Abstract
Obesity is now recognized as a multifaceted chronic disease that is intricately linked to metabolic, biochemical, and psychosocial dysfunction. In this article, we review the epidemiology of obesity, current understanding of its physiopathology, and the recommended staging system used to approach it as a chronic disease, and we include an overview of its health implications.
Keywords: obesity, cardiometabolic syndrome, fat mass disease, adiposopathy, diabesity
What is obesity?
The World Health Organization (WHO) defines obesity as the presence of a body mass index (BMI) greater than 30 kg/m2.1 However, this BMI-centric definition fails to capture the complexity of the condition. The Obesity Medicine Association (OMA) offers a more detailed perspective, describing obesity as a “chronic, progressive, relapsing, multifactorial, neurobehavioral disease” that results from an increase in body fat.2 According to this definition, excess body fat leads to dysfunction in adipose tissue that causes adverse metabolic (adiposopathy), biomechanical (fat mass disease), and psychosocial effects. Acknowledging obesity as a disease ensures a more comprehensive and scientific approach to its treatment and public health impact; it also requires providers to shift away from a biased blaming approach of “lack of willpower” and into the established framework of chronic disease management that requires appropriate staging and diagnostic and treatment tools as well as complication management and outcome measures.
Epidemiology: trends in obesity over time
Over the last three decades, obesity has escalated into a global epidemic, affecting populations across all regions and socioeconomic backgrounds. Currently, approximately 43% of the world’s population, or 2.5 billion adults, are classified as overweight, with 16% meeting the BMI-based criteria for obesity. Historically, overweight and obesity were seen primarily as issues affecting high-income countries. However, recent trends indicate a rapid rise in obesity rates within developing nations. For instance, China, home to one of the world’s largest populations and a developing nation, has witnessed a 90% increase in obesity prevalence over the past decade compared to a 6.7% rise in the United States (US) during the same period.3 Despite these regional differences, obesity prevalence has increased by at least 2% in every country globally, likely due to factors such as globalization, urbanization, changes in diet, and lifestyle shifts. However, the rise in obesity rates in Western countries are not to be dismissed as non-concerning. Obesity prevalence rates in the US have surged from 16% in 1995 to the current 34% (Figure 1),4 with projections indicating that the prevalence of obesity will reach up to 61% by 2050.
Figure 1.

United States prevalence of obesity in 1995 and 2003 illustrating a marked increase in obesity rates across the nation. For Pennsylvania and Kentucky, data from 2022 were utilized instead of 2023 due to insufficient data available for estimating 2023 figures.
In a US-based study, age-related differences show that obesity is most common in middle-aged adults (45-64 years), while younger populations, including adolescents and young adults (20-44 years), are experiencing a concerning rise in obesity, contributing to early-onset comorbidities. Ethnic and racial differences have been highlighted as well, with the most common adverse trends anticipated to affect individuals identifying as American Indian/Alaska Native or multiracial by 2050 (Figure 2).5 It is worth mentioning that these statistics are based on the crude BMI classification system and self-reporting, which likely underrepresents the actual impact of the disease.
Figure 2.
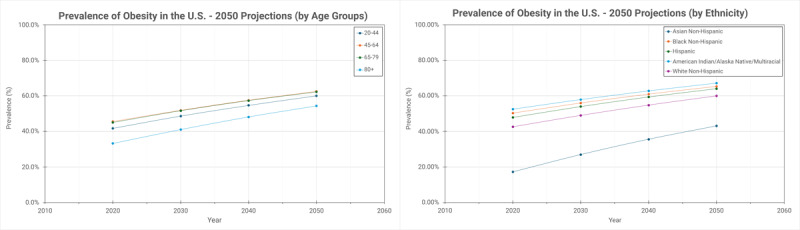
Projections of obesity prevalence from 2020 to 2050, stratified by age and ethnicity.
Pathophysiology
At its core, obesity results from an imbalance between energy intake and expenditure, where calories in excess of expenditure are stored primarily in adipose tissue. This construct is at the base of the trite recommendation of “eat less, exercise more” as the outdated tenets of weight management. What this reductive view fails to capture is the complexity of the systems that regulate energy balance, that include pathways involving communication between brain, gut, and peripheral tissue.6 These integrated systems control food intake, satiety signals, and energy expenditure while being heavily influenced by genetic, epigenetic, environmental, and sociocultural factors.
Central Regulation
The brain plays a critical role in regulating body weight through a complex neural system that is in constant communication with peripheral tissues through hormones produced in response to food intake and fasting states. These complex interactions can be better understood as four main pathways—the homeostatic, hedonic, limbic, and cognitive pathways—each referring to specific brain areas with specialized functions.
Homeostatic Center
The homeostatic center for appetite control is located in the hypothalamus, specifically within the arcuate nucleus. This region contains two distinct populations of neurons that produce peptides in response to peripheral signals, such as ghrelin and leptin. Orexigenic neurons, including those that release agouti-related peptide and neuropeptide Y, are stimulated by ghrelin released from the gastrointestinal (GI) system, stimulating appetite and increasing food intake.7 Conversely, anorexigenic neurons are activated by leptin produced by adipocytes and release pro-opiomelanocortin and alpha-melanocyte-stimulating hormones, which work to suppress appetite. These circuits are connected through redundant synapses and reciprocal inhibition, maintaining a tightly regulated balance. Disruptions in these pathways have been described as causes of severe monogenic forms of obesity.8
Hedonic Pathway
Food intake and appetite are not only homeostatic functions but are also pleasurable experiences regulated by the hedonic pathway that involves dopamine release within the hypothalamus as part of a positive reward feedback system. Although not uniform, data suggest that obesity, especially severe and prolonged, may induce downregulation of dopamine receptors in these areas, with increased need of food intake to achieve the same positive reward response as individuals without obesity.9
Limbic System
The limbic system, critical in the regulation of emotion, memory, and behavior, also partakes in food intake regulation, with the amygdala and hippocampus demonstrating increased activity in anticipation of food and enforcing the positive reward feedback from the hedonic pathways.
Cognitive Pathway
Finally, the termination of food intake is often dependent on the activation of the prefrontal cortex, which is crucial for impulse control and decision-making. Clearly, only a fraction of these pathways is under the influence of conscious control of patients, making lack of willpower an overestimated culprit in the complex process of obesity.
Peripheral Regulation
The main brain counterparts in weight regulation are the GI system and adipose tissue.
GI Tract
Sensory inputs from various cells, including smooth muscle, interstitial cells of Cajal, and the enteric nervous system, guarantee the sensing of meal volume and control the rate of gastric emptying.7 Additionally, gastric distension at the submucosal and muscular layer releases neurotransmitters through intrinsic (enteric) and extrinsic (vagal) neural networks that help regulate feeding behavior by transmitting these signals to the central nervous system and ultimately decreasing appetite after a meal is ingested, often regardless of calorie content.10 As food enters the small intestine, enteroendocrine cells release various hormones that influence gastric emptying and appetite control. Critically, glucagon-like peptide-1 (GLP-1) and cholecystokinin promote satiety, slow gastric emptying, and stimulate vagal afferent nerves that communicate with the central nervous system. Conversely, ghrelin, an orexigenic hormone, is secreted when the stomach is empty, stimulating hunger and promoting food intake by activating the vagus nerve. These hormones also regulate energy expenditure and fat storage, highlighting the interconnected nature of peripheral signals and metabolic balance, and have been the target of the newer, more potent anti-obesity medications.
Adipose Tissue
It is an active endocrine system that contributes to body weight regulation by secreting hormones such as leptin and adiponectin.11 Leptin signals to the brain to induce an anorexigenic response, reducing food intake and increasing energy expenditure when energy stores are sufficient. Adiponectin enhances insulin sensitivity and plays a role in lipid metabolism. Lower levels of adiponectin are associated with higher body fat and insulin resistance. Together, these peripheral pathways provide essential regulatory mechanisms for maintaining energy balance and body weight. In individuals with obesity, leptin resistance develops, blunting its effectiveness and exacerbating weight gain.12 Following weight loss, leptin production decreases in parallel with an increase in ghrelin level and improved metabolic efficiency, leading to a reduction in energy expenditure. This combination makes it challenging to maintain weight loss and often results in weight regain, a phenomenon known as “weight cycling.”13 These metabolic adaptations make it difficult to maintain long-term weight loss, as the body resists deviation from its higher baseline weight, and they increase the risk of obesity-associated comorbidities.
The Impact of Environment
These complex circuitries were developed through millennia of evolution and have served to ensure survival in times of famine and food scarcity. The current set point theory of obesity postulates that fat mass is the body’s defense against starvation and that the body protects it as such.14 The modern environment, however, has drastically changed toward what is deemed an obesogenic milieu, at a speed that has obviously outpaced our ability to adapt, with a consequent inappropriate regulation of fat mass and overall increasing obesity rates.15
Key environmental factors, including built environments, food availability and quality, and socioeconomic conditions, converge to shape dietary habits, energy intake, physical activity, and energy expenditure (Figure 3). The concept of “built environment” encompasses the infrastructure that influences individuals’ access to resources and their engagement within communities.16 For instance, neighborhoods characterized by limited access to recreational facilities and supermarkets often experience higher rates of obesity. Notably, “food deserts and food swamps”—respectively, areas lacking access to food in general or access to affordable and nutritious food—are prevalent in both rural and urban settings and are associated with obesity rates.17 The correlation between fast-food density and increased obesity prevalence has also become clear, highlighting the importance of the consumption of processed foods in the development and maintenance of higher body weight.18 Availability of healthier food options and recreational spaces to foster a better lifestyle appears to be critical to counteract current trends.
Figure 3.

A schematic representation of the main causes of obesity, encompassing neurobiology, environment factors, sociocultural influences, social determinants of health, genetics, and epigenetics. LEPR: leptin receptor; POMC: proopiomelanocortin; FTO: fat mass and obesity-associated (FTO) gene; ADIPOQ: adiponectin, C1Q and collagen domain containing Homo sapiens (human) gene; ADRB: adrenoceptor beta 3; APOE: apolipoprotein E gene; SDOH: social determinants of health; GI: gastrointestinal
On the other hand, these associations between environment and obesity often reflect broader social determinants of health, as less walkable neighborhoods typically house people at lower socioeconomic status.19 Residents of these areas frequently face barriers, such as lower disposable income, that negatively impact the ability to afford more expensive, healthier, unprocessed food options, as well as limited access to essential services, further perpetuating poor health outcomes.
Genetic and Epigenetic Factors
Although rare, monogenic forms of obesity are recognized and typically linked to mutations affecting homeostatic pathways such as the leptin-melanocortin system, which regulates appetite and energy balance.8 Much more common and impactful are genetic susceptibilities, where multiple genetic variations influence traits like appetite, fat storage, and metabolism. Based on twin, family, and adoption studies, it is estimated that 40% to 70% of BMI variability can be attributed to genetic influences.20 More recent large-scale linkage studies have identified over 1,000 genetic loci associated with obesity traits.21 Together, these loci contribute to a higher fat mass set point or an increased susceptibility to obesity. Interestingly, the impact of some genes, like those linked to the FTO (fat mass and obesity associated) locus, can be mitigated by lifestyle changes. For example, it has recently been reported that physical activity and a balanced diet can reduce the risk associated with certain obesity-related genes by 30% to 40%.22
In addition to these genetic influences, epigenetics—reversible changes to gene expression—can also play a role. Environmental factors such as diet, exercise, bariatric surgery, and in utero experience can modify DNA methylation patterns, which further affect weight regulation processes.
Impact of obesity on patient health
Obesity significantly impacts the physical, mental, and social well-being of an individual through what are commonly described as two main mechanisms of “fat mass disease” and “adiposopathy.”
The first refers to the adverse negative effects of excessive weight on bodily function. Adiposopathy encompasses the metabolic dysregulation that occurs in the setting of increased fat deposition, especially in the visceral and organ compartments.11 Under normal circumstances, adipocytes (mostly located in the subcutaneous space) release adipokines-hormones that influence metabolism, immune function, and inflammation. In patients with obesity, the profile of adipokine production from mostly visceral adipose tissue shifts, favoring proinflammatory adipokines such as tumor necrosis factor-alpha (TNF-a), interleukin-6 (IL-6), and leptin, while the secretion of anti-inflammatory adipokines like adiponectin is diminished.7 This imbalance in hormone production drives systemic inflammation and results in adverse endocrine and immune responses that directly lead to insulin resistance, certain cancers, cardiovascular diseases, liver dysfunction, and more.
Fat Mass Disease
Osteoarthritis
Osteoarthritis (OA), a degenerative joint disease, is frequently seen in patients with obesity, who are nearly seven times more likely to develop knee OA than individuals of normal weight.23 Excess weight places mechanical strain on joints, accelerating cartilage wear and further contributing to joint degeneration. Modest weight loss of even 5% can greatly improve joint pain, while at least 10% of body weight loss is needed for clinical improvements such as slowing down structural deterioration and enhancing mobility.24
Obstructive Sleep Apnea
Obstructive sleep apnea (OSA)_is characterized by repeated episodes of upper airway collapse during sleep, leading to intermittent hypoxemia and disrupted sleep. Excess adipose tissue in the neck and chest increases the likelihood of airway obstruction by applying external pressure to the thoracic cavity, leading to OSA. This mechanical disruption, coupled with resultant hypoxia, elevates stress hormone levels, which in turn negatively impact weight regulation.25 Weight loss is the cornerstone of treatment.
Adiposopathy
Type 2 Diabetes
Approximately one-third of people with obesity develop type 2 diabetes (T2DM), driven by insulin resistance and progressive pancreatic β-cell dysfunction.26 The prognosis in individuals with obesity and T2DM, termed “diabesity,”27 has traditionally been poor, as the condition significantly decreases life expectancy and quality of life and increases healthcare costs.28 The newer metabolic drugs enhancing incretin signals—glucagon-like peptide-1 (GLP-1) and GLP1/glucose-dependent insulinotropic polypeptide (GIP) agonist—have revolutionized treatment for these patients, significantly impacting morbidity and mortality. These agents not only improve glycemic control and promote weight loss, but they also have favorable cardiovascular outcomes, renal protection, and overall mortality.29,30,31,32
Liver Disease
Excess fat deposition in non-adipose tissues, particularly the liver, leads to metabolic dysfunction-associated fatty liver disease (MAFLD), which affects approximately 75% of individuals with obesity and is currently the most common chronic liver disease.33 MAFLD can progress from steatosis to more severe forms, such as fibrosis, cirrhosis, or even hepatocellular carcinoma, as the liver’s ability to regulate glucose and lipid metabolism is progressively impaired. Newer metabolic drugs, such as incretin receptor agonist, have shown promise in the long-term treatment of MAFLD.34
Cancer
Obesity is strongly associated with an increased risk of developing various cancers, including breast, endometrial, colorectal, pancreatic, kidney, and liver cancer. The underlying mechanisms involve a complex interplay of metabolic and inflammatory processes with adiposopathy-associated chronic inflammation triggering the production of reactive oxygen species, which can damage cellular DNA and interfere with its repair mechanisms, increasing the risk of mutations and cancerous cell development.35 Furthermore, obesity-related increase in insulin-like growth factors and insulin promotes cancer cell proliferation and inhibition of cell death. These latter mechanisms are particularly relevant in breast and colorectal cancer, where insulin resistance and hyperinsulinemia stimulate signaling pathways that further support tumor growth.36 In post-menopausal women, increased estrogen production from the extra adipose tissue can represent a growth stimulus for breast and endometrial cancer. Adipocytes produced leptin also promotes cancer cell growth by activating pathways involved in cell survival and proliferation, contributing to the aggressiveness of tumors in individuals with obesity.37
Cardiovascular Diseases
Obesity-induced metabolic derangements increase the risk for cardiovascular diseases (CVD). Excessive visceral fat exacerbates insulin resistance, dyslipidemia, and inflammation, which are all main drivers of atherosclerosis, increasing the likelihood of heart attacks and strokes.38 Additional linkage of obesity with hypertension and T2DM further exacerbates these cardiovascular risks. Beyond metabolic disturbances, obesity also affects the heart’s structure, function, and electrical conduction. Increased epicardial fat, systemic inflammation, and oxidative stress can induce atrial enlargement and atrial fibrillation.39 Furthermore, increase in body weight places a higher demand on the heart, leading to left ventricular hypertrophy and eventually heart failure, specifically heart failure with preserved ejection fraction.40,41
Psychological and Societal Impact
Up to 40% of adults with obesity have experienced weight stigma, which involves social devaluation and negative interactions due to excess body weight. This stigma can lead to harmful attitudes, stereotypes, prejudice, and discrimination.42 These experiences are scattered through a person’s lifetime, often starting in childhood and progressively solidifying in adolescence and adulthood.43 Women report even higher rates of weight stigma.44 Biases against people with obesity can translate into workplace discrimination, reduced earning potential, mental health issues, and decreased quality of life. Sadly, healthcare professionals at all levels, from medical students to specialists, are often not only unaware of these specific issues but can also contribute to stigmatization, creating an additional barrier to appropriate care for patients with obesity.45 Negative experiences with weight stigma often lead to internalized self judgement, known as internalized weight bias, which further fuels a cycle of low self-esteem, depression, avoidance of health care, and worse health outcomes.46
A Better staging system
As mentioned, a purely BMI-based definition and staging of obesity, although practical for epidemiological purposes, is reductive and not functional for the appropriate clinical management of a complex, chronic disease. Firstly, BMI does not account for gender, ethnic, or racial variability,47 nor for individual variabilities in body composition, such as with elite athletes, where an elevated BMI is related to increased muscle mass and not excess fat. Although the latter can be overcome with body composition analysis techniques (underwater weighing, dual-energy X-ray absorptiometry [DEXA scanning], computed tomography, and magnetic resonance imaging), these methods are impractical for daily clinical use and continue to offer only a limited assessment of the complex disease of obesity as the thresholds of excess adiposity can occur at varying body weights.
Scientific societies have proposed a more comprehensive staging approach that combines anthropometric measures with presence and degree of associated negative health consequences.48,49 For example, the Edmonton Obesity Staging System (EOSS) categorizes obesity based on its clinical impact on multiple dimensions (Figure 4). Stage 0 represents individuals with obesity but without any apparent obesity-related health complications—what would be considered “patient at risk” in similar classifications of chronic diseases, such as Heart Failure—while Stage 4 includes those with end-stage disease, such as severe cardiovascular disease, organ failure, or debilitating conditions. This approach is valuable in providing a framework for the appropriate diagnostic approach, as it requires the investigation of presence and degree of associated comorbidities in patients with obesity, and also in guiding treatment strategies, which allows clinicians to prioritize interventions based on the severity of the disease. It moves away from the simplistic view of obesity as a numerical BMI category, recognizing how the variability in excess body fat affects different individuals (Table 1).25,50,51,52,53,54,55 This approach also helps practitioners recognize that weight is only part of the diagnostic criteria and reminds them of the treatment goals that should be targeted.
Figure 4.

The Edmonton Obesity Staging System (EOSS) categorizes obesity into four progressive stages based on medical, mental, and functional health, ranging from absence of disease to end-stage disease. Medical health includes progression of chronic diseases such as T2DM and hypertension; mental health spans from minimal stress to severe depression linked to obesity; and functional health assess quality of life, from mild discomfort to limitations in daily activities. Treatment tiers (A, B, and C) align staging with lifestyle intervention to be incorporated at all stages of obesity (A), medical intervention (B) to be started at Stage 2, and surgical options (C) to be considered for Stage 3 and 4.
Table 1.
Relative risk factor and (%) weight loss required for therapeutic benefits for complications related to obesity.25,50,51,52,53,54,55 T2DM: type 2 diabetes mellitus; MAFLD: metabolic dysfunction-associated fatty liver disease; CAD: coronary artery disease; OSA: obstructive sleep apnea
|
| |||
|---|---|---|---|
| COMPLICATIONS | RELATIVE RISK FACTOR | (%) WEIGHT LOSS REQUIRED FOR THERAPEUTIC BENEFIT | |
|
| |||
| MALE | FEMALE | ||
|
| |||
| T2DM | 6.74 | 12.41 | > 2.5 (improvement) 10-15 (remission) |
|
| |||
| Hypertension | 1.84 | 2.42 | 5 |
|
| |||
| CAD | 1.72 | 3.10 | > 10 |
|
| |||
| Osteoarthritis | 4.20 | 1.96 | > 5-10 |
|
| |||
| OSA | 2.00 | 2.00 | > 10 |
|
| |||
| MAFLD | 5.51* | 5.51* | > 10 |
|
| |||
| Breast cancer | - | 1.13 | - |
|
| |||
| Colorectal cancer | 1.95 | 1.66 | - |
|
| |||
| Endometrial cancer | - | 3.22 | - |
|
| |||
| Kidney cancer | 1.82 | 2.64 | - |
|
| |||
| Ovarian cancer | - | 1.28 | - |
|
| |||
| Pancreatic cancer | 2.29 | 1.60 | - |
|
| |||
* Calculated odds ratio
Approaching Obesity
In approaching obesity, it is critical to acknowledge it as a chronic disease and offer this framework to patients as well. Reframing obesity in this way will help reduce stigma and foster a more constructive relationship between the patient and the providers. Moreover, it allows for a broader focus that goes beyond weight or BMI as markers of success to encompass the physical, mental, and functional health of the patient.
Given this broader view, effective obesity management requires an interprofessional and collaborative approach that addresses the medical complexities of the condition, integrating the expertise of dietitians, social workers, fitness and mental health specialists as well as medical and surgical providers in developing individual care plans. Finally, societal determinants of obesity need to be considered as well. Patients from lower socioeconomic backgrounds often face significant barriers, including limited access to healthy food, safe spaces for physical activity, and basic healthcare needs. To ensure equitable and effective care, healthcare teams must also account for social determinants of health that uniquely affect each patient.
Obesity prevalence continues to rise, with significant impact on patients’ lives and health. Recognized as a chronic disease in 2013, it’s high time the medical community embraces the implications of such a definition and meets the challenge it represents.
Key Points
Obesity is a complex, chronic disease that goes beyond the simple notion of “lack of willpower.”
Physicians should shift from a body mass index-centric diagnostic criteria to a more comprehensive classification system, such as the Edmonton Obesity Staging System.
Overweight and obesity lead to complex physiological changes that occur across various body systems.
The comorbidities associated with obesity necessitate lifestyle changes, medical interventions, and/or surgical options to achieve therapeutic benefits.
Approaching obesity in a nonbiased and collaborative manner is pivotal to provide comprehensive medical, mental, and functional benefits.
CME Credit opportunity
Houston Methodist is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Houston Methodist designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Click to earn CME credit: learn.houstonmethodist.org/ MDCVJ-21.2.
Competing Interests
The authors have no competing interests to declare.
References
- 1.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000:894:i-xii, 1-253. PMID: 11234459 [PubMed] [Google Scholar]
- 2.Fitch AK, Bays HE. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obese Pillars. 2022. Jan 15:1:100004. doi: 10.1016/j.obpill.2021.100004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022. Aug:133:155217. doi: 10.1016/j.metabol.2022.155217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC [Internet]. Atlanta, GA: Centers for Disease Control and Prevention; c2024. BRFSS Prevalence & Trends Data: Home; 2023. [cited 2024 Dec 15]. Available from: https://www.cdc.gov/brfss/brfssprevalence/index.html [Google Scholar]
- 5.Joynt Maddox KE, Elkind MSV, Aparicio HJ, et al. Forecasting the Burden of Cardiovascular Disease and Stroke in the United States Through 2050-Prevalence of Risk Factors and Disease: A Presidential Advisory From the American Heart Association. Circulation. 2024. Jul 23;150(4):e65-e88. doi: 10.1161/CIR.0000000000001256 [DOI] [PubMed] [Google Scholar]
- 6.Hall KD, Hammond RA, Rahmandad H. Dynamic interplay among homeostatic, hedonic, and cognitive feedback circuits regulating body weight. Am J Public Health. 2014. Jul;104(7):1169-75. doi: 10.2105/AJPH.2014.301931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busebee B, Ghusn W, Cifuentes L, Acosta A. Obesity: A Review of Pathophysiology and Classification. Mayo Clin Proc. 2023. Dec;98(12):1842-1857. doi: 10.1016/j.mayocp.2023.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Choi JH. Pathophysiology and clinical characteristics of hypothalamic obesity in children and adolescents. Ann Pediatr Endocrinol Metab. 2013. Dec;18(4):161-7. doi: 10.6065/apem.2013.18.4.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro G, Maia A, Cotovio G, Oliveira FPM, Costa DC, Oliveira-Maia AJ. Striatal dopamine D2-like receptors availability in obesity and its modulation by bariatric surgery: a systematic review and meta-analysis. Sci Rep. 2023. Mar 27;13(1):4959. doi: 10.1038/s41598-023-31250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wachsmuth HR, Weninger SN, Duca FA. Role of the gut-brain axis in energy and glucose metabolism. Exp Mol Med. 2022. Apr;54(4):377-392. doi: 10.1038/s12276-021-00677-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011. Jun 21;57(25):2461-73. doi: 10.1016/j.jacc.2011.02.038 [DOI] [PubMed] [Google Scholar]
- 12.Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients. 2019;11(11):2704. doi: 10.3390/nu11112704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endotext [Internet]. Bethesda, MD: National Library of Medicine; c2024. Woolf EK, Cabre HE, Niclou AN, Redman LM. Body Weight Regulation; 2024. Jun 13 [cited 2024 Dec 15]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK278932/ [Google Scholar]
- 14.Garvey WT. Is Obesity or Adiposity-Based Chronic Disease Curable: The Set Point Theory, the Environment, and Second-Generation Medications. Endocr Pract. 2022. Feb;28(2):214-222. doi: 10.1016/j.eprac.2021.11.082 [DOI] [PubMed] [Google Scholar]
- 15.Ravussin E, Bouchard C. Human genomics and obesity: finding appropriate drug targets. Eur J Pharmacol. 2000. Dec 27;410(2-3):131-145. doi: 10.1016/s0014-2999(00)00811-6 [DOI] [PubMed] [Google Scholar]
- 16.Lake A, Townshend T. Obesogenic environments: exploring the built and food environments. J R Soc Promot Health. 2006. Nov;126(6):262-7. doi: 10.1177/1466424006070487 [DOI] [PubMed] [Google Scholar]
- 17.Ghosh-Dastidar B, Cohen D, Hunter G, et al. Distance to store, food prices, and obesity in urban food deserts. Am J Prev Med. 2014. Nov;47(5):587-95. doi: 10.1016/j.amepre.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall KD, Ayuketah A, Brychta R, et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019. Jul 2;30(1):67-77.e3. doi: 10.1016/j.cmet.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anekwe CV, Jarrell AR, Townsend MJ, Gaudier GI, Hiserodt JM, Stanford FC. Socioeconomics of Obesity. Curr Obes Rep. 2020. Sep;9(3):272-279. doi: 10.1007/s13679-020-00398-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray MS, Loos RJF, McCaffery JM, et al. NIH working group report-using genomic information to guide weight management: From universal to precision treatment. Obesity (Silver Spring). 2016. Jan;24(1):14-22. doi: 10.1002/oby.21381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen H. Is Obesity A Disease or A Behavior Abnormality? Did the AMA Get It Right? Mo Med. 2014. Mar-Apr;111(2):104-108. PMID: 30323513 [PMC free article] [PubMed] [Google Scholar]
- 22.Kilpeläinen TO, Qi L, Brage S, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011. Nov;8(11):e1001116. doi: 10.1371/journal.pmed.1001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King LK, March L, Anandacoomarasamy A. Obesity & osteoarthritis. Indian J Med Res. 2013;138(2):185-93. PMID: 24056594 [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent HK, Heywood K, Connelly J, Hurley RW. Obesity and weight loss in the treatment and prevention of osteoarthritis. PM R. 2012. May;4(5 Suppl):S59-67. doi: 10.1016/j.pmrj.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010. Mar;137(3):711-9. doi: 10.1378/chest.09-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin X, Qiu T, Li L, et al. Pathophysiology of obesity and its associated diseases. Acta Pharm Sin B. 2023. Jun;13(6):2403-2424. doi: 10.1016/j.apsb.2023.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaelidou M, Pappachan JM, Jeeyavudeen MS. Management of diabesity: Current concepts. World J Diabetes. 2023. Apr 15;14(4):396-411. doi: 10.4239/wjd.v14.i4.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farag YMK, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2011. Jan;26(1):28-35. doi: 10.1093/ndt/gfq576 [DOI] [PubMed] [Google Scholar]
- 29.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016. Nov 10;375(19):1834-1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 30.Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021. Mar 18;384(11):989-1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 31.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N Engl J Med. 2023. Dec 14;389(24):2221-2232. doi: 10.1056/NEJMoa2307563 [DOI] [PubMed] [Google Scholar]
- 32.Perkovic V, Tuttle KR, Rossing P, et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N Engl J Med. 2024. Jul 11;391(2):109-121. doi: 10.1056/NEJMoa2403347 [DOI] [PubMed] [Google Scholar]
- 33.Ding Y, Deng Q, Yang M, Niu H, Wang Z, Xia S. Clinical Classification of Obesity and Implications for Metabolic Dysfunction-Associated Fatty Liver Disease and Treatment. Diabetes Metab Syndr Obes. 2023. Oct 25:16:3303-3329. doi: 10.2147/DMSO.S431251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ordóñez-Vázquez AL, Beltrán-Gall SM, Pal SC, Méndez-Sánchez N. Editorial: Treatment with Dual Incretin Receptor Agonists to Maintain Normal Glucose Levels May Also Maintain Normal Weight and Control Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Med Sci Monit. 2022. Sep 12:28:e938365. doi: 10.12659/MSM.938365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masenga SK, Kabwe LS, Chakulya M, Kirabo A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int J Mol Sci. 2023. Apr 26;24(9):7898. doi: 10.3390/ijms24097898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001. Nov;131(11 Suppl):3109S-20S. doi: 10.1093/jn/131.11.3109S [DOI] [PubMed] [Google Scholar]
- 37.Andò S, Gelsomino L, Panza S, et al. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers (Basel). 2019. Jan 9;11(1):62. doi: 10.3390/cancers11010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002. Mar 5;105(9):1135-43. doi: 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 39.Shu H, Cheng J, Li N, et al. Obesity and atrial fibrillation: a narrative review from arrhythmogenic mechanisms to clinical significance. Cardiovasc Diabetol. 2023. Jul 29;22(1):192. doi: 10.1186/s12933-023-01913-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc Res. 2023. Feb 3;118(18):3434-3450. doi: 10.1093/cvr/cvac120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jamaly S, Carlsson L, Peltonen M, Jacobson P, Karason K. Surgical obesity treatment and the risk of heart failure. Eur Heart J. 2019. Jul 1;40(26):2131-2138. doi: 10.1093/eurheartj/ehz295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020. Apr;26(4):485-497. doi: 10.1038/s41591-020-0803-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring). 2009. May;17(5):941-64. doi: 10.1038/oby.2008.636 [DOI] [PubMed] [Google Scholar]
- 44.Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond). 2009. Mar;33(3):289-95. doi: 10.1038/ijo.2009.2 [DOI] [PubMed] [Google Scholar]
- 45.Phelan SM, Bauer KW, Bradley D, et al. A model of weight-based stigma in health care and utilization outcomes: Evidence from the learning health systems network. Obesity Sci Pract. 2021. Aug 27;8(2):139-146. doi: 10.1002/osp4.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puhl RM, Himmelstein MS, Pearl RL. Weight stigma as a psychosocial contributor to obesity. Am Psychol. 2020. Feb-Mar;75(2):274-289. doi: 10.1037/amp0000538 [DOI] [PubMed] [Google Scholar]
- 47.Stanford FC, Lee M, Hur C. Race, Ethnicity, Sex, and Obesity: Is It Time to Personalize the Scale? Mayo Clin Proc. 2019. Feb;94(2):362-363. doi: 10.1016/j.mayocp.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract. 2016. Jul:22 Suppl 3:1-203. doi: 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 49.Canning KL, Brown RE, Wharton S, Sharma AM, Kuk JL. Edmonton Obesity Staging System Prevalence and Association with Weight Loss in a Publicly Funded Referral-Based Obesity Clinic. J Obes. 2015:2015:619734. doi: 10.1155/2015/619734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kushner RF, Ryan DH. Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. JAMA. 2014. Sep 3;312(9):943-52. doi: 10.1001/jama.2014.10432 [DOI] [PubMed] [Google Scholar]
- 51.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009. Mar 25:9:88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taheri E, Moslem A, Mousavi-Jarrahi A, et al. Predictors of metabolic-associated fatty liver disease (MAFLD) in adults: a population-based study in Northeastern Iran. Gastroenterol Hepatol Bed Bench. 2021. Fall;14(Suppl1):S102-S111. [PMC free article] [PubMed] [Google Scholar]
- 53.Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018. Feb 10;391(10120):541-551. doi: 10.1016/S0140-6736(17)33102-1 [DOI] [PubMed] [Google Scholar]
- 54.Ryan DH, Yockey SR. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr Obes Rep. 2017. Jun;6(2):187-194. doi: 10.1007/s13679-017-0262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pack QR, Rodriguez-Escudero JP, Thomas RJ, et al. The prognostic importance of weight loss in coronary artery disease: a systematic review and meta-analysis. Mayo Clin Proc. 2014. Oct;89(10):1368-77. doi: 10.1016/j.mayocp.2014.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]


