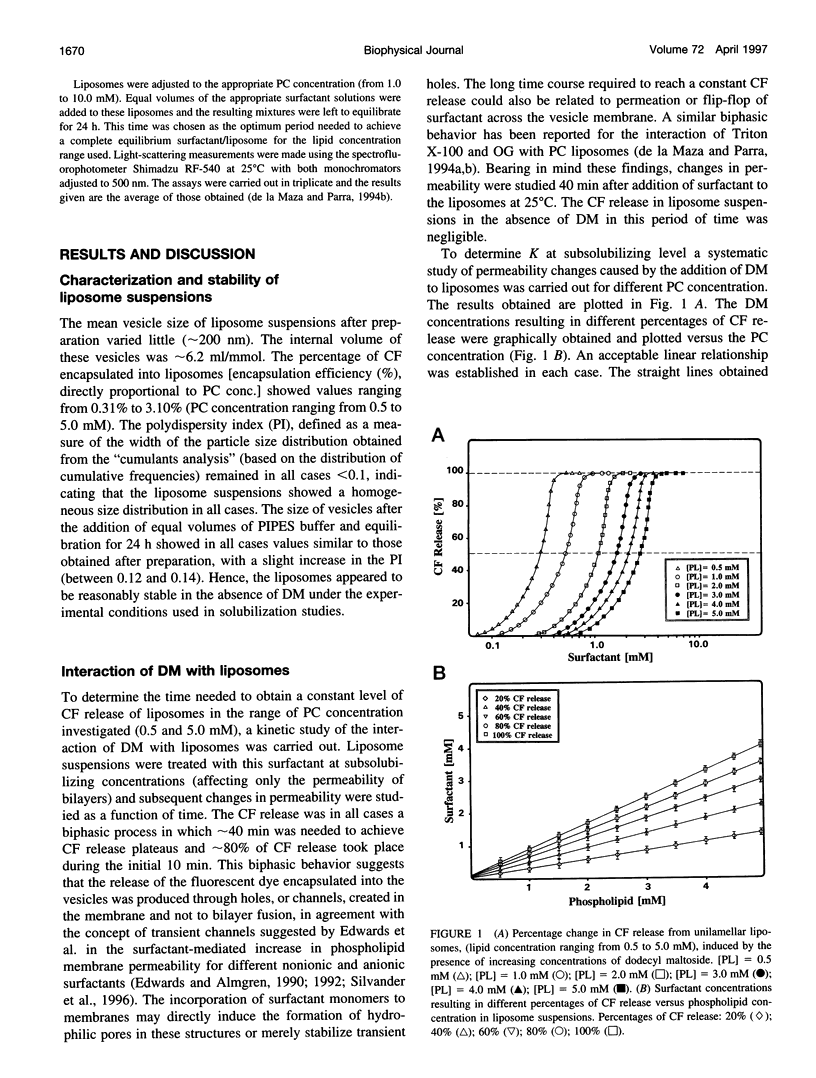
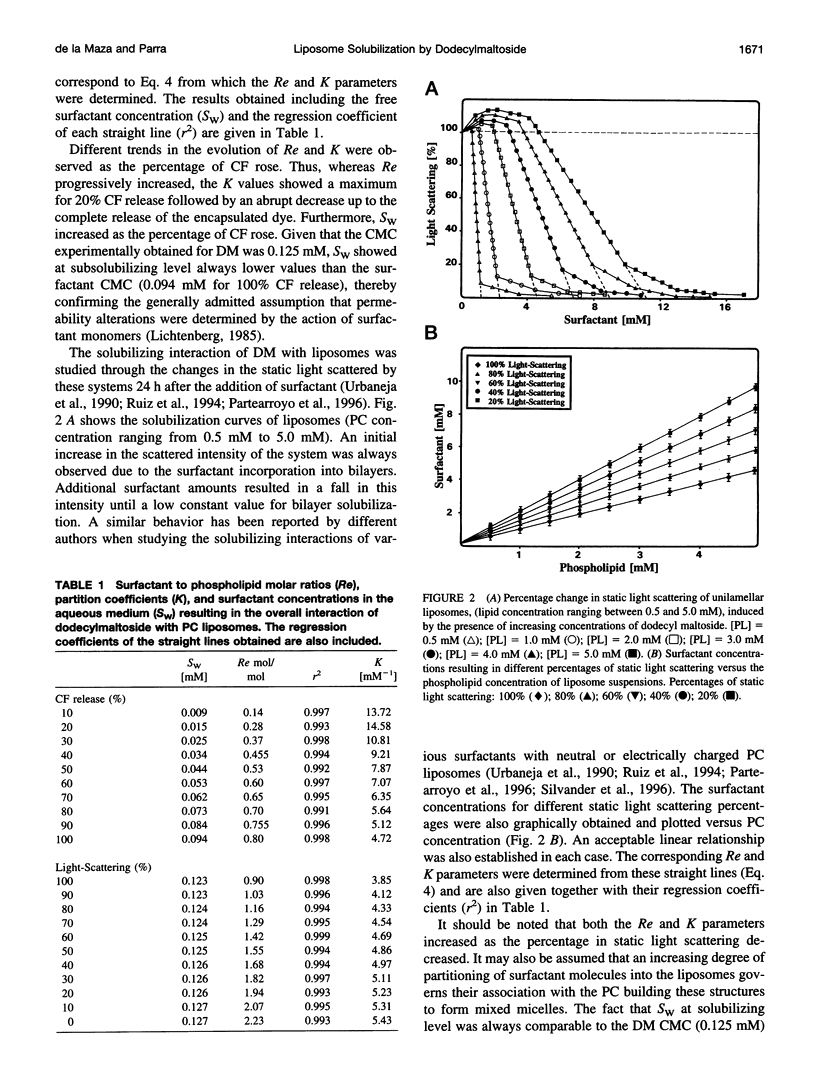
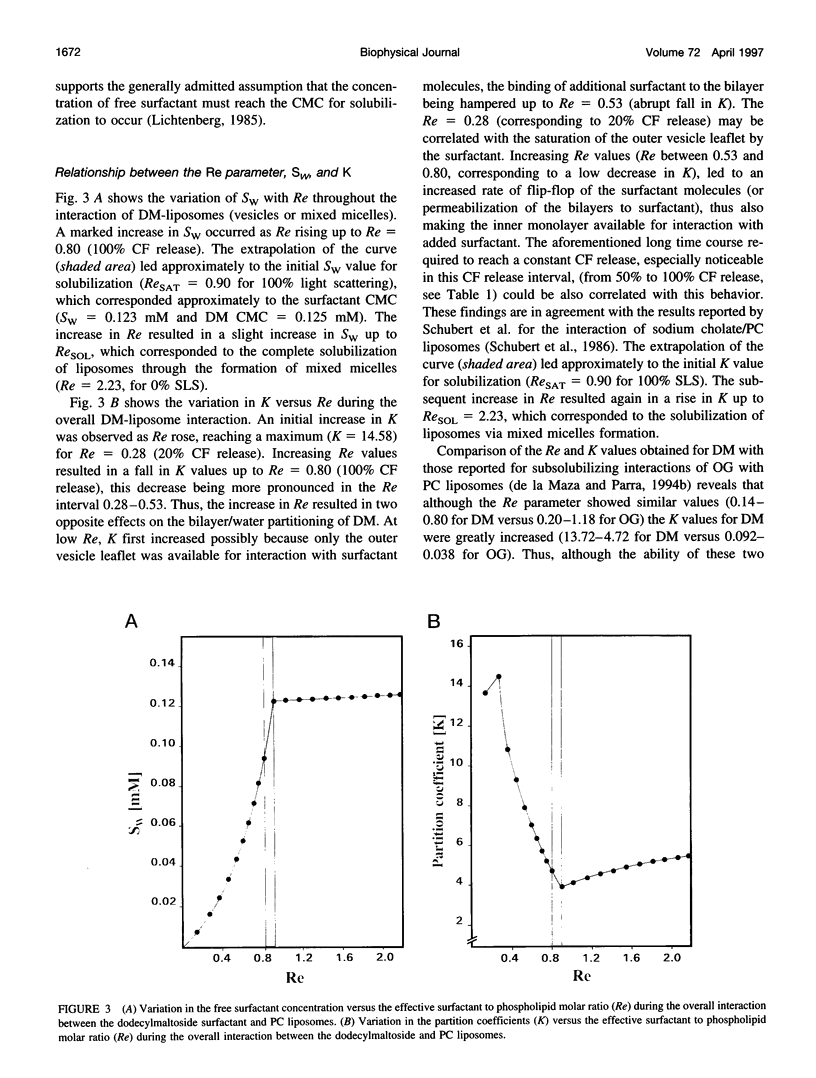
Abstract
The interaction of the nonionic surfactant dodecylmaltoside (DM) with phosphatidylcholine liposomes was investigated. Permeability alterations were detected as a change in 5(6)-carboxyfluorescein released from the interior of vesicles and bilayer solubilization as a decrease in the static light scattered by liposome suspensions. This surfactant showed higher capacity to saturate and solubilize PC liposomes and greater affinity with these structures than those reported for the octyl glucoside. At subsolubilizing level an initial maximum in the bilayer/water partitioning (K) followed by an abrupt decrease of this parameter occurred as the effective molar ratio of surfactant to phospholipid in bilayers (Re) rose. However, at solubilizing level a direct dependence was established between both parameters. A direct correlation took place in the initial interaction steps (Re up to 0.28) between the growth of vesicles, their fluidity, and Re. A similar direct dependence was established during solubilization (Re range from 0.9 to 1.7) between the decrease in both the surfactant-PC aggregate size, the light scattering of the system, and Re (composition of aggregates). The fact that the free DM concentration at subsolubilizing and solubilizing levels showed values lower than and similar to its critical micelle concentration indicates that permeability alterations and solubilization were determined, respectively, by the action of surfactant monomer and by the formation of mixed micelles.
Full text
PDF

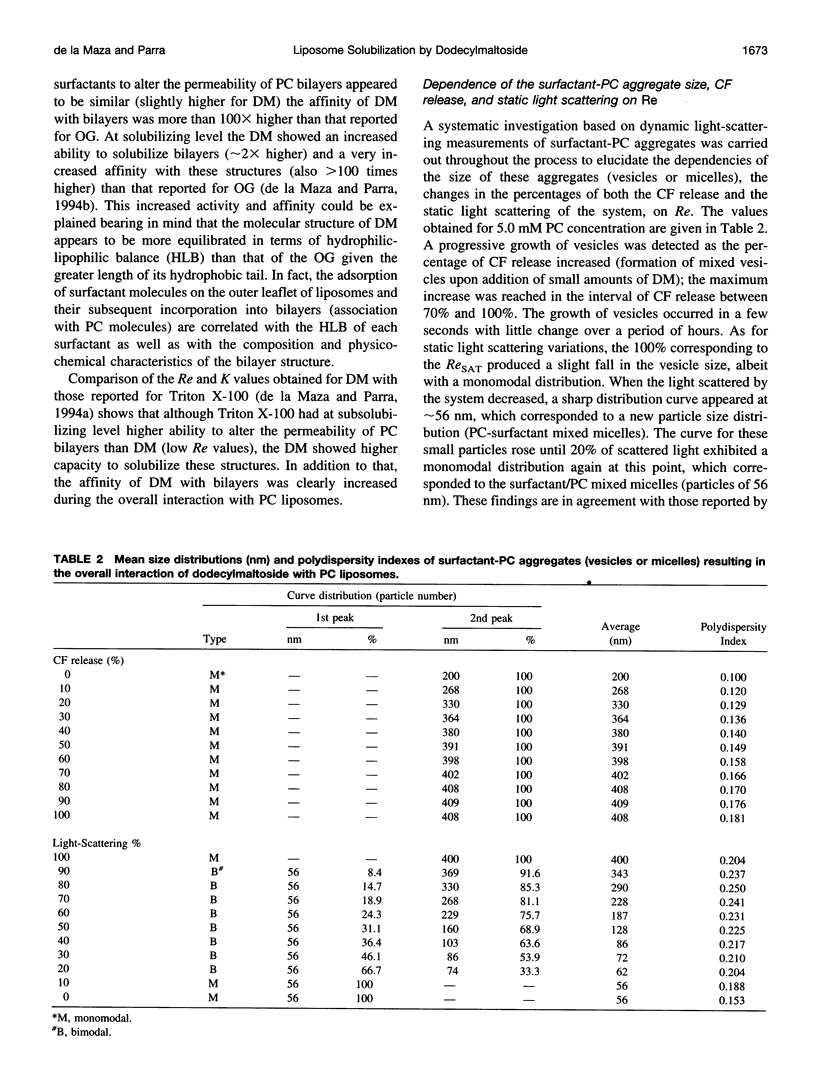
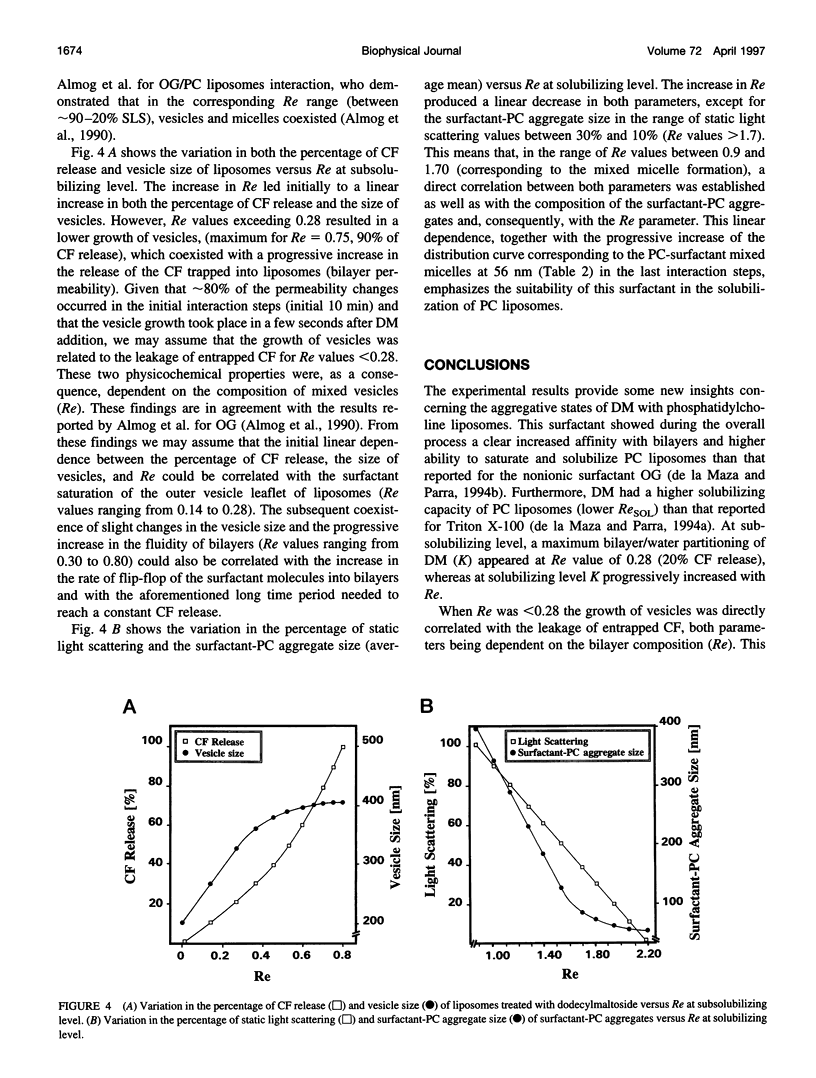

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almog S., Litman B. J., Wimley W., Cohen J., Wachtel E. J., Barenholz Y., Ben-Shaul A., Lichtenberg D. States of aggregation and phase transformations in mixtures of phosphatidylcholine and octyl glucoside. Biochemistry. 1990 May 15;29(19):4582–4592. doi: 10.1021/bi00471a012. [DOI] [PubMed] [Google Scholar]
- Begoña Ruiz M., Prado A., Goñi F. M., Alonso A. An assessment of the biochemical applications of the non-ionic surfactant Hecameg. Biochim Biophys Acta. 1994 Aug 3;1193(2):301–306. doi: 10.1016/0005-2736(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Bolli R., Nałecz K. A., Azzi A. The aggregation state of bovine heart cytochrome c oxidase and its kinetics in monomeric and dimeric form. Arch Biochem Biophys. 1985 Jul;240(1):102–116. doi: 10.1016/0003-9861(85)90012-8. [DOI] [PubMed] [Google Scholar]
- De la Maza A., Parra J. L. Vesicle-micelle structural transition of phosphatidylcholine bilayers and Triton X-100. Biochem J. 1994 Nov 1;303(Pt 3):907–914. doi: 10.1042/bj3030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry C. J., Sudan H. L., Abutidze K., Mellor I. R., Barnard E. A., Usherwood P. N. Reconstitution of glutamate receptor proteins purified from Xenopus central nervous system into artificial bilayers. Mol Pharmacol. 1993 Jul;44(1):142–152. [PubMed] [Google Scholar]
- Kragh-Hansen U., le Maire M., Nöel J. P., Gulik-Krzywicki T., Møller J. V. Transitional steps in the solubilization of protein-containing membranes and liposomes by nonionic detergent. Biochemistry. 1993 Feb 16;32(6):1648–1656. doi: 10.1021/bi00057a032. [DOI] [PubMed] [Google Scholar]
- Levy D., Gulik A., Seigneuret M., Rigaud J. L. Phospholipid vesicle solubilization and reconstitution by detergents. Symmetrical analysis of the two processes using octaethylene glycol mono-n-dodecyl ether. Biochemistry. 1990 Oct 9;29(40):9480–9488. doi: 10.1021/bi00492a022. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim Biophys Acta. 1985 Dec 19;821(3):470–478. doi: 10.1016/0005-2736(85)90052-5. [DOI] [PubMed] [Google Scholar]
- Lummis S. C., Martin I. L. Solubilization, purification, and functional reconstitution of 5-hydroxytryptamine3 receptors from N1E-115 neuroblastoma cells. Mol Pharmacol. 1992 Jan;41(1):18–23. [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta. 1986 Jun 13;858(1):161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Schubert R., Beyer K., Wolburg H., Schmidt K. H. Structural changes in membranes of large unilamellar vesicles after binding of sodium cholate. Biochemistry. 1986 Sep 9;25(18):5263–5269. doi: 10.1021/bi00366a042. [DOI] [PubMed] [Google Scholar]
- Suarez M. D., Revzin A., Narlock R., Kempner E. S., Thompson D. A., Ferguson-Miller S. The functional and physical form of mammalian cytochrome c oxidase determined by gel filtration, radiation inactivation, and sedimentation equilibrium analysis. J Biol Chem. 1984 Nov 25;259(22):13791–13799. [PubMed] [Google Scholar]
- Urbaneja M. A., Alonso A., Gonzalez-Mañas J. M., Goñi F. M., Partearroyo M. A., Tribout M., Paredes S. Detergent solubilization of phospholipid vesicle. Effect of electric charge. Biochem J. 1990 Sep 1;270(2):305–308. doi: 10.1042/bj2700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A., Dencher N. A., Gräber P. Co-reconstitution of plasma membrane H(+)-ATPase from yeast and bacteriorhodopsin into liposomes. ATP hydrolysis as a function of external and internal pH. Eur J Biochem. 1993 Jun 1;214(2):563–568. doi: 10.1111/j.1432-1033.1993.tb17954.x. [DOI] [PubMed] [Google Scholar]
- de Foresta B., Henao F., Champeil P. Kinetic characterization of the perturbation by dodecylmaltoside of sarcoplasmic reticulum Ca(2+)-ATPase. Eur J Biochem. 1992 Nov 1;209(3):1023–1034. doi: 10.1111/j.1432-1033.1992.tb17378.x. [DOI] [PubMed] [Google Scholar]
- de la Maza A., Parra J. L. Structural phase transitions involved in the interaction of phospholipid bilayers with octyl glucoside. Eur J Biochem. 1994 Dec 15;226(3):1029–1038. doi: 10.1111/j.1432-1033.1994.t01-1-01029.x. [DOI] [PubMed] [Google Scholar]