Abstract
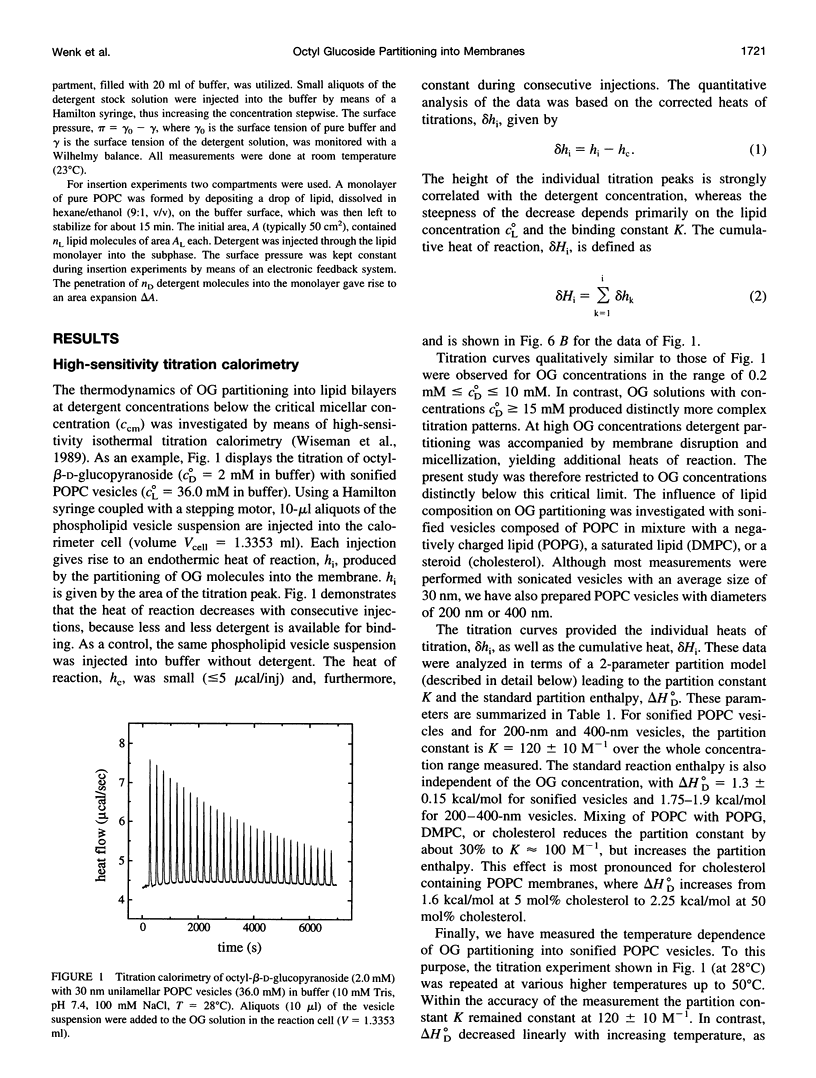
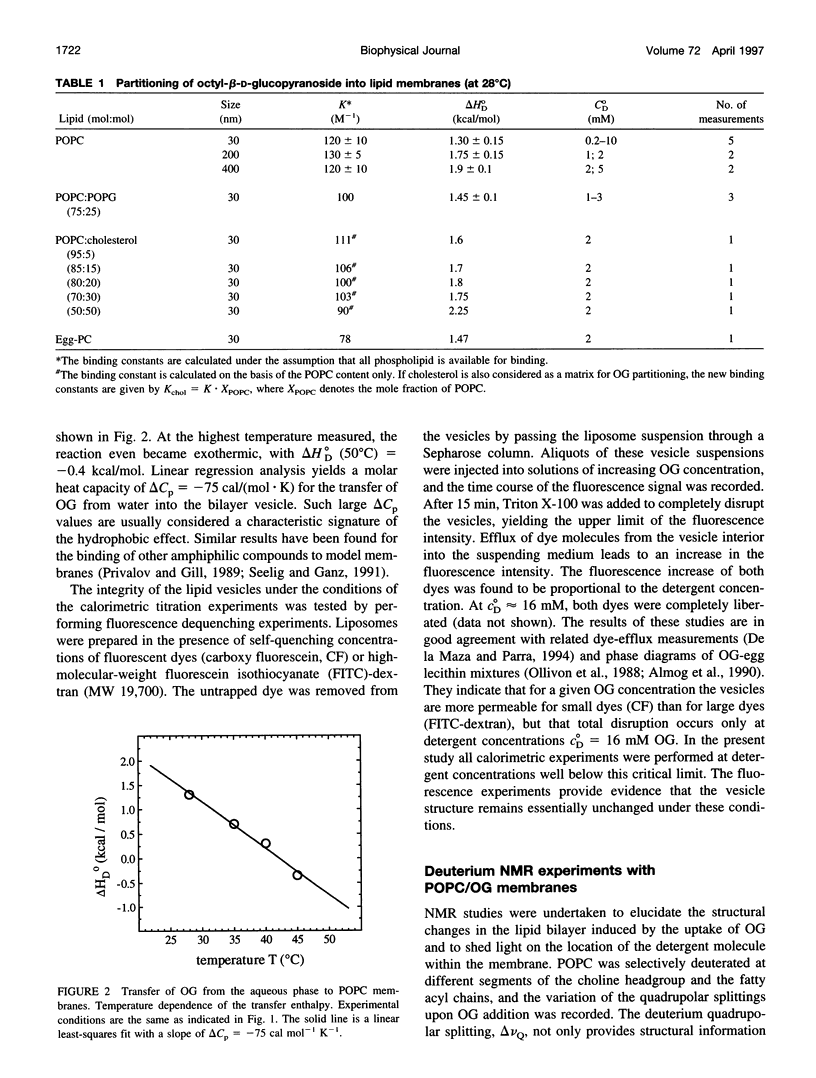
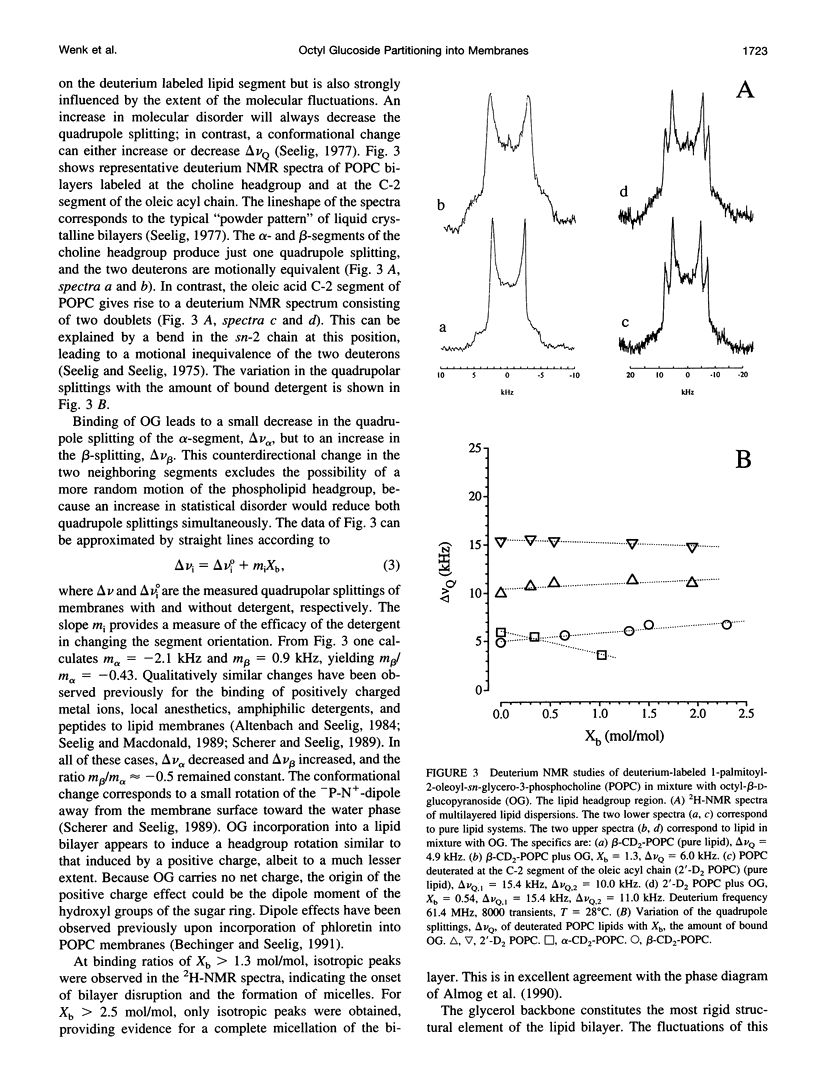
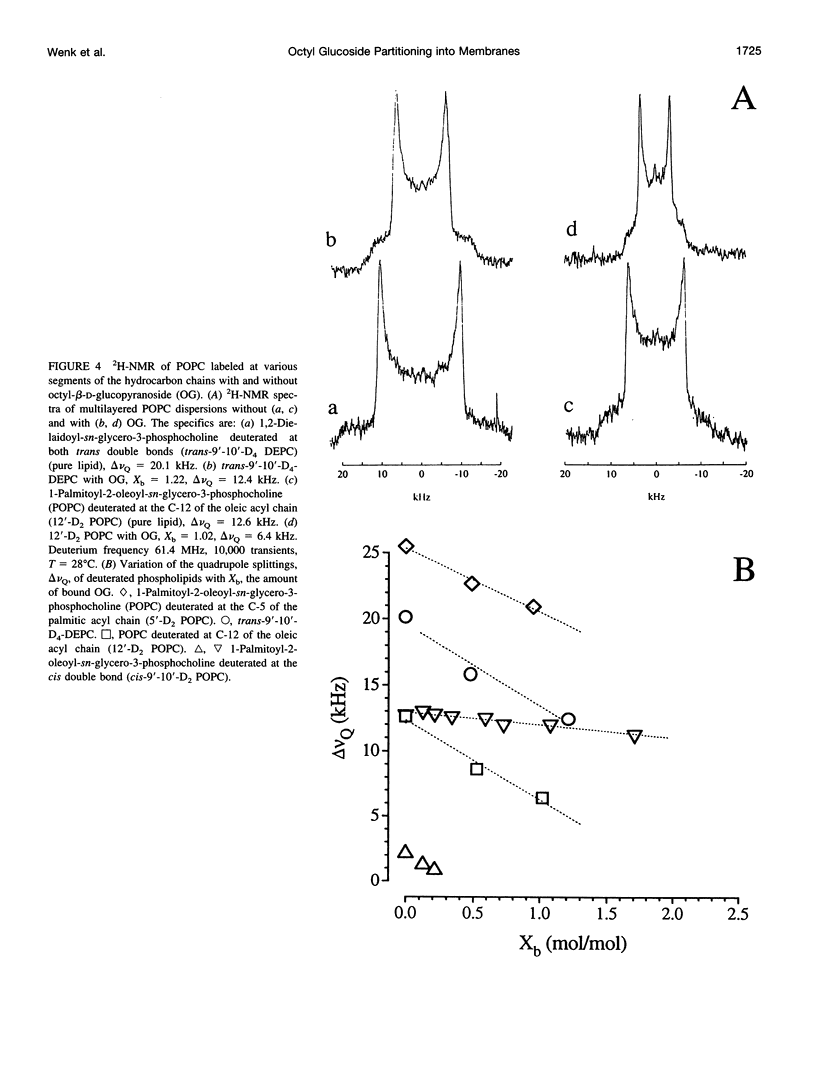
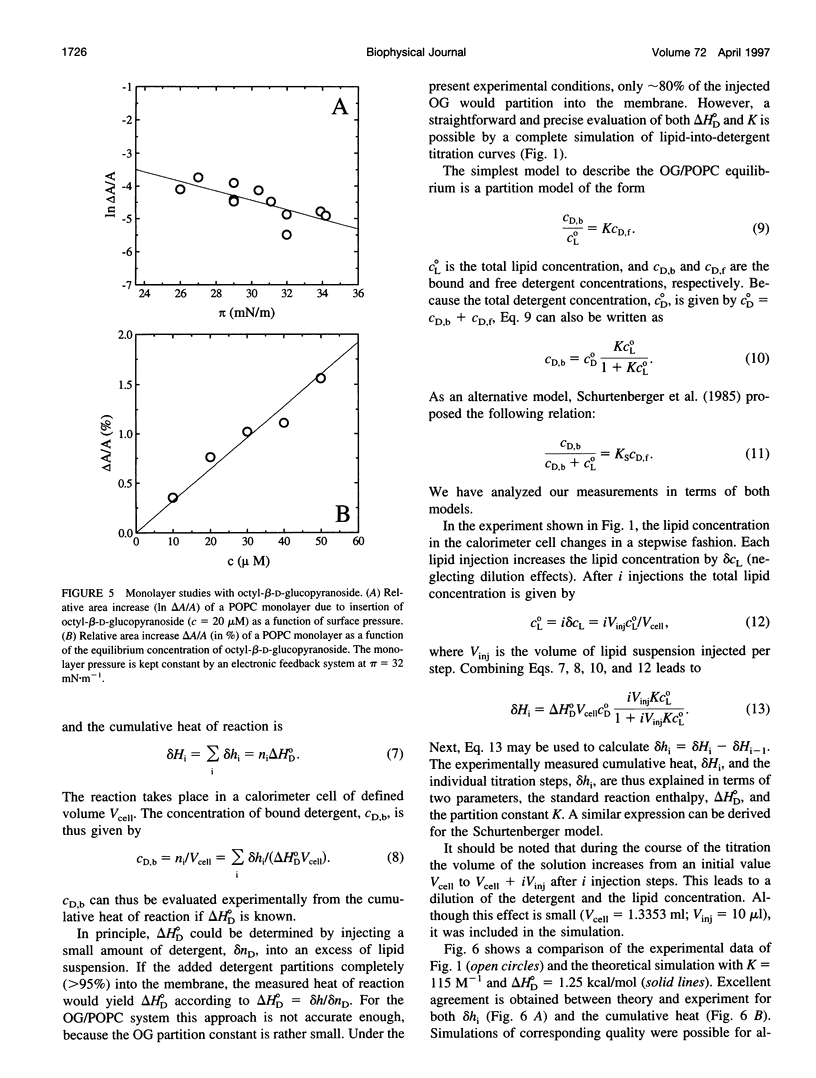
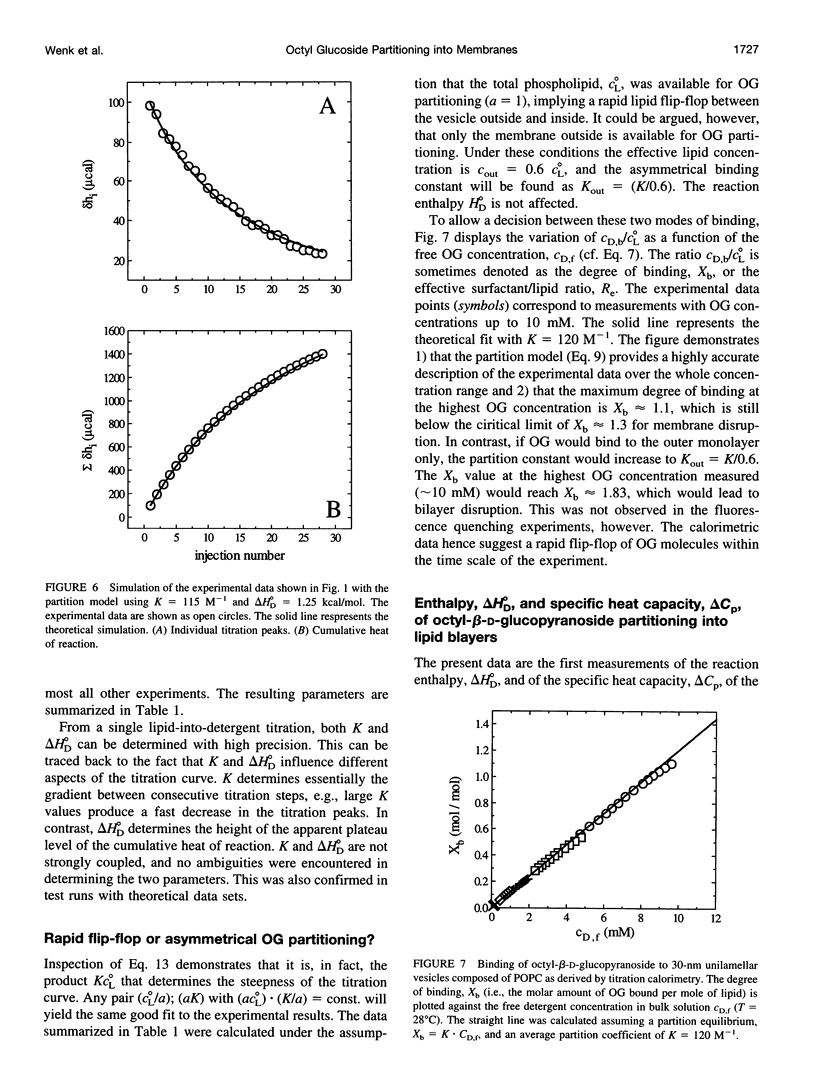
The interaction of the nonionic detergent octyl-beta-D-glucopyranoside (OG) with lipid bilayers was studied with high-sensitivity isothermal titration calorimetry (ITC) and solid-state 2H-NMR spectroscopy. The transfer of OG from the aqueous phase to lipid bilayers composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) can be investigated by employing detergent at concentrations below the critical micellar concentration; it can be defined by a surface partition equilibrium with a partition coefficient of K = 120 +/- 10 M-1, a molar binding enthalpy of delta H degrees D = 1.3 +/- 0.15 kcal/mol, and a free energy of binding of delta G degrees D = -5.2 kcal/mol. The heat of transfer is temperature dependent, with a molar heat capacity of delta CP = -75 cal K-1 mol-1. The large heat capacity and the near-zero delta H are typical for a hydrophobic binding equilibrium. The partition constant K decreased to approximately 100 M-1 for POPC membranes mixed with either negatively charged lipids or cholesterol, but was independent of membrane curvature. In contrast, a much larger variation was observed in the partition enthalpy. delta H degrees D increased by about 50% for large vesicles and by 75% for membranes containing 50 mol% cholesterol. Structural changes in the lipid bilayer were investigated with solid-state 2H-NMR. POPC was selectively deuterated at the headgroup segments and at different positions of the fatty acyl chains, and the measurement of the quadrupolar splittings provided information on the conformation and the order of the bilayer membrane. Addition of OG had almost no influence on the lipid headgroup region, even at concentrations close to bilayer disruption. In contrast, the fluctuations of fatty acyl chain segments located in the inner part of the bilayer increased strongly with increasing OG concentration. The 2H-NMR results demonstrate that the headgroup region is the most stable structural element of the lipid membrane, remaining intact until the disordering of the chains reaches a critical limit. The perturbing effect of OG is thus different from that of another nonionic detergent, octaethyleneglycol mono-n-dodecylether (C12E8), which produces a general disordering at all levels of the lipid bilayer. The OG-POPC interaction was also investigated with POPC monolayers, using a Langmuir trough. In the absence of lipid, the measurement of the Gibbs adsorption isotherm for pure OG solutions yielded an OG surface area of AS = 51 +/- 3 A2. On the other hand, the insertion area AI of OG in a POPC monolayer was determined by a monolayer expansion technique as AI = 58 +/- 10 A2. The similar area requirements with AS approximately AI indicate an almost complete insertion of OG into the lipid monolayer. The OG partition constant for a POPC monolayer at 32 mN/m was Kp approximately 320 M-1 and thus was larger than that for a POPC bilayer.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almog S., Litman B. J., Wimley W., Cohen J., Wachtel E. J., Barenholz Y., Ben-Shaul A., Lichtenberg D. States of aggregation and phase transformations in mixtures of phosphatidylcholine and octyl glucoside. Biochemistry. 1990 May 15;29(19):4582–4592. doi: 10.1021/bi00471a012. [DOI] [PubMed] [Google Scholar]
- Altenbach C., Seelig J. Ca2+ binding to phosphatidylcholine bilayers as studied by deuterium magnetic resonance. Evidence for the formation of a Ca2+ complex with two phospholipid molecules. Biochemistry. 1984 Aug 14;23(17):3913–3920. doi: 10.1021/bi00312a019. [DOI] [PubMed] [Google Scholar]
- Bechinger B., Seelig J. Interaction of electric dipoles with phospholipid head groups. A 2H and 31P NMR study of phloretin and phloretin analogues in phosphatidylcholine membranes. Biochemistry. 1991 Apr 23;30(16):3923–3929. doi: 10.1021/bi00230a017. [DOI] [PubMed] [Google Scholar]
- Blume A. A comparative study of the phase transitions of phospholipid bilayers and monolayers. Biochim Biophys Acta. 1979 Oct 19;557(1):32–44. doi: 10.1016/0005-2736(79)90087-7. [DOI] [PubMed] [Google Scholar]
- Boguslavsky V., Rebecchi M., Morris A. J., Jhon D. Y., Rhee S. G., McLaughlin S. Effect of monolayer surface pressure on the activities of phosphoinositide-specific phospholipase C-beta 1, -gamma 1, and -delta 1. Biochemistry. 1994 Mar 15;33(10):3032–3037. doi: 10.1021/bi00176a036. [DOI] [PubMed] [Google Scholar]
- Eidelman O., Blumenthal R., Walter A. Composition of octyl glucoside-phosphatidylcholine mixed micelles. Biochemistry. 1988 Apr 19;27(8):2839–2846. doi: 10.1021/bi00408a027. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Williams M. A., Tinoco J. Surface areas of 1-palmitoyl phosphatidylcholines and their interactions with cholesterol. Biochem J. 1987 Jul 15;245(2):455–462. doi: 10.1042/bj2450455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. L., Schmidt C. F., Lichtenberg D., Litman B. J., Albert A. D. Solubilization of phosphatidylcholine bilayers by octyl glucoside. Biochemistry. 1982 Sep 14;21(19):4576–4582. doi: 10.1021/bi00262a010. [DOI] [PubMed] [Google Scholar]
- Lasch J. Interaction of detergents with lipid vesicles. Biochim Biophys Acta. 1995 Jul 17;1241(2):269–292. doi: 10.1016/0304-4157(95)00010-o. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim Biophys Acta. 1985 Dec 19;821(3):470–478. doi: 10.1016/0005-2736(85)90052-5. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D., Robson R. J., Dennis E. A. Solubilization of phospholipids by detergents. Structural and kinetic aspects. Biochim Biophys Acta. 1983 May 24;737(2):285–304. doi: 10.1016/0304-4157(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta. 1986 Jun 13;858(1):161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- Mimms L. T., Zampighi G., Nozaki Y., Tanford C., Reynolds J. A. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981 Feb 17;20(4):833–840. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- Ollivon M., Eidelman O., Blumenthal R., Walter A. Micelle-vesicle transition of egg phosphatidylcholine and octyl glucoside. Biochemistry. 1988 Mar 8;27(5):1695–1703. doi: 10.1021/bi00405a047. [DOI] [PubMed] [Google Scholar]
- Otten D., Löbbecke L., Beyer K. Stages of the bilayer-micelle transition in the system phosphatidylcholine-C12E8 as studied by deuterium- and phosphorous-NMR, light scattering, and calorimetry. Biophys J. 1995 Feb;68(2):584–597. doi: 10.1016/S0006-3495(95)80220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitzsch R. M., McLaughlin S. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 1993 Oct 5;32(39):10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- Racker E. Reconstitution of membrane processes. Methods Enzymol. 1979;55:699–711. doi: 10.1016/0076-6879(79)55078-2. [DOI] [PubMed] [Google Scholar]
- Richieri G. V., Ogata R. T., Kleinfeld A. M. Thermodynamics of fatty acid binding to fatty acid-binding proteins and fatty acid partition between water and membranes measured using the fluorescent probe ADIFAB. J Biol Chem. 1995 Jun 23;270(25):15076–15084. doi: 10.1074/jbc.270.25.15076. [DOI] [PubMed] [Google Scholar]
- Scherer P. G., Seelig J. Electric charge effects on phospholipid headgroups. Phosphatidylcholine in mixtures with cationic and anionic amphiphiles. Biochemistry. 1989 Sep 19;28(19):7720–7728. doi: 10.1021/bi00445a030. [DOI] [PubMed] [Google Scholar]
- Schindler H., Seelig J. Deuterium order parameters in relation to thermodynamic properties of a phospholiped bilayer. A statistical mechanical interpretation. Biochemistry. 1975 Jun 3;14(11):2283–2287. doi: 10.1021/bi00682a001. [DOI] [PubMed] [Google Scholar]
- Schurtenberger P., Mazer N., Waldvogel S., Känzig W. Preparation of monodisperse vesicles with variable size by dilution of mixed micellar solutions of bile salt and phosphatidylcholine. Biochim Biophys Acta. 1984 Aug 8;775(1):111–114. doi: 10.1016/0005-2736(84)90241-4. [DOI] [PubMed] [Google Scholar]
- Seelig A., Alt T., Lotz S., Hölzemann G. Binding of substance P agonists to lipid membranes and to the neurokinin-1 receptor. Biochemistry. 1996 Apr 9;35(14):4365–4374. doi: 10.1021/bi952434q. [DOI] [PubMed] [Google Scholar]
- Seelig A. Local anesthetics and pressure: a comparison of dibucaine binding to lipid monolayers and bilayers. Biochim Biophys Acta. 1987 May 29;899(2):196–204. doi: 10.1016/0005-2736(87)90400-7. [DOI] [PubMed] [Google Scholar]
- Seelig A., Macdonald P. M. Binding of a neuropeptide, substance P, to neutral and negatively charged lipids. Biochemistry. 1989 Mar 21;28(6):2490–2496. doi: 10.1021/bi00432a021. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. Bilayers of dipalmitoyl-3-sn-phosphatidylcholine. Conformational differences between the fatty acyl chains. Biochim Biophys Acta. 1975 Sep 16;406(1):1–5. doi: 10.1016/0005-2736(75)90037-1. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. Effect of a single cis double bond on the structures of a phospholipid bilayer. Biochemistry. 1977 Jan 11;16(1):45–50. doi: 10.1021/bi00620a008. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. The dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry. 1974 Nov 5;13(23):4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Seelig J., Ganz P. Nonclassical hydrophobic effect in membrane binding equilibria. Biochemistry. 1991 Sep 24;30(38):9354–9359. doi: 10.1021/bi00102a031. [DOI] [PubMed] [Google Scholar]
- Seelig J., Waespe-Sarcevic N. Molecular order in cis and trans unsaturated phospholipid bilayers. Biochemistry. 1978 Aug 8;17(16):3310–3315. doi: 10.1021/bi00609a021. [DOI] [PubMed] [Google Scholar]
- Stubbs G. W., Smith H. G., Jr, Litman B. J. Alkyl glucosides as effective solubilizing agents for bovine rhodopsin. A comparison with several commonly used detergents. Biochim Biophys Acta. 1976 Feb 19;426(1):46–56. doi: 10.1016/0005-2736(76)90428-4. [DOI] [PubMed] [Google Scholar]
- Tamm L. K., Seelig J. Lipid solvation of cytochrome c oxidase. Deuterium, nitrogen-14, and phosphorus-31 nuclear magnetic resonance studies on the phosphocholine head group and on cis-unsaturated fatty acyl chains. Biochemistry. 1983 Mar 15;22(6):1474–1483. doi: 10.1021/bi00275a023. [DOI] [PubMed] [Google Scholar]
- Ueno M. Partition behavior of a nonionic detergent, octyl glucoside, between membrane and water phases, and its effect on membrane permeability. Biochemistry. 1989 Jun 27;28(13):5631–5634. doi: 10.1021/bi00439a044. [DOI] [PubMed] [Google Scholar]
- Vinson P. K., Talmon Y., Walter A. Vesicle-micelle transition of phosphatidylcholine and octyl glucoside elucidated by cryo-transmission electron microscopy. Biophys J. 1989 Oct;56(4):669–681. doi: 10.1016/S0006-3495(89)82714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk M. R., Fahr A., Reszka R., Seelig J. Paclitaxel partitioning into lipid bilayers. J Pharm Sci. 1996 Feb;85(2):228–231. doi: 10.1021/js950120i. [DOI] [PubMed] [Google Scholar]
- Wiseman T., Williston S., Brandts J. F., Lin L. N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989 May 15;179(1):131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- de la Maza A., Parra J. L. Structural phase transitions involved in the interaction of phospholipid bilayers with octyl glucoside. Eur J Biochem. 1994 Dec 15;226(3):1029–1038. doi: 10.1111/j.1432-1033.1994.t01-1-01029.x. [DOI] [PubMed] [Google Scholar]