Abstract
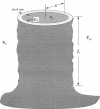
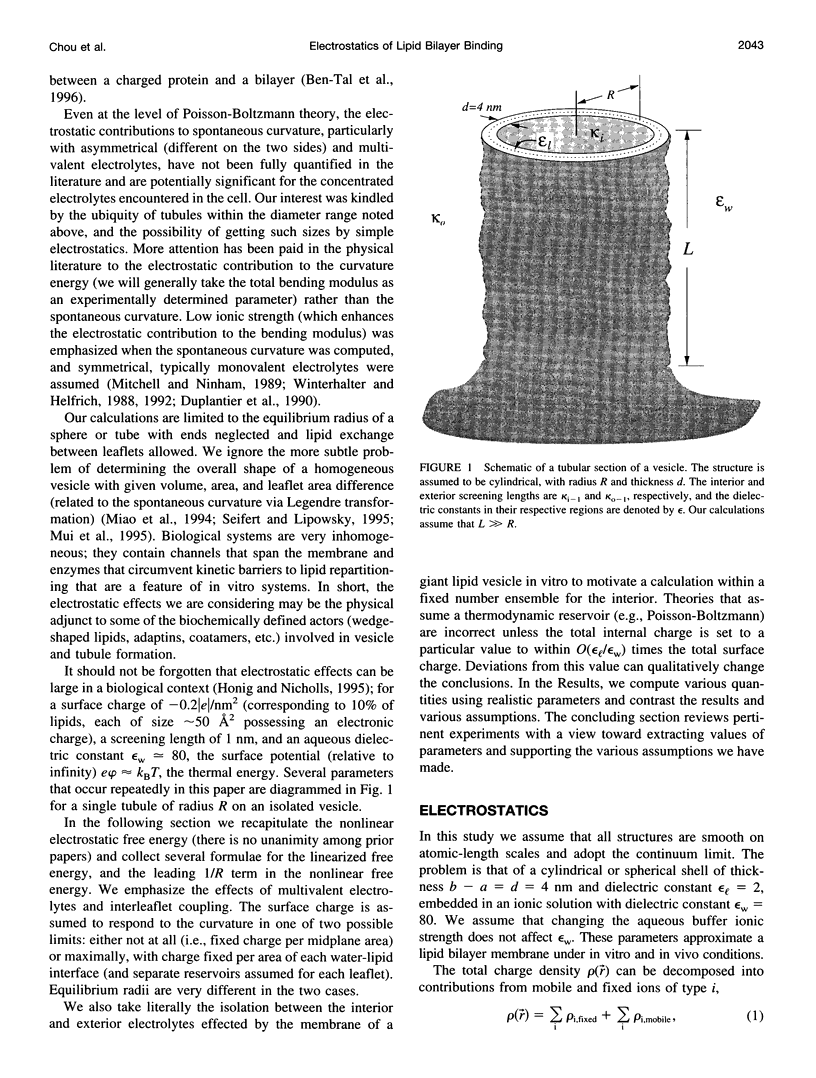
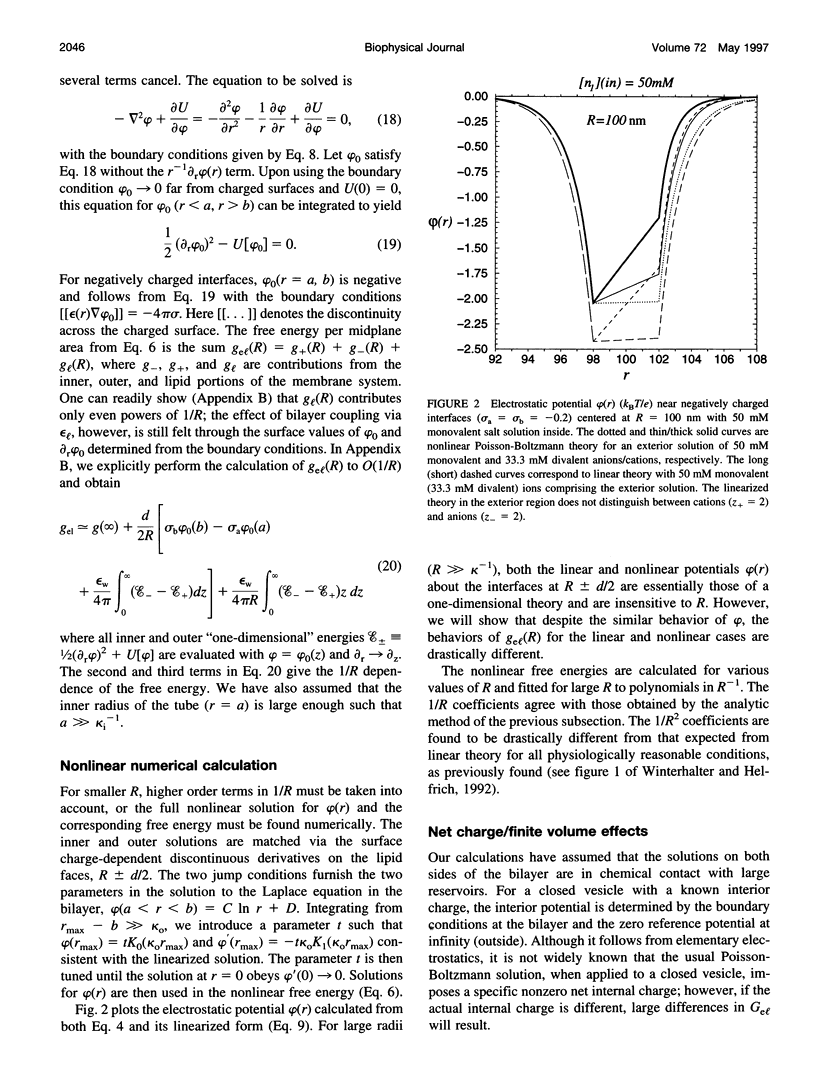
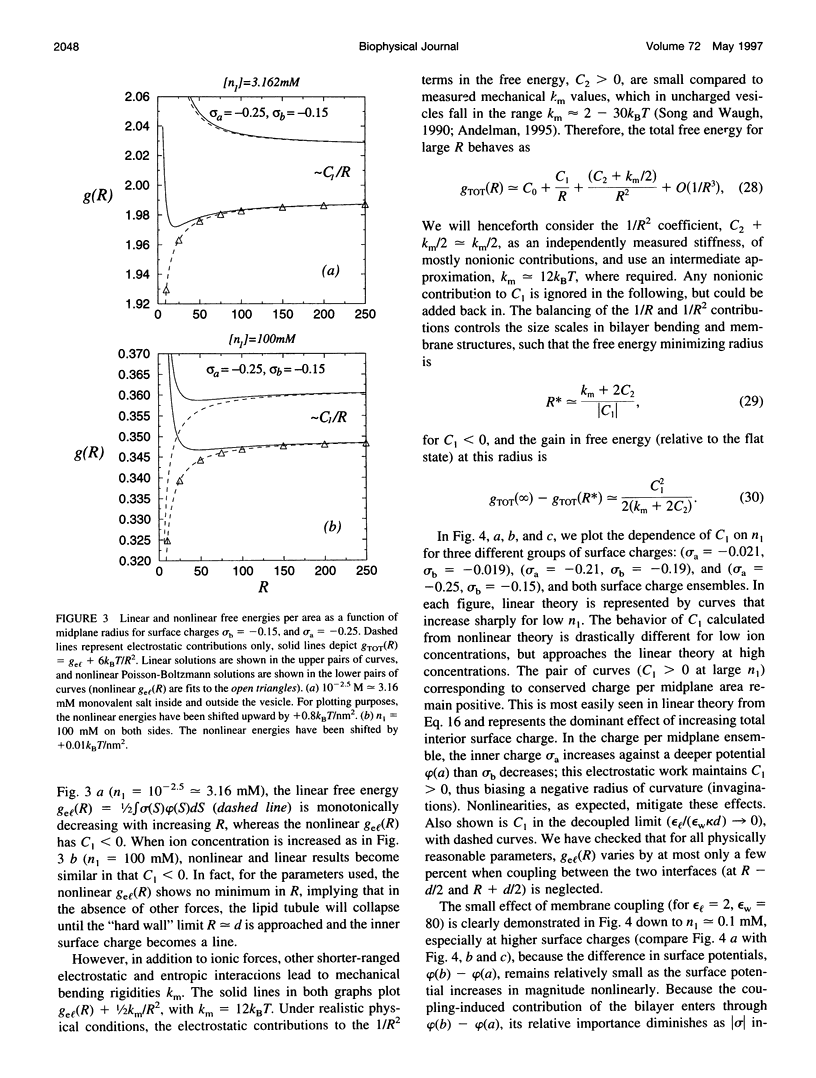
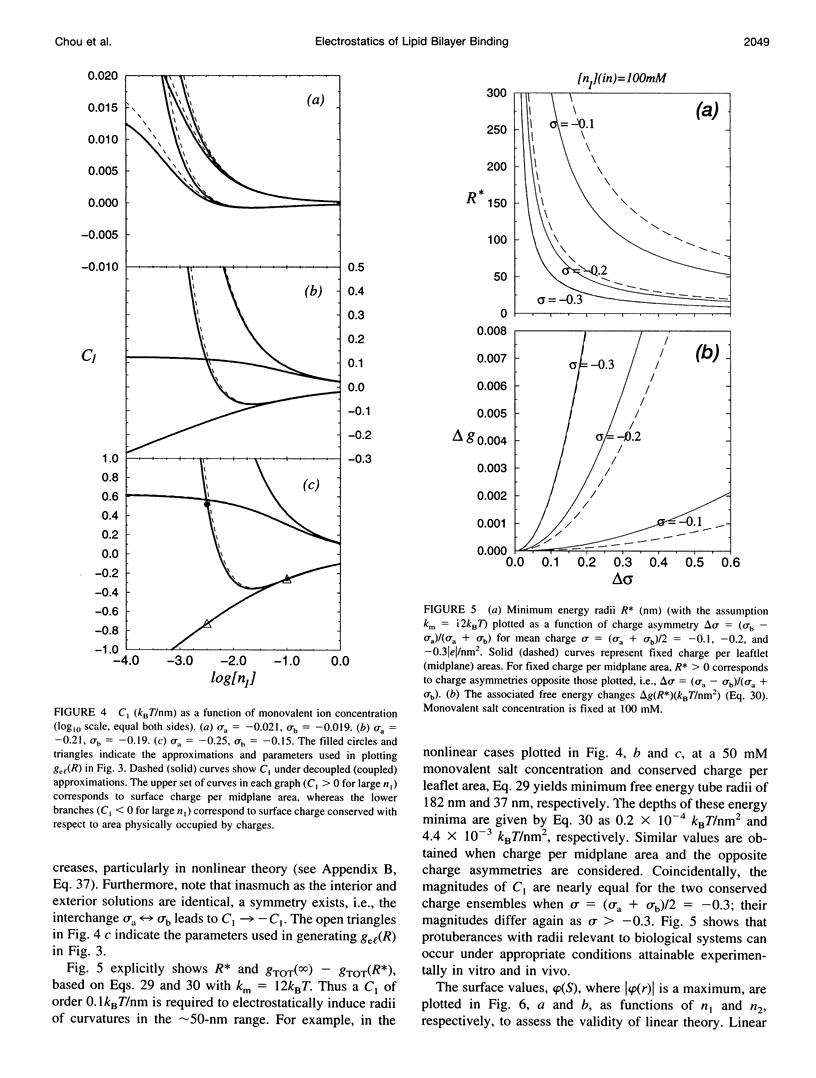
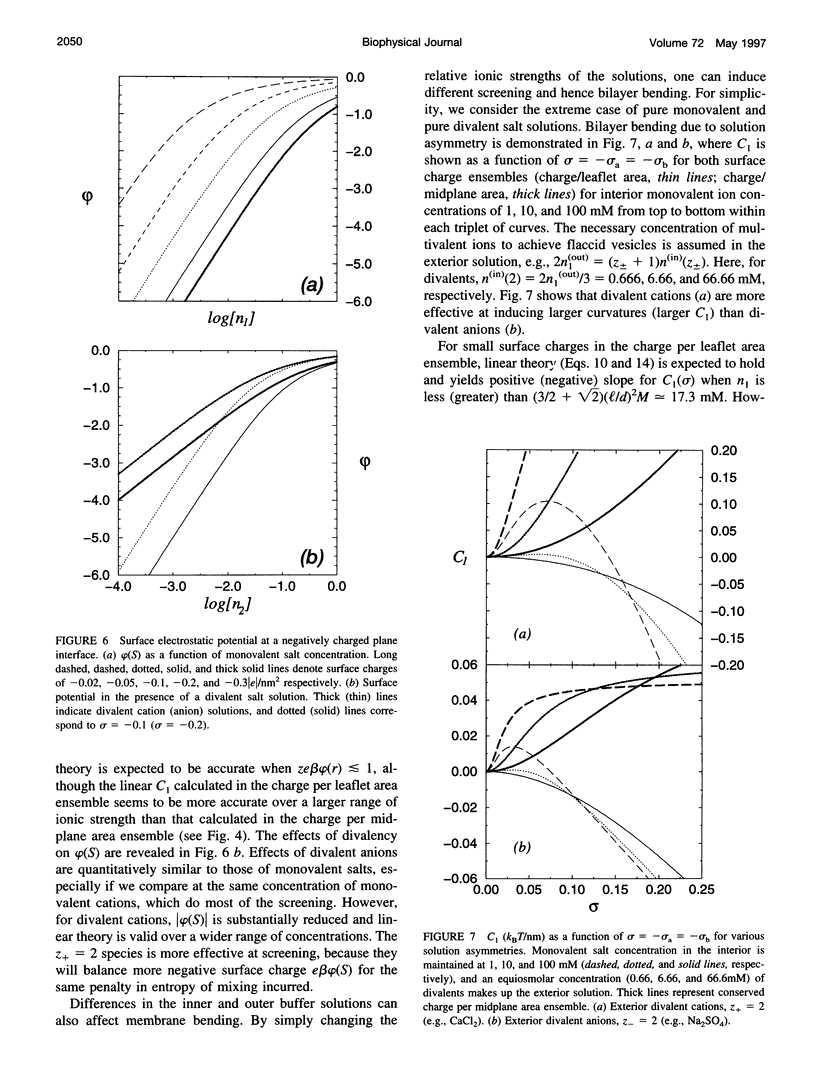
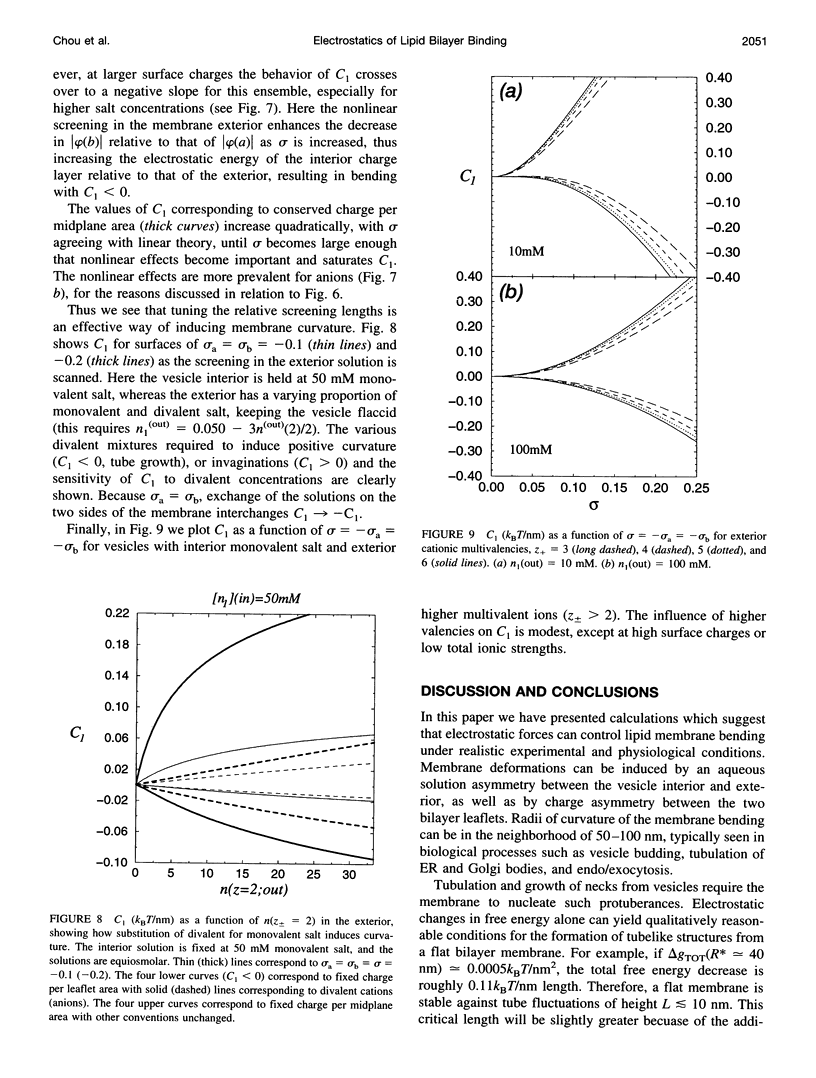
The electrostatic contribution to spontaneous membrane curvature is calculated within Poisson-Boltzmann theory under a variety of assumptions and emphasizing parameters in the physiological range. Asymmetrical surface charges can be fixed with respect to bilayer midplane area or with respect to the lipid-water area, but induce curvatures of opposite signs. Unequal screening layers on the two sides of a vesicle (e.g., multivalent cationic proteins on one side and monovalent salt on the other) also induce bending. For reasonable parameters, tubules formed by electrostatically induced bending can have radii in the 50-100-nm range, often seen in many intracellular organelles. Thus membrane associated proteins may induce curvature and subsequent budding, without themselves being intrinsically curved. Furthermore, we derive the previously unexplored effects of respecting the strict conservation of charge within the interior of a vesicle. The electrostatic component of the bending modulus is small under most of our conditions and is left as an experimental parameter. The large parameter space of conditions is surveyed in an array of graphs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Tal N., Honig B., Peitzsch R. M., Denisov G., McLaughlin S. Binding of small basic peptides to membranes containing acidic lipids: theoretical models and experimental results. Biophys J. 1996 Aug;71(2):561–575. doi: 10.1016/S0006-3495(96)79280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Munro S. Cholesterol and the Golgi apparatus. Science. 1993 Sep 3;261(5126):1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- Chow W. S., Barber J. Salt-dependent changes of 9-aminoacridine fluorescence as a measure of charge densities of membrane surfaces. J Biochem Biophys Methods. 1980 Sep;3(3):173–185. doi: 10.1016/0165-022x(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Cluett E. B., Wood S. A., Banta M., Brown W. J. Tubulation of Golgi membranes in vivo and in vitro in the absence of brefeldin A. J Cell Biol. 1993 Jan;120(1):15–24. doi: 10.1083/jcb.120.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Emr S. D., McPherson P. S., Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996 Mar 15;271(5255):1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- Duplantier B, Goldstein RE, Romero-Rochn V, V, Pesci AI. Geometrical and topological aspects of electric double layers near curved surfaces. Phys Rev Lett. 1990 Jul 23;65(4):508–511. doi: 10.1103/PhysRevLett.65.508. [DOI] [PubMed] [Google Scholar]
- Eibl H., Blume A. The influence of charge on phosphatidic acid bilayer membranes. Biochim Biophys Acta. 1979 Jun 2;553(3):476–488. doi: 10.1016/0005-2736(79)90303-1. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Maxfield F. R. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995 Aug;7(4):552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism: the molecular basis of nonbilayer phases. Annu Rev Biophys Biophys Chem. 1985;14:211–238. doi: 10.1146/annurev.bb.14.060185.001235. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Redelmeier T. E., Wong K. F., Rodrigueza W., Cullis P. R. Phospholipid asymmetry in large unilamellar vesicles induced by transmembrane pH gradients. Biochemistry. 1989 May 16;28(10):4181–4187. doi: 10.1021/bi00436a009. [DOI] [PubMed] [Google Scholar]
- Hui S. W. Lipid molecular shape and high curvature structures. Biophys J. 1993 Oct;65(4):1361–1362. doi: 10.1016/S0006-3495(93)81186-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Taraschi T. F., Janes N. Support for the shape concept of lipid structure based on a headgroup volume approach. Biophys J. 1993 Oct;65(4):1429–1432. doi: 10.1016/S0006-3495(93)81206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky R. Domain-induced budding of fluid membranes. Biophys J. 1993 Apr;64(4):1133–1138. doi: 10.1016/S0006-3495(93)81479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Miao L, Seifert U, Wortis M, Döbereiner HG. Budding transitions of fluid-bilayer vesicles: The effect of area-difference elasticity. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1994 Jun;49(6):5389–5407. doi: 10.1103/physreve.49.5389. [DOI] [PubMed] [Google Scholar]
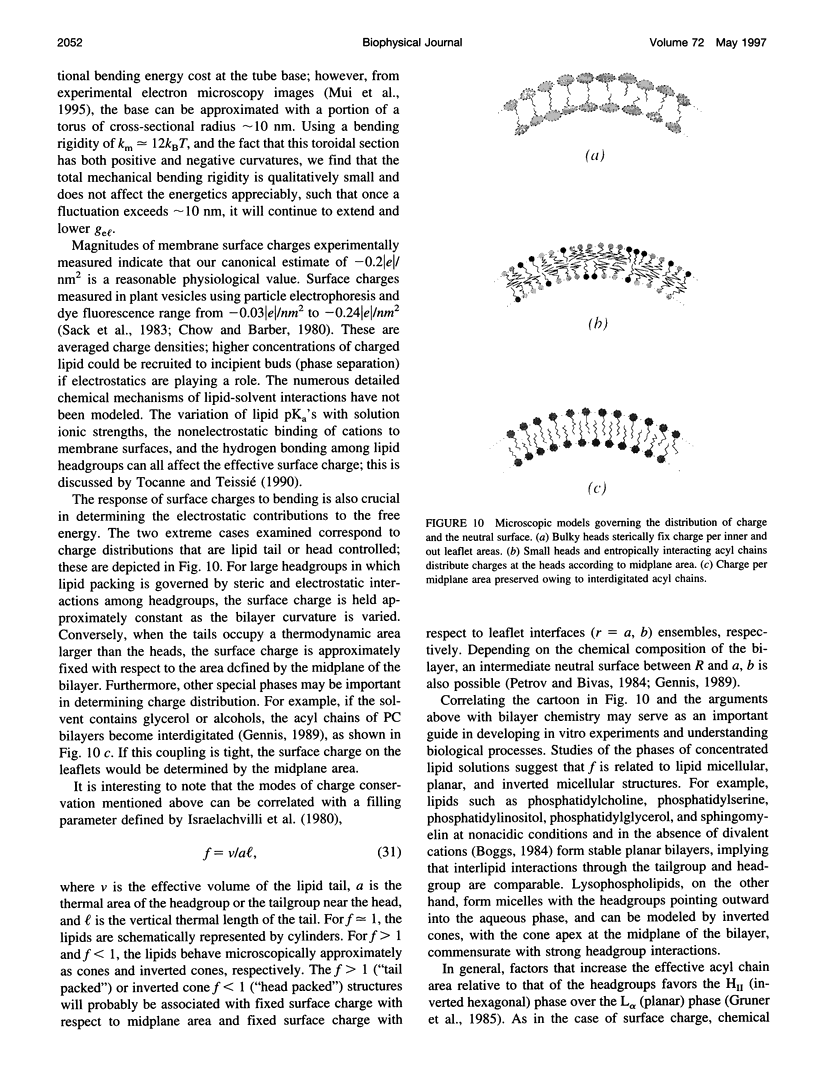
- Mui B. L., Döbereiner H. G., Madden T. D., Cullis P. R. Influence of transbilayer area asymmetry on the morphology of large unilamellar vesicles. Biophys J. 1995 Sep;69(3):930–941. doi: 10.1016/S0006-3495(95)79967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A., Clermont Y. Three-dimensional electron microscopy: structure of the Golgi apparatus. Eur J Cell Biol. 1990 Apr;51(2):189–200. [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Schekman R., Orci L. Coat proteins and vesicle budding. Science. 1996 Mar 15;271(5255):1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Shraiman B. I. On the role of assembly kinetics in determining the structure of clathrin cages. Biophys J. 1997 Feb;72(2 Pt 1):953–957. doi: 10.1016/s0006-3495(97)78729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Waugh R. E. Bending rigidity of SOPC membranes containing cholesterol. Biophys J. 1993 Jun;64(6):1967–1970. doi: 10.1016/S0006-3495(93)81566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Waugh R. E. Bilayer membrane bending stiffness by tether formation from mixed PC-PS lipid vesicles. J Biomech Eng. 1990 Aug;112(3):235–240. doi: 10.1115/1.2891178. [DOI] [PubMed] [Google Scholar]
- Terasaki M., Chen L. B., Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986 Oct;103(4):1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocanne J. F., Teissié J. Ionization of phospholipids and phospholipid-supported interfacial lateral diffusion of protons in membrane model systems. Biochim Biophys Acta. 1990 Feb 28;1031(1):111–142. doi: 10.1016/0304-4157(90)90005-w. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]