/μL.
Specificity and Selectivity of the Immunosensor Confirmed the Biosensor’s Capability to Detect CCSP-2 Biomarker
To analyze the specificity and selectivity of the immunosensor probe, two distinct experiments were conducted: one in the absence of cell extracts (to assess specificity) and another in the presence of cell extracts (to assess selectivity). In both cases, a significant difference in the ΔRct/Rcti value was observed between the positive control (Ag) and the blank (PBS buffer), while no significant difference was noted between the negative analyte (BSA) and the blank (PBS buffer), as determined by one-way ANOVA statistical analysis (Figure 5a,b). This primarily confirms the immunosensor’s specificity for detecting Ag over other nonspecific proteins. However, further studies with additional nonspecific proteins are needed to fully validate the sensor’s specificity.
Figure 5.
(a–c) Specificity and selectivity analysis of the sensor toward Ag. (a, b) One-way ANOVA analysis: (a) Target analytes only without cell extracts, (b) Target analytes with cell extracts. (c) Two-way ANOVA analysis. ns: P > 0.05 (no statistical difference); *: P ≤ 0.05; **: P ≤ 0.001.
The selectivity of the immunosensor probe toward Ag was evaluated using two-way ANOVA, with the results from Figure 5a,b combined and presented in Figure 5c. While there were some changes in ΔRct/Rcti values in the presence and absence of cell extracts, likely due to interference from different biomolecules present in the cell extracts, these changes were not statistically significant confirmed by two-way ANOVA analysis. Importantly, no significant differences were observed between the target analyte groups (Blank, BSA, Ag) and the target analytes with interferences (Caco-2 cell extracts), confirming the biosensor’s ability to selectively detect Ag even in the presence of interfering molecules.
Comparison of Electrochemical Biosensors for Ag Detection
Table 1 provides a detailed comparison of electrochemical biosensors for Ag detection, featuring reported approaches from the literature and the newly developed label-free impedimetric immunosensor. This comparison considers detection techniques, sensitivity, advantages, and limitations. Although further optimization could enhance the sensitivity and specificity of our immunosensor, its cost-effectiveness, simplicity, and rapid response underscore its potential as a scalable, point-of-care diagnostic tool, especially for accessible, real-time monitoring applications.
Table 1. Comparative Analysis of Electrochemical Biosensors for Ag Detection.
| biosensor | detection technique | limit of detection | advantages | disadvantages | refs |
|---|---|---|---|---|---|
| zeolite-modified gap-fingered dielectrodes | linear sweep voltammetry (LSV) | 3 nM | low detection limit. | complex surface modification steps. | (36) |
| nanomaterial (zeolite) enhances biomolecule immobilization. | requires validation with clinical samples for practical use. | ||||
| sustainable nanomaterial source (rice husk ash). | |||||
| E-FECS (Electric Field Effect Colorectal Sensor) | field-effect transistor (FET) | 10 ag/mL | high sensitivity. | complex and costly fabrication. | (37) |
| wide dynamic range (1 fg/mL–10 ng/mL). | uncertain performance across cancer stages. | ||||
| blood-based noninvasive detection. | high cost due to complex setup. | ||||
| high specificity (86.7%) for CRC diagnosis. | |||||
| label-free impedimetric immunosensor | electrochemical impedance spectroscopy (EIS) | 0.71 ng/μL | label-free detection. | moderate sensitivity. | this work |
| simple, scalable surface modifications for commercial production. | limited validation with human samples. | ||||
| cost-effective, rapid response. | |||||
| potential for low-cost, point-of-care deployment. |
Conclusions
A simple, cost-effective, and easy-to-fabricate label-free impedimetric immunosensor has been developed for the detection of CCSP-2, a potential biomarker for colorectal cancer (CRC). The immunosensor design involved functionalizing the gold (Au) surface with cysteine-modified recombinant protein G (RPGCys), immobilizing antibodies, and subsequently blocking with BSA to prevent fouling. The electrochemical impedance spectroscopy (EIS) technique was successfully applied to measure the CRC biomarker CCSP-2 by calculating the changes in relative charge transfer resistance (ΔRct/Rcti) before and after testing. A calibration plot between logarithm of Ag concentration and ΔRct/Rcti value demonstrated a strong linear correlation (R2 = 0.95) over a concentration range of 10–100 ng/μL, reaching a plateau after 100 ng/μL. The lower limit of detection (LoD) was calculated as 0.71 ng/μL. According to literature, CCSP-2 biomarker is overexpressed by approximately 78-fold in colon cancer cells compared to normal cells. However, there remains a gap in research regarding the clinical threshold concentration of Ag for distinguishing between cancer patients and healthy individuals. Further studies are needed to establish reliable clinical thresholds for Ag across different stages of colorectal cancer.
Statistical analysis of specificity and selectivity studies revealed significant specificity of the immunosensor toward Ag over other nonspecific molecule, both in the absence and presence of interfering molecules (e.g., Caco-2 cell extracts). The successful detection of Ag by this label-free impedimetric immunosensor, with good sensitivity and specificity, paves the way for noninvasive and cost-effective early stage diagnosis of CRC. Further research is required to evaluate the immunosensor’s performance for Ag detection in human serum.
Moreover, this immunosensor design for CCSP-2 biomarker can be integrated with other immunosensor designs targeting significant serological CRC biomarkers for simultaneous detection on a microchip platform. This approach could yield a reliable and robust immunosensor for early stage CRC screening, enhancing sensitivity, specificity, cost-effectiveness, portability, and noninvasiveness.
Acknowledgments
The authors are grateful to the financial support of the NSF-PFI grant number 2122627. C.R.C. acknowledges the STARs Award (2021) from the University of Texas System.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmeasuresciau.4c00073.
Brief description of recombinant protein G cys (N-term) protein (RPGCys) and colon cancer secreted protein-2 (CCSP-2); cell culture and extraction preparation protocol for Caco-2 cell line (Figure S1); cyclic voltammograms of cleaned gold electrode and current density calculation (Figure S2); Randles equivalent circuit analysis for EIS data fitting (Figure S3); AFM images of bare gold electrode, immunosensor probe, and Ag modified immunosensor probe (Figure S4) (PDF)
Author Contributions
CRediT: Ruma Paul conceptualization, formal analysis, investigation, methodology; Yermary Morales-Lozada conceptualization, methodology, writing - review & editing; Andrea R. Hernandez investigation; Sourav Roy formal analysis, writing - review & editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca-Cancer J. Clin. 2018, 68 (6), 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Frick C.; Rumgay H.; Vignat J.; Ginsburg O.; Nolte E.; Bray F.; Soerjomataram I. Quantitative Estimates of Preventable and Treatable Deaths from 36 Cancers Worldwide: A Population-Based Study. Lancet Global Health 2023, 11 (11), e1700–e1712. 10.1016/S2214-109X(23)00406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favoriti P.; Carbone G.; Greco M.; Pirozzi F.; Pirozzi R. E. M.; Corcione F. Worldwide Burden of Colorectal Cancer: A Review. Updates Surg. 2016, 68 (1), 7–11. 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- Cancer of the Colon and Rectum - Cancer Stat Facts; SEER. https://seer.cancer.gov/statfacts/html/colorect.html. (accessed September 16, 2024).
- Labianca R.; Beretta G. D.; Kildani B.; Milesi L.; Merlin F.; Mosconi S.; Pessi M. A.; Prochilo T.; Quadri A.; Gatta G.; de Braud F.; Wils J. Colon Cancer. Crit. Rev. Oncol. Hematol. 2010, 74 (2), 106–133. 10.1016/j.critrevonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Brenner H.; Kloor M.; Pox C. P. Colorectal Cancer. Lancet 2014, 383 (9927), 1490–1502. 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- Rex D. K.; Boland R. C.; Dominitz J. A.; Giardiello F. M.; Johnson D. A.; Kaltenbach T.; Levin T. R.; Lieberman D.; Robertson D. J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 112 (7), 1016–1030. 10.1038/ajg.2017.174. [DOI] [PubMed] [Google Scholar]
- Wolf A. M. D.; Fontham E. T. H.; Church T. R.; Flowers C. R.; Guerra C. E.; LaMonte S. J.; Etzioni R.; McKenna M. T.; Oeffinger K. C.; Shih Y.-C. T.; Walter L. C.; Andrews K. S.; Brawley O. W.; Brooks D.; Fedewa S. A.; Manassaram-Baptiste D.; Siegel R. L.; Wender R. C.; Smith R. A. Colorectal Cancer Screening for Average-Risk Adults: 2018 Guideline Update from the American Cancer Society. Ca-Cancer J. Clin. 2018, 68 (4), 250–281. 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- Tonini V.; Zanni M. Why Is Early Detection of Colon Cancer Still Not Possible in 2023?. World J. Gastroenterol. 2024, 30 (3), 211–224. 10.3748/wjg.v30.i3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A.; Geiger S.; Keil A.; Bias H.; Schatz P.; deVos T.; Dhein J.; Zimmermann M.; Tauber R.; Wiedenmann B. Improving Compliance to Colorectal Cancer Screening Using Blood and Stool Based Tests in Patients Refusing Screening Colonoscopy in Germany. BMC Gastroenterol. 2014, 14 (1), 183 10.1186/1471-230X-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin B.; Platzer P.; Fink S. P.; Reese L.; Nosrati A.; Willson J. K. V.; Wilson K.; Markowitz S. Colon Cancer Secreted Protein-2 (CCSP-2), a Novel Candidate Serological Marker of Colon Neoplasia. Oncogene 2005, 24 (4), 724–731. 10.1038/sj.onc.1208134. [DOI] [PubMed] [Google Scholar]
- González B.; Fece de la Cruz F.; Samuelsson J. K.; Alibés A.; Alonso S. Epigenetic and Transcriptional Dysregulation of VWA2 Associated with a MYC-Driven Oncogenic Program in Colorectal Cancer. Sci. Rep. 2018, 8 (1), 11097 10.1038/s41598-018-29378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer J. M.; Karlsen K. R.; Neiss W. F.; Paulsson M.; Wagener R. Expression of the AMACO (VWA2 Protein) Ortholog in Zebrafish. Gene Expression Patterns 2010, 10 (1), 53–59. 10.1016/j.gep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Aycan M.; Yildiz M.. Polymerase Chain Reaction Research; IntechOpen, 2024. [Google Scholar]
- Xiong Y.; Leng Y.; Li X.; Huang X.; Xiong Y. Emerging Strategies to Enhance the Sensitivity of Competitive ELISA for Detection of Chemical Contaminants in Food Samples. TrAC, Trends Anal. Chem. 2020, 126, 115861 10.1016/j.trac.2020.115861. [DOI] [Google Scholar]
- Carvalho M. S. N.; de Luna Costa A. S.; Santana A. V. A.; de Luna Batista R. S..; de Mesquita Neto F. P.; de Lima J. C.; Cabral A. B. The Nobel Prize in Physiology 1977: A Literature Review of the Radioimmunoassay Technique. Res. Soc. Dev. 2023, 12 (6), e14712642090 10.33448/rsd-v12i6.42090. [DOI] [Google Scholar]
- Magaki S.; Hojat S. A.; Wei B.; So A.; Yong W. H.. An Introduction to the Performance of Immunohistochemistry. In Biobanking: Methods and Protocols; Yong W. H., Ed.; Springer: New York, NY, 2019; Vol. 1897, pp 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell E. A.; Ernst D. N.; Hingorani R. Multiparameter Flow Cytometry: Advances in High Resolution Analysis. Immune Network 2013, 13 (2), 43–54. 10.4110/in.2013.13.2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F.; Zhou Z.; Zhou H. S. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167 (3), 037525 10.1149/2.0252003JES. [DOI] [Google Scholar]
- Wu J.; Liu H.; Chen W.; Ma B.; Ju H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1 (5), 346–360. 10.1038/s44222-023-00032-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanko V.; Kuralay F. Label-Free Electrochemical Biosensor Platforms for Cancer Diagnosis: Recent Achievements and Challenges. Biosensors 2023, 13 (3), 333 10.3390/bios13030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colorectal Cancer | Labcorp Oncology. https://oncology.labcorp.com/colorectal-cancer. (accessed 2024–10–21).
- Mollarasouli F.; Kurbanoglu S.; Ozkan S. A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9 (3), 86 10.3390/bios9030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikkaveeraiah B. V.; Bhirde A. A.; Morgan N. Y.; Eden H. S.; Chen X. Electrochemical Immunosensors for Detection of Cancer Protein Biomarkers. ACS Nano 2012, 6 (8), 6546–6561. 10.1021/nn3023969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzanfar S.; Alam F.; Pala N.; Wang C. Review—A Review of Electrochemical Aptasensors for Label-Free Cancer Diagnosis. J. Electrochem. Soc. 2020, 167 (6), 067511 10.1149/1945-7111/ab7f20. [DOI] [Google Scholar]
- Zamani M.; M Klapperich C.; L Furst A. Recent Advances in Gold Electrode Fabrication for Low-Resource Setting Biosensing. Lab Chip 2023, 23 (5), 1410–1419. 10.1039/D2LC00552B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer-Eriksson A. E.; Kleywegt G. J.; Uhlén M.; Jones T. A. Crystal Structure of the C2 Fragment of Streptococcal Protein G in Complex with the Fc Domain of Human IgG. Structure 1995, 3 (3), 265–278. 10.1016/S0969-2126(01)00157-5. [DOI] [PubMed] [Google Scholar]
- Kim H.; Kang D.-Y.; Goh H.-J.; Oh B.-K.; Singh R. P.; Oh S.-M.; Choi J.-W. Analysis of Direct Immobilized Recombinant Protein G on a Gold Surface. Ultramicroscopy 2008, 108 (10), 1152–1156. 10.1016/j.ultramic.2008.04.049. [DOI] [PubMed] [Google Scholar]
- Fowler J. M.; Stuart M. C.; Wong D. K. Y. Self-Assembled Layer of Thiolated Protein G as an Immunosensor Scaffold. Anal. Chem. 2007, 79 (1), 350–354. 10.1021/ac061175f. [DOI] [PubMed] [Google Scholar]
- Walker N. L.; Dick J. E. Leakless, Bipolar Reference Electrodes: Fabrication, Performance, and Miniaturization. Anal. Chem. 2021, 93 (29), 10065–10074. 10.1021/acs.analchem.1c00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J. N. Y.; Pimentel G. J. C.; Poker J. A.; Merces L.; Paschoalino W. J.; Vieira L. C. S.; Castro A. C. H.; Alves W. A.; Ayres L. B.; Kubota L. T.; Santhiago M.; Garcia C. D.; Piazzetta M. H. O.; Gobbi A. L.; Shimizu F. M.; Lima R. S. Single-Response Duplexing of Electrochemical Label-Free Biosensor from the Same Tag. Adv. Healthcare Mater. 2024, 13 (11), 2303509 10.1002/adhm.202303509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Ayala R.; López-Nieves M.; Berlingeri E. S. C.; Cabrera C. R.; Cunci L.; González C. I.; Escobar P. F. Test Strip Platform Spin-Off for Telomerase Activity Detection: Development of an Electrochemical Biosensor. ACS Omega 2022, 7 (11), 9964–9972. 10.1021/acsomega.2c00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randviir E. P.; E Banks C. Electrochemical Impedance Spectroscopy: An Overview of Bioanalytical Applications. Anal. Methods 2013, 5 (5), 1098–1115. 10.1039/C3AY26476A. [DOI] [PubMed] [Google Scholar]
- Lazanas A. C.; Prodromidis M. I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3 (3), 162–193. 10.1021/acsmeasuresciau.2c00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster D. A.; Pry T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29 (1), S49–S52. [PMC free article] [PubMed] [Google Scholar]
- Gao M.; Sun Y.; Wang Q.; Ma S.; Guo X.; Zhou L.; Chen Y.; Marimuthu K.; Gopinath S. C. B. Nanosensing Colon Cancer Biomarker on Zeolite-Modified Gap-Fingered Dielectrodes. Biotechnol. Appl. Biochem. 2022, 69 (5), 1885–1892. 10.1002/bab.2254. [DOI] [PubMed] [Google Scholar]
- Jeun M.; Lee H. J.; Park S.; Do E.; Choi J.; Sung Y.-N.; Hong S.-M.; Kim S.-Y.; Kim D.-H.; Kang J. Y.; Son H.-N.; Joo J.; Song E. M.; Hwang S. W.; Park S. H.; Yang D.-H.; Ye B. D.; Byeon J.-S.; Choe J.; Yang S.-K.; Moinova H.; Markowitz S. D.; Lee K. H.; Myung S.-J. A Novel Blood-Based Colorectal Cancer Diagnostic Technology Using Electrical Detection of Colon Cancer Secreted Protein-2. Adv. Sci. 2019, 6 (11), 1802115 10.1002/advs.201802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.