Abstract
For immobilized nitroxide spin-labels with a well-defined interprobe geometry, resolved dipolar splittings can be observed in continuous wave electron paramagnetic resonance (CW-EPR) spectra for interelectron distances as large as 30 A using perdeuterated probes. In this work, algorithms are developed for calculating CW-EPR spectra of immobilized, dipolar coupled nitroxides, and then used to define the limits of sensitivity to the interelectron distance as a function of geometry and microwave frequency. Secondly, the CW-EPR spectra of N epsilon-spin-labeled coenzyme NAD+ bound to microcrystalline, tetrameric glyceraldehyde-3-phosphate dehydrogenase (GAPDH) have been collected at 9.8, 34, and 94 GHz. These data have been analyzed, using a combination of simulated annealing and global analysis, to obtain a unique fit to the data. The values of the intermitroxide distance and the five angles defining the relative orientation of the two nitroxides are in reasonable agreement with a molecular model built from the known crystal structure. Finally, the effect of rigid body isotropic rotational diffusion on the CW-EPR spectra of dipolar coupled nitroxides has been investigated using an algorithm based on Brownian dynamics trajectories. These calculations demonstrate the sensitivity of CW-EPR spectra to dipolar coupling in the presence of rigid body rotational diffusion.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbach C., Marti T., Khorana H. G., Hubbell W. L. Transmembrane protein structure: spin labeling of bacteriorhodopsin mutants. Science. 1990 Jun 1;248(4959):1088–1092. doi: 10.1126/science.2160734. [DOI] [PubMed] [Google Scholar]
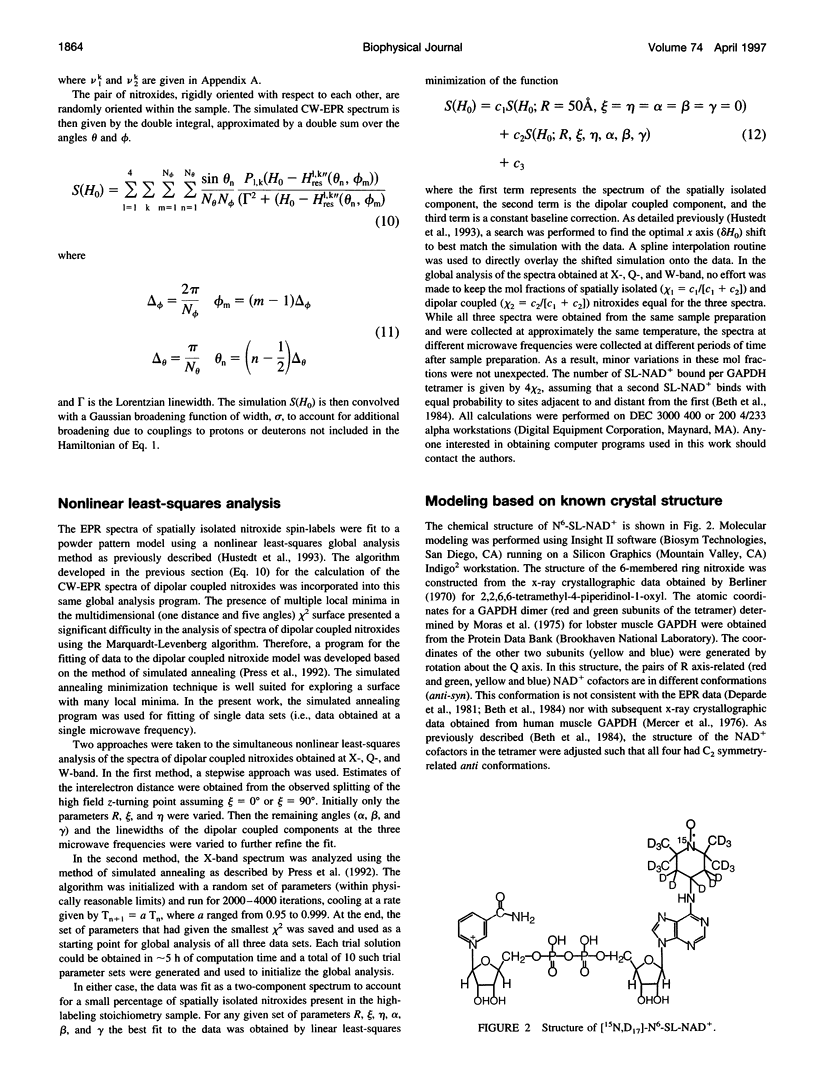
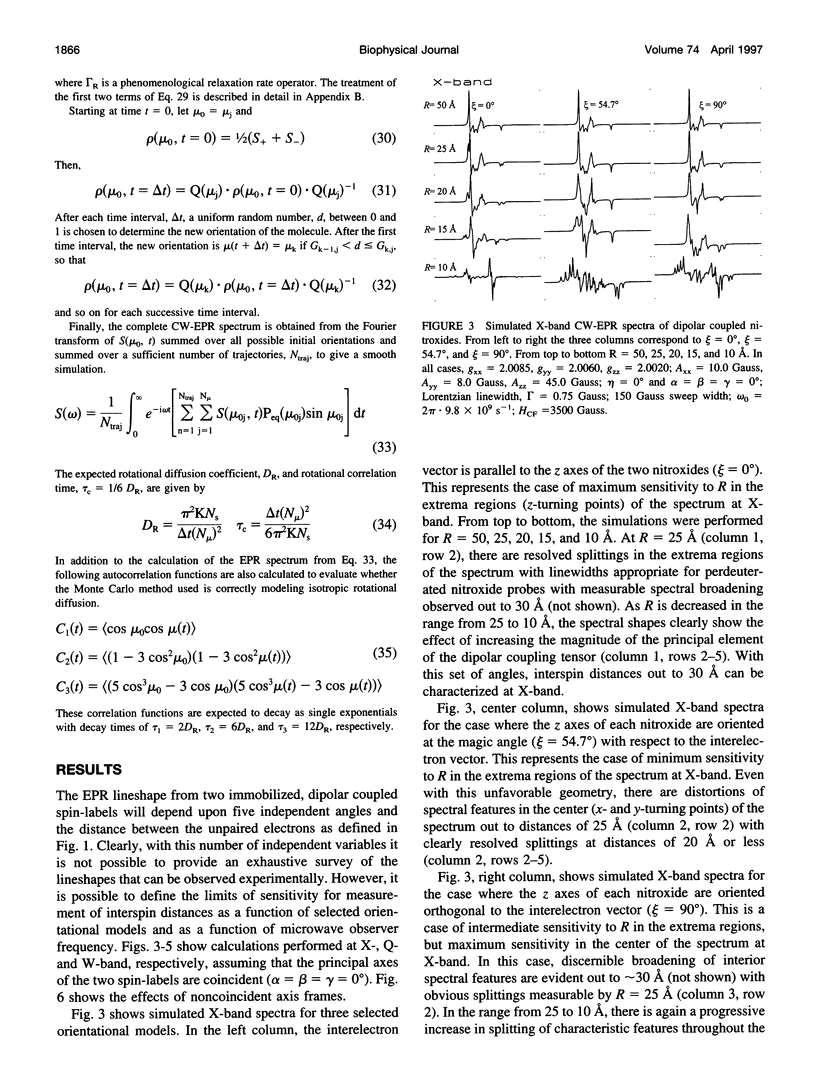
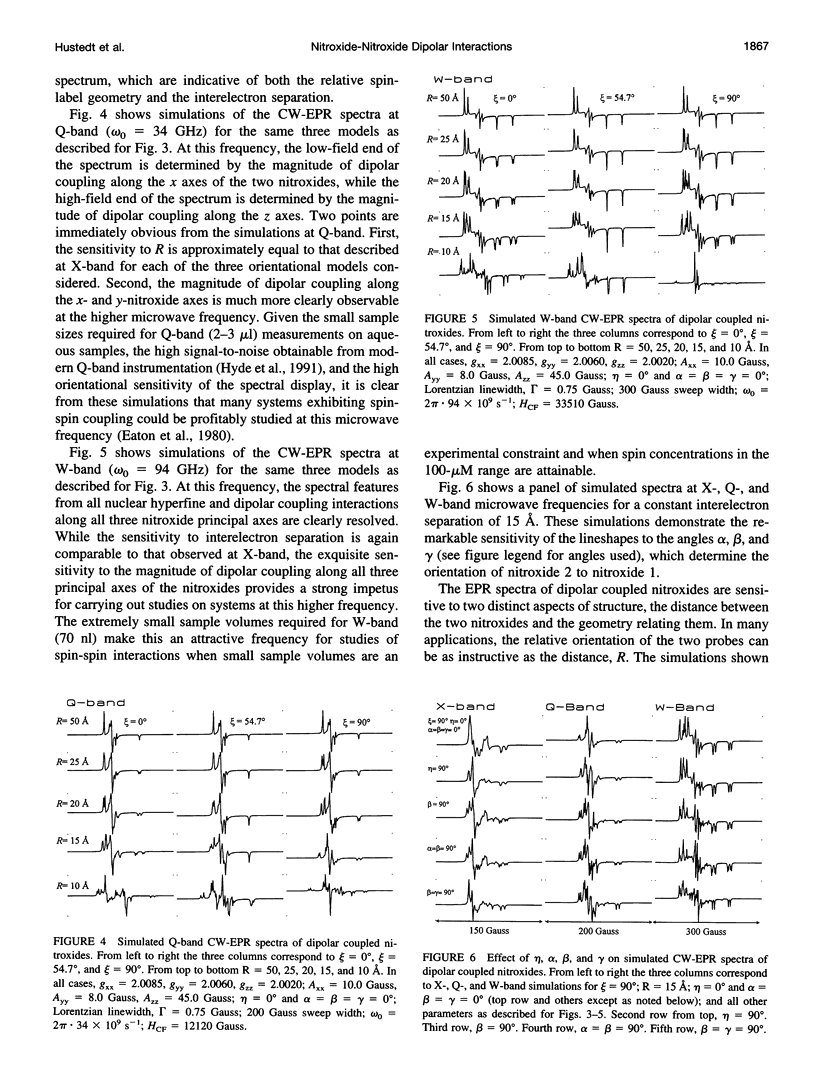
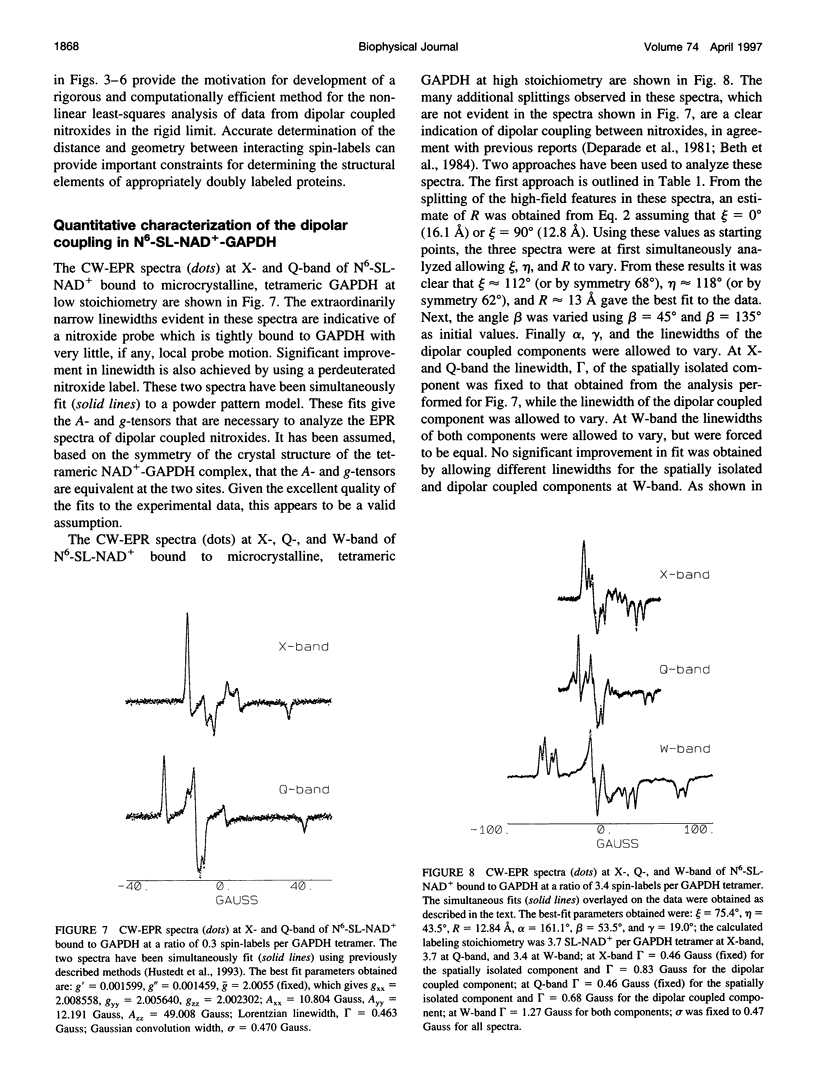
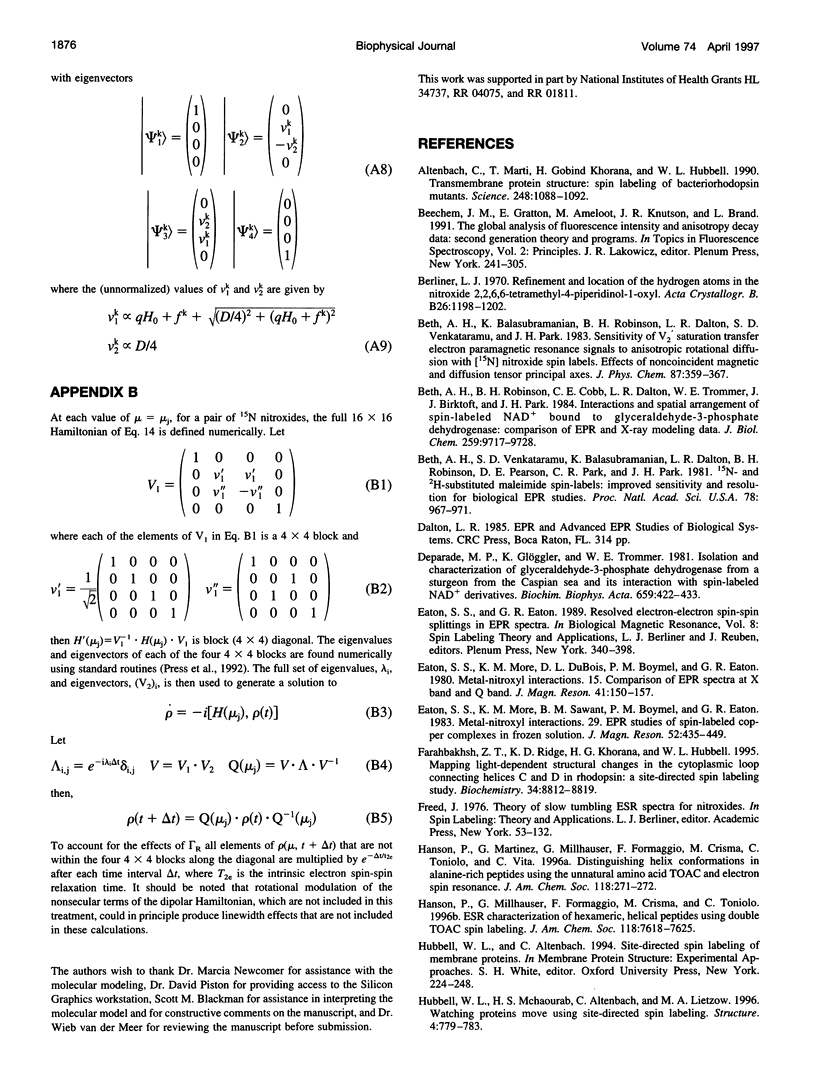
- Beth A. H., Robinson B. H., Cobb C. E., Dalton L. R., Trommer W. E., Birktoft J. J., Park J. H. Interactions and spatial arrangement of spin-labeled NAD+ bound to glyceraldehyde-3-phosphate dehydrogenase. Comparison of EPR and X-ray modeling data. J Biol Chem. 1984 Aug 10;259(15):9717–9728. [PubMed] [Google Scholar]
- Beth A. H., Venkataramu S. D., Balasubramanian K., Dalton L. R., Robinson B. H., Pearson D. E., Park C. R., Park J. H. 15N- and 2H-substituted maleimide spin labels: improved sensitivity and resolution for biological EPR studies. Proc Natl Acad Sci U S A. 1981 Feb;78(2):967–971. doi: 10.1073/pnas.78.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deparade M. P., Glöggler K., Trommer W. E. Isolation and properties of glyceraldehyde-3-phosphate dehydrogenase from a sturgeon from the Caspian Sea and its interaction with spin-labeled NAD+ derivatives. Biochim Biophys Acta. 1981 Jun 15;659(2):422–433. doi: 10.1016/0005-2744(81)90068-1. [DOI] [PubMed] [Google Scholar]
- Farahbakhsh Z. T., Ridge K. D., Khorana H. G., Hubbell W. L. Mapping light-dependent structural changes in the cytoplasmic loop connecting helices C and D in rhodopsin: a site-directed spin labeling study. Biochemistry. 1995 Jul 11;34(27):8812–8819. doi: 10.1021/bi00027a033. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., Mchaourab H. S., Altenbach C., Lietzow M. A. Watching proteins move using site-directed spin labeling. Structure. 1996 Jul 15;4(7):779–783. doi: 10.1016/s0969-2126(96)00085-8. [DOI] [PubMed] [Google Scholar]
- Hustedt E. J., Cobb C. E., Beth A. H., Beechem J. M. Measurement of rotational dynamics by the simultaneous nonlinear analysis of optical and EPR data. Biophys J. 1993 Mar;64(3):614–621. doi: 10.1016/S0006-3495(93)81420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Rutz J. M., Klebba P. E., Feix J. B. A site-directed spin-labeling study of ligand-induced conformational change in the ferric enterobactin receptor, FepA. Biochemistry. 1994 Nov 15;33(45):13274–13283. doi: 10.1021/bi00249a014. [DOI] [PubMed] [Google Scholar]
- Mchaourab H. S., Lietzow M. A., Hideg K., Hubbell W. L. Motion of spin-labeled side chains in T4 lysozyme. Correlation with protein structure and dynamics. Biochemistry. 1996 Jun 18;35(24):7692–7704. doi: 10.1021/bi960482k. [DOI] [PubMed] [Google Scholar]
- Mercer W. D., Winn S. I., Watson H. C. Twinning in crystals of human skeletal muscle D-glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1976 Jun 14;104(1):277–283. doi: 10.1016/0022-2836(76)90013-9. [DOI] [PubMed] [Google Scholar]
- Miick S. M., Martinez G. V., Fiori W. R., Todd A. P., Millhauser G. L. Short alanine-based peptides may form 3(10)-helices and not alpha-helices in aqueous solution. Nature. 1992 Oct 15;359(6396):653–655. doi: 10.1038/359653a0. [DOI] [PubMed] [Google Scholar]
- Millhauser G. L. Selective placement of electron spin resonance spin labels: new structural methods for peptides and proteins. Trends Biochem Sci. 1992 Nov;17(11):448–452. doi: 10.1016/0968-0004(92)90486-s. [DOI] [PubMed] [Google Scholar]
- Moras D., Olsen K. W., Sabesan M. N., Buehner M., Ford G. C., Rossmann M. G. Studies of asymmetry in the three-dimensional structure of lobster D-glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1975 Dec 10;250(23):9137–9162. doi: 10.2210/pdb1gpd/pdb. [DOI] [PubMed] [Google Scholar]
- Oh K. J., Zhan H., Cui C., Hideg K., Collier R. J., Hubbell W. L. Organization of diphtheria toxin T domain in bilayers: a site-directed spin labeling study. Science. 1996 Aug 9;273(5276):810–812. doi: 10.1126/science.273.5276.810. [DOI] [PubMed] [Google Scholar]
- Rabenstein M. D., Shin Y. K. Determination of the distance between two spin labels attached to a macromolecule. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8239–8243. doi: 10.1073/pnas.92.18.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. K., Levinthal C., Levinthal F., Hubbell W. L. Colicin E1 binding to membranes: time-resolved studies of spin-labeled mutants. Science. 1993 Feb 12;259(5097):960–963. doi: 10.1126/science.8382373. [DOI] [PubMed] [Google Scholar]
- Smirnov A. I., Smirnova T. I., Morse P. D., 2nd Very high frequency electron paramagnetic resonance of 2,2,6,6-tetramethyl-1-piperidinyloxy in 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine liposomes: partitioning and molecular dynamics. Biophys J. 1995 Jun;68(6):2350–2360. doi: 10.1016/S0006-3495(95)80417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff H. J., Hubbell W. L. Calculation of electron paramagnetic resonance spectra from Brownian dynamics trajectories: application to nitroxide side chains in proteins. Biophys J. 1996 Oct;71(4):2201–2212. doi: 10.1016/S0006-3495(96)79421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J., He M. M., Hubbell W. L., Kaback H. R. Site-directed spin labeling demonstrates that transmembrane domain XII in the lactose permease of Escherichia coli is an alpha-helix. Biochemistry. 1996 Oct 1;35(39):12915–12918. doi: 10.1021/bi9608774. [DOI] [PubMed] [Google Scholar]
- Voss J., Hubbell W. L., Kaback H. R. Distance determination in proteins using designed metal ion binding sites and site-directed spin labeling: application to the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12300–12303. doi: 10.1073/pnas.92.26.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J., Salwiński L., Kaback H. R., Hubbell W. L. A method for distance determination in proteins using a designed metal ion binding site and site-directed spin labeling: evaluation with T4 lysozyme. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12295–12299. doi: 10.1073/pnas.92.26.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer B. W., Raymer M. A., Wagoner S. L., Hackney R. L., Beechem J. M., Gratton E. Designing matrix models for fluorescence energy transfer between moving donors and acceptors. Biophys J. 1993 Apr;64(4):1243–1263. doi: 10.1016/S0006-3495(93)81490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


