Abstract
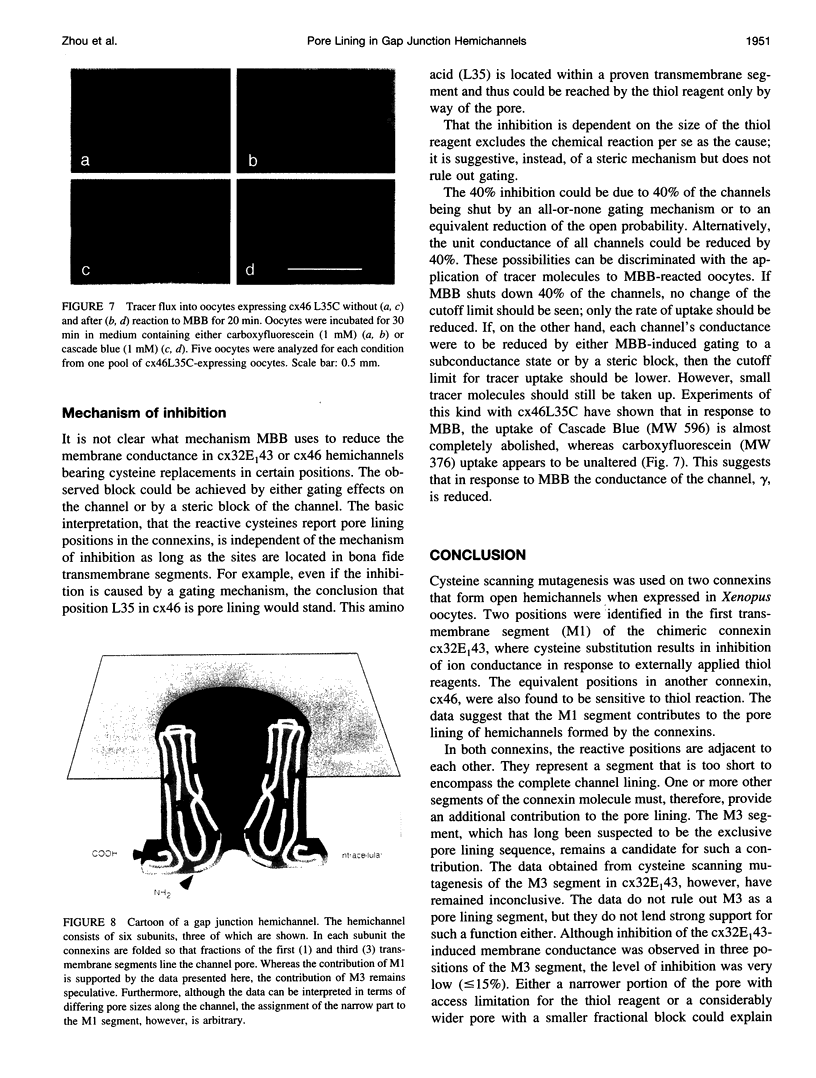
The ability of certain connexins to form open hemichannels has been exploited to study the pore structure of gap junction (hemi)channels. Cysteine scanning mutagenesis was applied to cx46 and to a chimeric connexin, cx32E(1)43, which both form patent hemichannels when expressed in Xenopus oocytes. The thiol reagent maleimido-butyryl-biocytin was used to probe 12 cysteine replacement mutants in the first transmembrane segment and two in the amino-terminal segment. Maleimido-butyryl-biocytin was found to inhibit channel activity with cysteines in two equivalent positions in both connexins: I33C and M34C in cx32E(1)43 and I34C and L35C in cx46. These two positions in the first transmembrane segment are thus accessible from the extracellular space and consequently appear to contribute to the pore lining. The data also suggest that the pore structure is complex and may involve more than one transmembrane segment.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akabas M. H., Stauffer D. A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992 Oct 9;258(5080):307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Safars M., Wilchek M. Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: studies on purified proteins and erythrocyte membranes. Anal Biochem. 1987 Mar;161(2):262–271. doi: 10.1016/0003-2697(87)90450-7. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Goodenough D. A. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull. 1978 Sep;16(3):1–486. [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar D. L., Goodenough D. A., Makowski L., Phillips W. C. Gap junction structures. I. Correlated electron microscopy and x-ray diffraction. J Cell Biol. 1977 Aug;74(2):605–628. doi: 10.1083/jcb.74.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X. B., Hou Y. X., Jensen T. J., Riordan J. R. Mapping of cystic fibrosis transmembrane conductance regulator membrane topology by glycosylation site insertion. J Biol Chem. 1994 Jul 15;269(28):18572–18575. [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Dahl G., Levine E., Rabadan-Diehl C., Werner R. Cell/cell channel formation involves disulfide exchange. Eur J Biochem. 1991 Apr 10;197(1):141–144. doi: 10.1111/j.1432-1033.1991.tb15892.x. [DOI] [PubMed] [Google Scholar]
- Dahl G., Nonner W., Werner R. Attempts to define functional domains of gap junction proteins with synthetic peptides. Biophys J. 1994 Nov;67(5):1816–1822. doi: 10.1016/S0006-3495(94)80663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G., Werner R., Levine E., Rabadan-Diehl C. Mutational analysis of gap junction formation. Biophys J. 1992 Apr;62(1):172–182. doi: 10.1016/S0006-3495(92)81803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Atomic scale structure and functional models of voltage-gated potassium channels. Biophys J. 1992 Apr;62(1):238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol. 1993 Jul;102(1):59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L. Xenopus connexin38 forms hemi-gap-junctional channels in the nonjunctional plasma membrane of Xenopus oocytes. Biophys J. 1996 Aug;71(2):742–748. doi: 10.1016/S0006-3495(96)79273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Paul D. L., Jesaitis L. Topological distribution of two connexin32 antigenic sites in intact and split rodent hepatocyte gap junctions. J Cell Biol. 1988 Nov;107(5):1817–1824. doi: 10.1083/jcb.107.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger J. A., Bruzzone R., Jenkins N. A., Gilbert D. J., Copeland N. G., Paul D. L. Four novel members of the connexin family of gap junction proteins. Molecular cloning, expression, and chromosome mapping. J Biol Chem. 1992 Jan 25;267(3):2057–2064. [PubMed] [Google Scholar]
- Hertzberg E. L., Disher R. M., Tiller A. A., Zhou Y., Cook R. G. Topology of the Mr 27,000 liver gap junction protein. Cytoplasmic localization of amino- and carboxyl termini and a hydrophilic domain which is protease-hypersensitive. J Biol Chem. 1988 Dec 15;263(35):19105–19111. [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- John S. A., Revel J. P. Connexon integrity is maintained by non-covalent bonds: intramolecular disulfide bonds link the extracellular domains in rat connexin-43. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1312–1318. doi: 10.1016/0006-291x(91)91037-d. [DOI] [PubMed] [Google Scholar]
- Karlin A., Akabas M. H. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995 Dec;15(6):1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Kumar N. M., Gilula N. B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986 Sep;103(3):767–776. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu T. F., Lazrak A., Peracchia C., Goldberg G. S., Lampe P. D., Johnson R. G. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996 Aug;134(4):1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Lü Q., Miller C. Silver as a probe of pore-forming residues in a potassium channel. Science. 1995 Apr 14;268(5208):304–307. doi: 10.1126/science.7716526. [DOI] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol. 1977 Aug;74(2):629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milks L. C., Kumar N. M., Houghten R., Unwin N., Gilula N. B. Topology of the 32-kd liver gap junction protein determined by site-directed antibody localizations. EMBO J. 1988 Oct;7(10):2967–2975. doi: 10.1002/j.1460-2075.1988.tb03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. L., Ebihara L., Takemoto L. J., Swenson K. I., Goodenough D. A. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991 Nov;115(4):1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. L. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986 Jul;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabadan-Diehl C., Dahl G., Werner R. A connexin-32 mutation associated with Charcot-Marie-Tooth disease does not affect channel formation in oocytes. FEBS Lett. 1994 Aug 29;351(1):90–94. doi: 10.1016/0014-5793(94)00819-1. [DOI] [PubMed] [Google Scholar]
- Rahman S., Evans W. H. Topography of connexin32 in rat liver gap junctions. Evidence for an intramolecular disulphide linkage connecting the two extracellular peptide loops. J Cell Sci. 1991 Nov;100(Pt 3):567–578. doi: 10.1242/jcs.100.3.567. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Nicholson B. J., Yancey S. B. Molecular organization of gap junctions. Fed Proc. 1984 Sep;43(12):2672–2677. [PubMed] [Google Scholar]
- Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989 Dec;3(6):665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Willecke K., Hennemann H., Dahl E., Jungbluth S., Heynkes R. The diversity of connexin genes encoding gap junctional proteins. Eur J Cell Biol. 1991 Oct;56(1):1–7. [PubMed] [Google Scholar]
- Xu M., Akabas M. H. Amino acids lining the channel of the gamma-aminobutyric acid type A receptor identified by cysteine substitution. J Biol Chem. 1993 Oct 15;268(29):21505–21508. [PubMed] [Google Scholar]
- Yancey S. B., John S. A., Lal R., Austin B. J., Revel J. P. The 43-kD polypeptide of heart gap junctions: immunolocalization, topology, and functional domains. J Cell Biol. 1989 Jun;108(6):2241–2254. doi: 10.1083/jcb.108.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. The topological structure of connexin 26 and its distribution compared to connexin 32 in hepatic gap junctions. J Membr Biol. 1994 Apr;139(1):15–29. doi: 10.1007/BF00232671. [DOI] [PubMed] [Google Scholar]






