Abstract
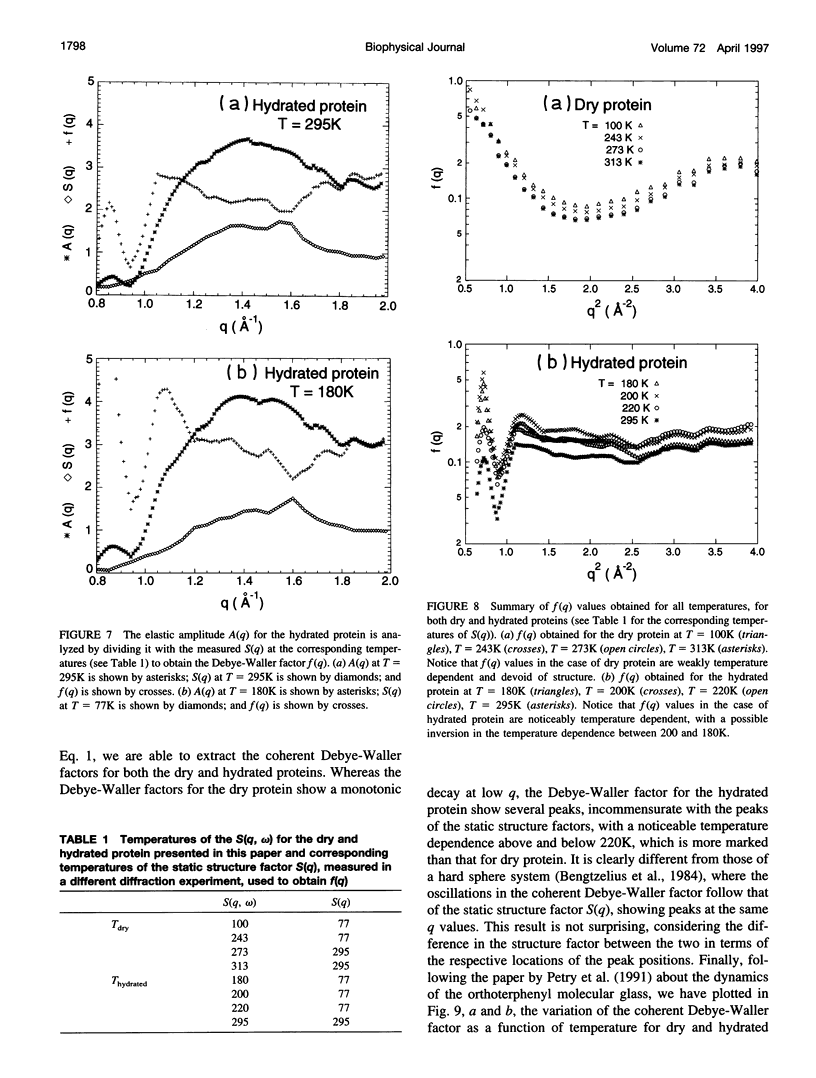
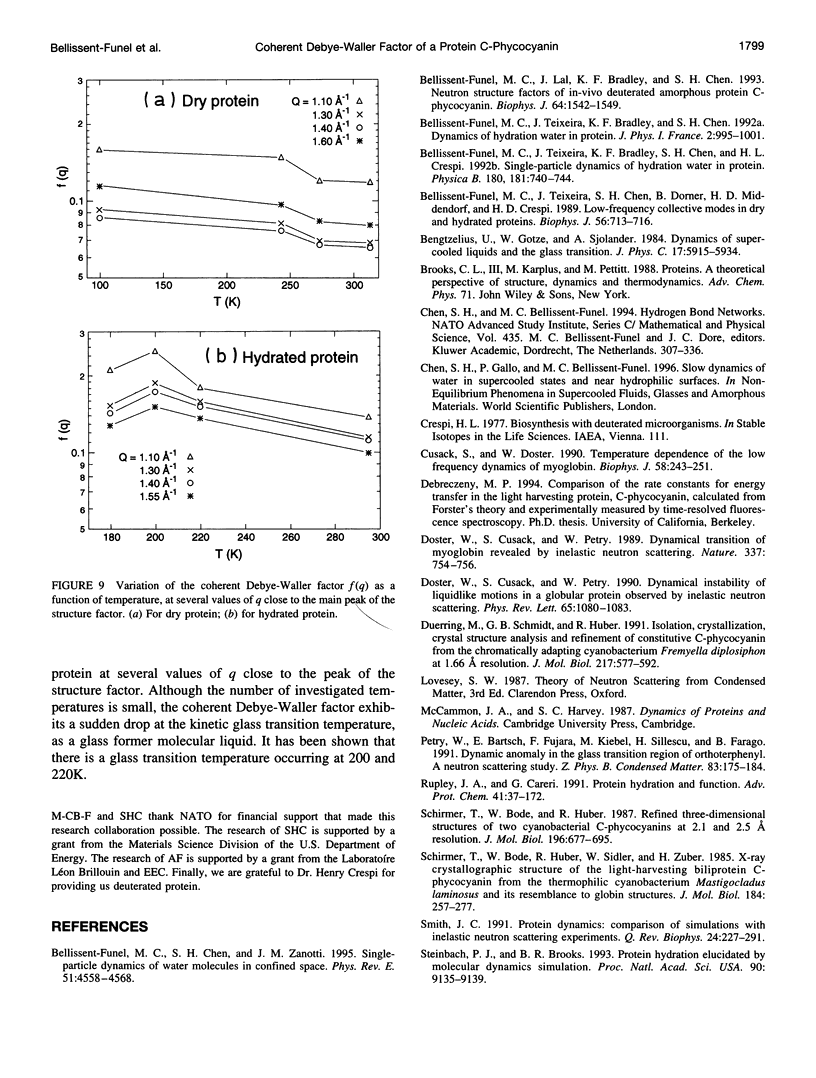
Quasielastic neutron scattering measurements of dry and 35% D2O hydrated amorphous protein powder of C-phycocyanin were made as a function of temperature ranging from 313K down to 100K. The protein is grown from blue-green algae cultured in D2O and is deuterated up to 99%. The scattering is thus dominated by coherent scattering. Within the best energy resolution of the time-of-flight instrument, which is 28 mueV FWHM, the scattering appears entirely elastic. For this reason we are able to extract a coherent Debye-Waller factor by making an independent measurement of the static structure factor. We observe a considerable difference in the q dependence of the Debye-Waller factor between the dry and hydrated proteins; furthermore, there is an interesting temperature dependence of the Debye-Waller factor that is quite different from that predicted for dense hard-sphere liquids.
Full text
PDF

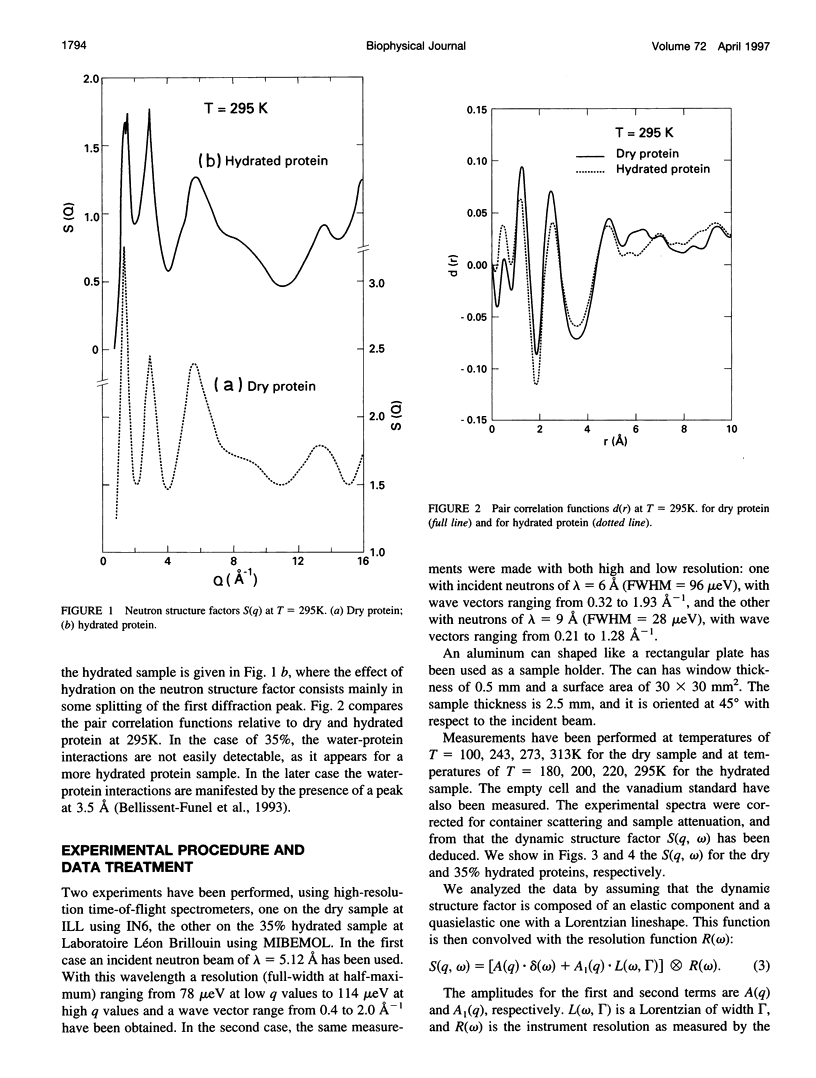
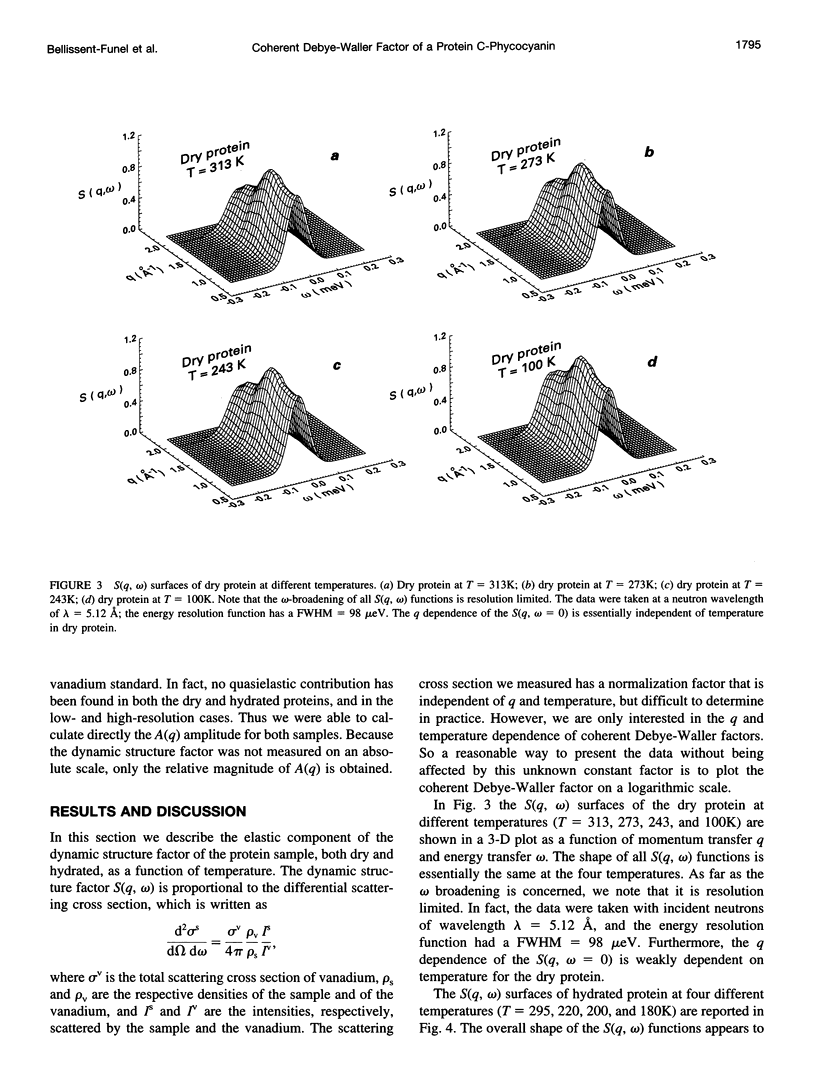
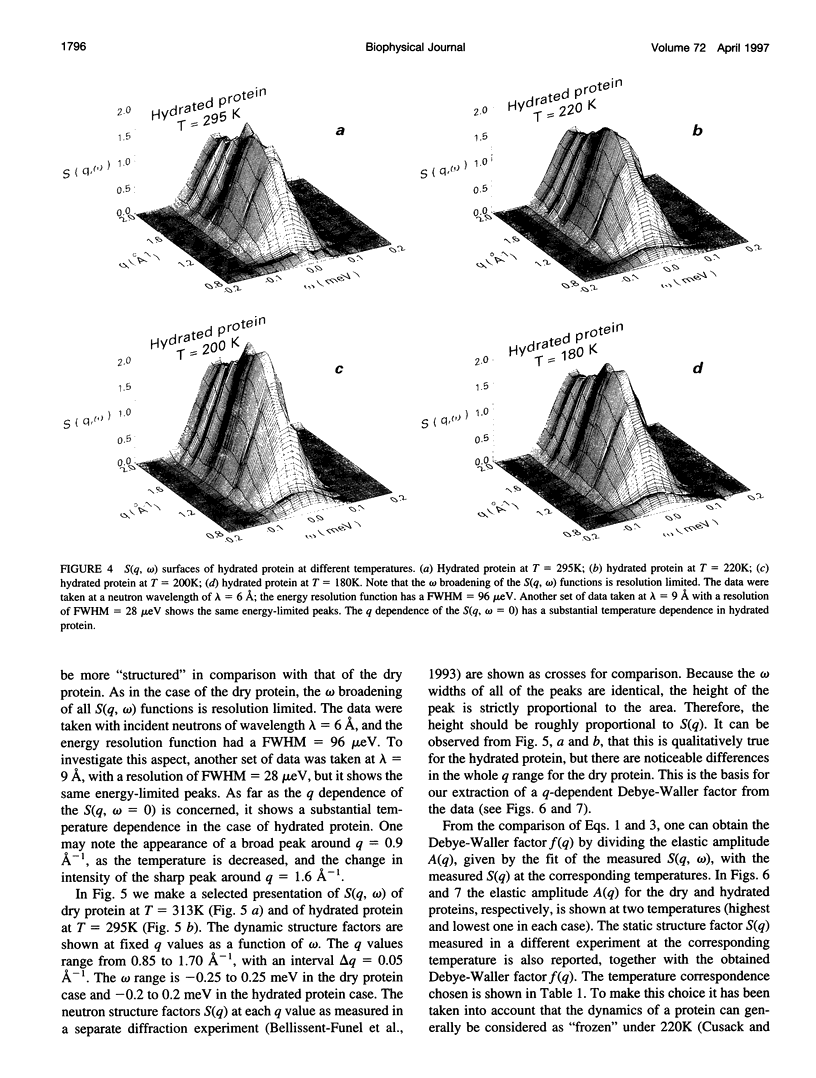
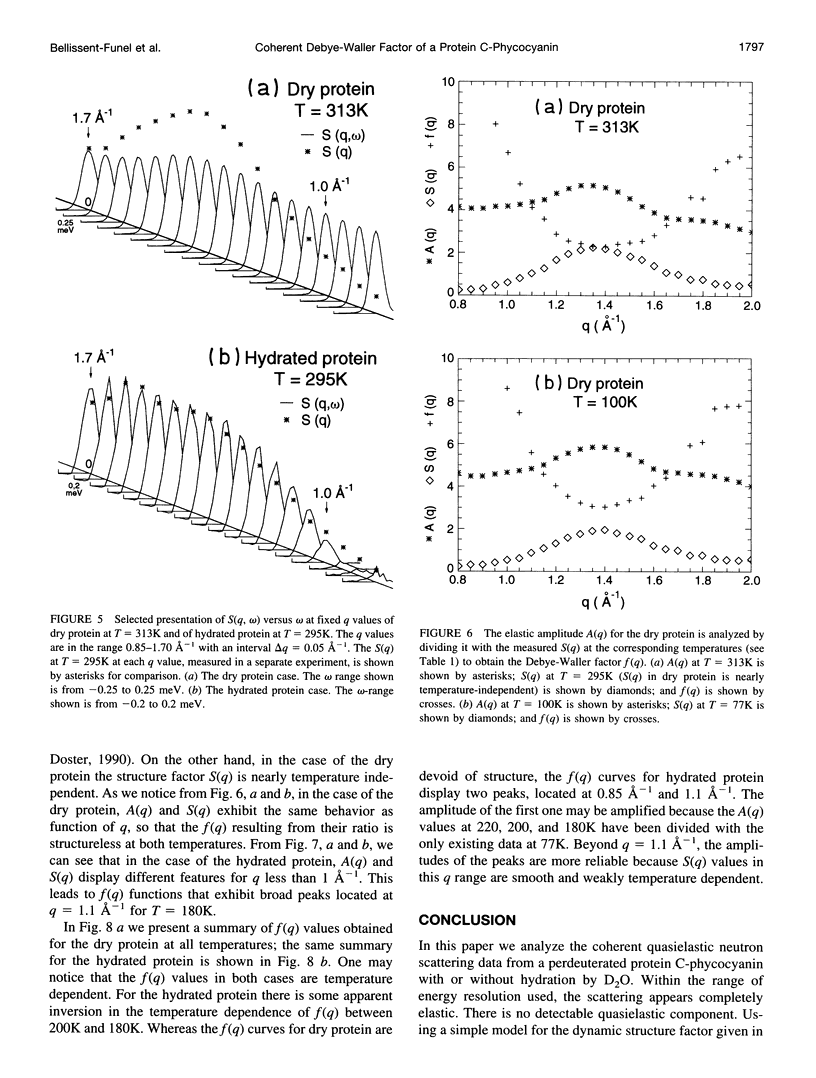
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellissent-Funel M. C., Lal J., Bradley K. F., Chen S. H. Neutron structure factors of in-vivo deuterated amorphous protein C-phycocyanin. Biophys J. 1993 May;64(5):1542–1549. doi: 10.1016/S0006-3495(93)81523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellissent-Funel M. C., Teixeira J., Chen S. H., Dorner B., Middendorf H. D., Crespi H. L. Low-frequency collective modes in dry and hydrated proteins. Biophys J. 1989 Oct;56(4):713–716. doi: 10.1016/S0006-3495(89)82718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellissent-Funel M, Chen SH, Zanotti J. Single-particle dynamics of water molecules in confined space. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1995 May;51(5):4558–4569. doi: 10.1103/physreve.51.4558. [DOI] [PubMed] [Google Scholar]
- Cusack S., Doster W. Temperature dependence of the low frequency dynamics of myoglobin. Measurement of the vibrational frequency distribution by inelastic neutron scattering. Biophys J. 1990 Jul;58(1):243–251. doi: 10.1016/S0006-3495(90)82369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Doster W, Cusack S, Petry W. Dynamic instability of liquidlike motions in a globular protein observed by inelastic neutron scattering. Phys Rev Lett. 1990 Aug 20;65(8):1080–1083. doi: 10.1103/PhysRevLett.65.1080. [DOI] [PubMed] [Google Scholar]
- Duerring M., Schmidt G. B., Huber R. Isolation, crystallization, crystal structure analysis and refinement of constitutive C-phycocyanin from the chromatically adapting cyanobacterium Fremyella diplosiphon at 1.66 A resolution. J Mol Biol. 1991 Feb 5;217(3):577–592. doi: 10.1016/0022-2836(91)90759-y. [DOI] [PubMed] [Google Scholar]
- Rupley J. A., Careri G. Protein hydration and function. Adv Protein Chem. 1991;41:37–172. doi: 10.1016/s0065-3233(08)60197-7. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R. Refined three-dimensional structures of two cyanobacterial C-phycocyanins at 2.1 and 2.5 A resolution. A common principle of phycobilin-protein interaction. J Mol Biol. 1987 Aug 5;196(3):677–695. doi: 10.1016/0022-2836(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R., Sidler W., Zuber H. X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J Mol Biol. 1985 Jul 20;184(2):257–277. doi: 10.1016/0022-2836(85)90379-1. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Brooks B. R. Protein hydration elucidated by molecular dynamics simulation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9135–9139. doi: 10.1073/pnas.90.19.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]



