Abstract
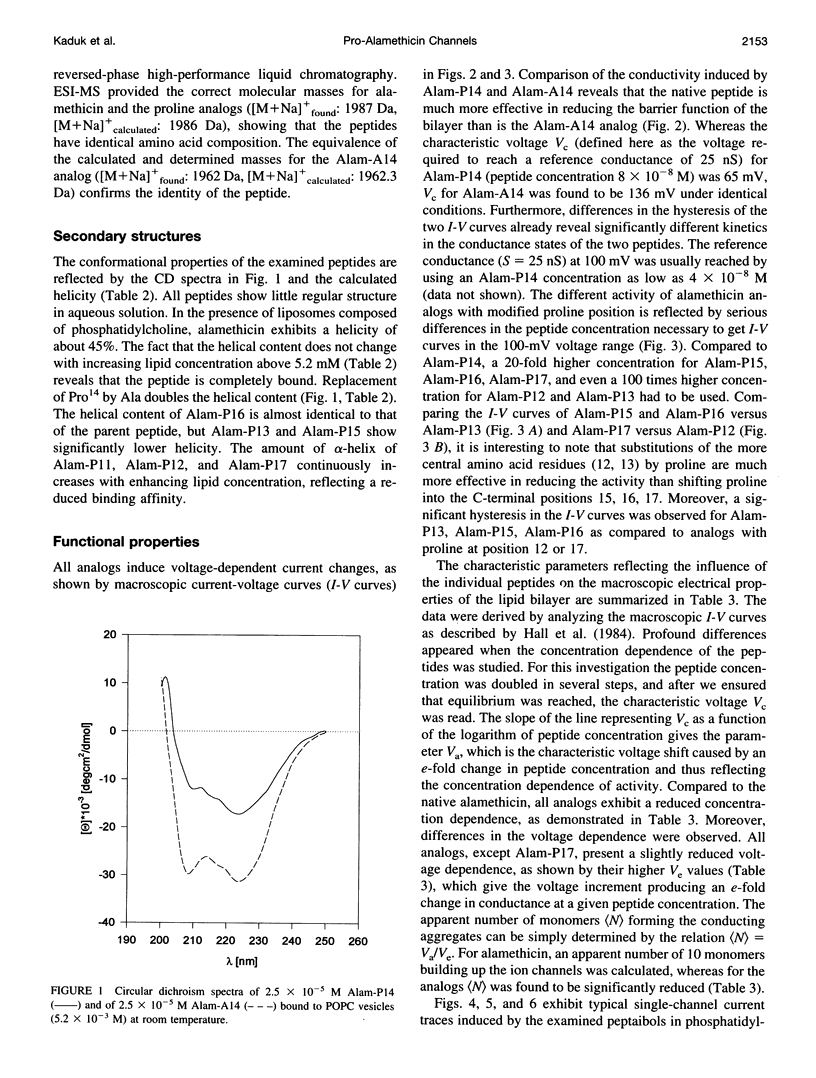
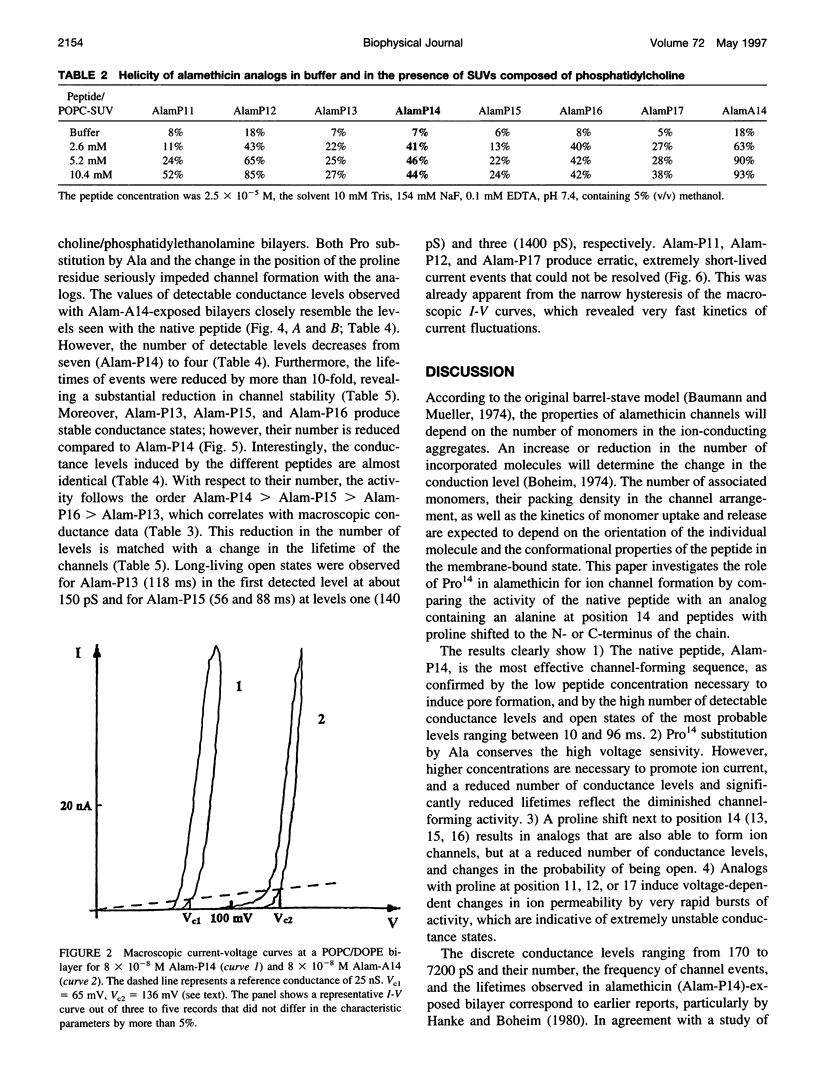
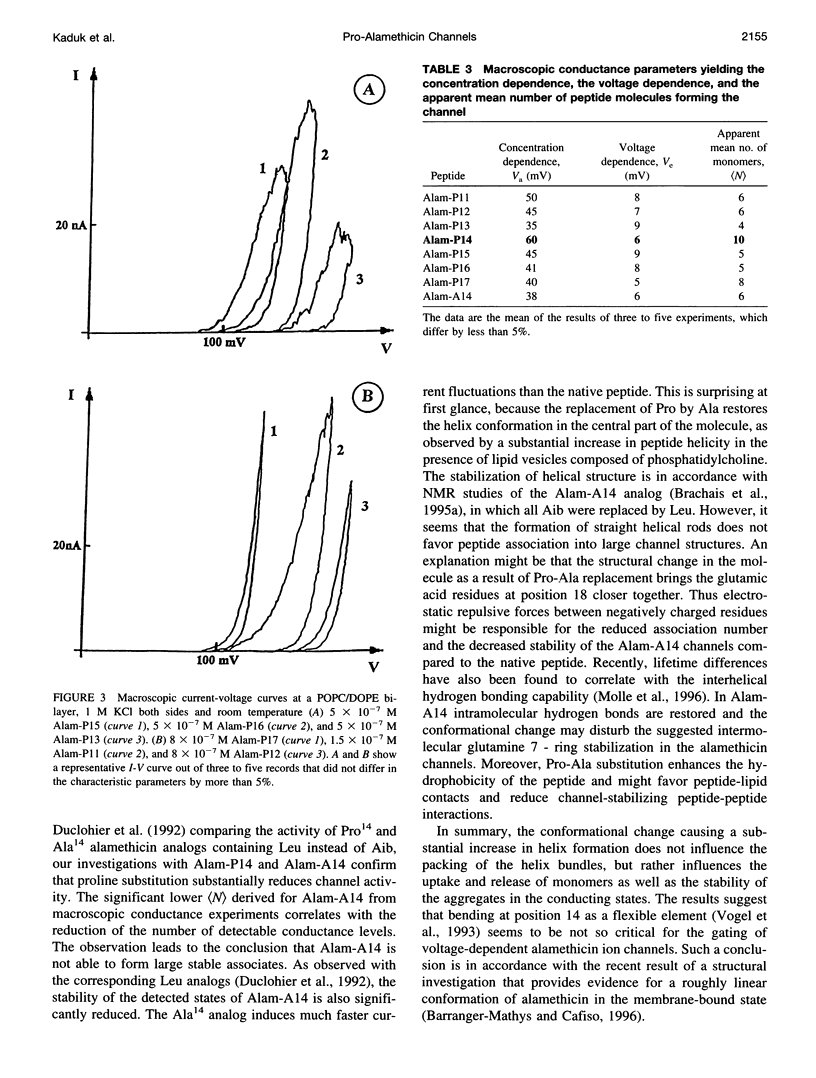
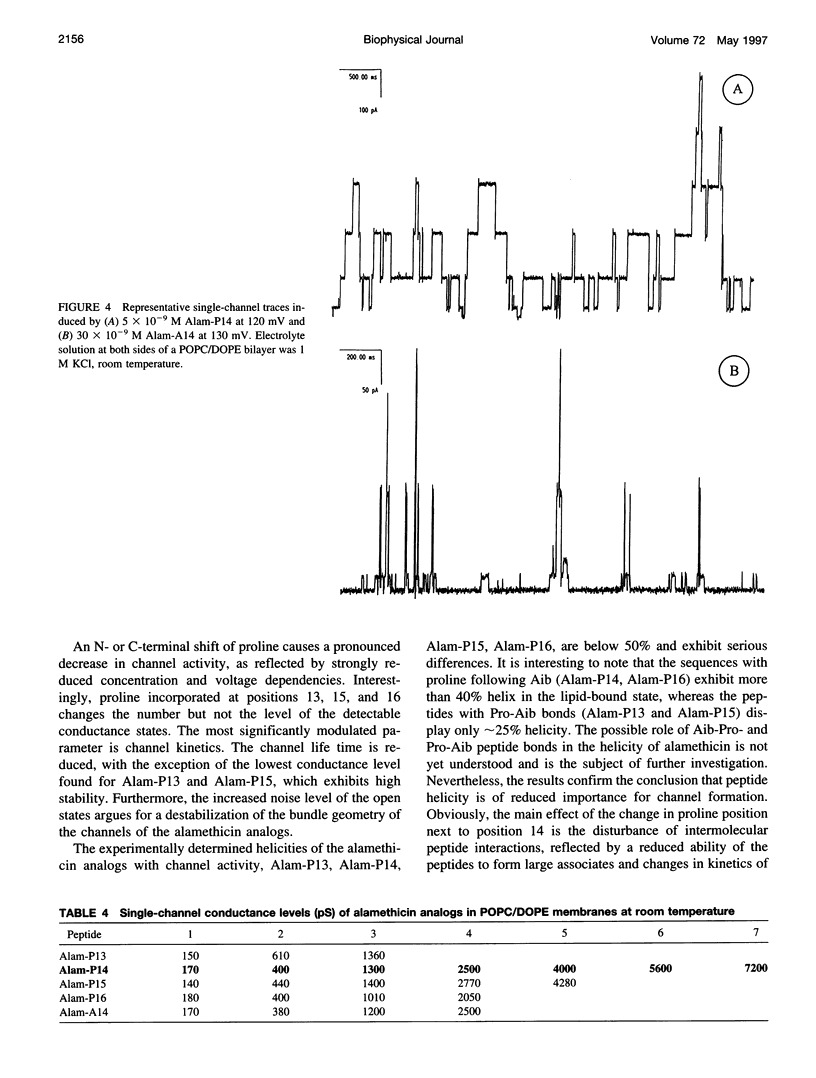
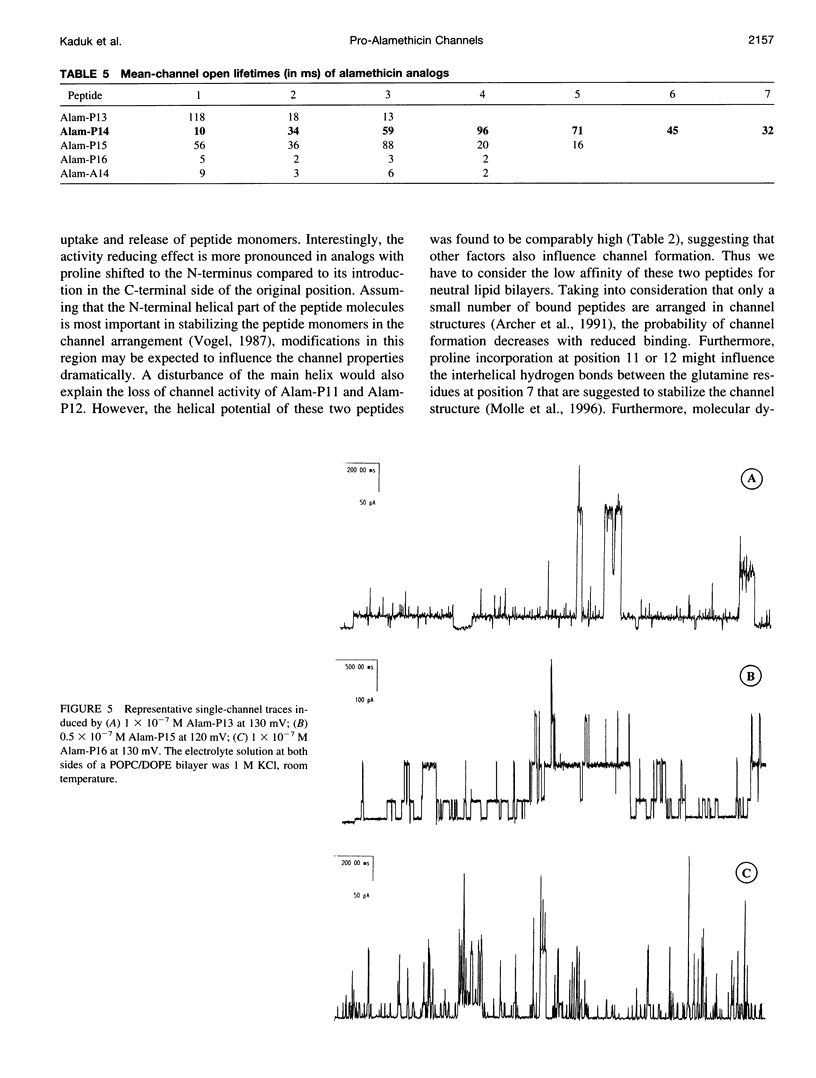
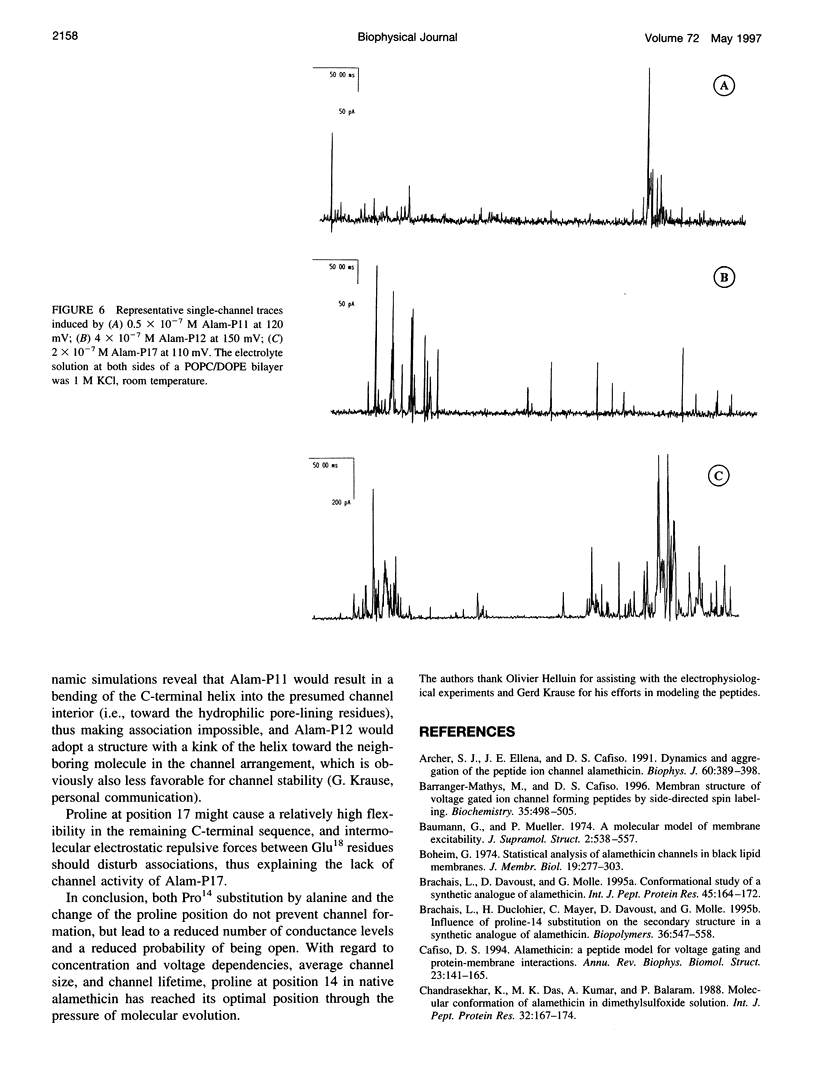
Alamethicin, a 20-residue peptaibol, induces voltage-dependent ion channels in lipid bilayers according to the barrel-stave model. To study relationships between the proline-14-induced kink region and the channel-forming behavior of the peptide, a set of alamethicin analogs with proline incorporated at positions 11, 12, 13, 14, 15, 16, and 17, respectively, as well as an analog with alanine instead of proline at position 14 were synthesized. Macroscopic conductance experiments show that the voltage dependence of the peptides is conserved although slightly influenced, but the apparent mean number of monomers forming the channels is significantly reduced when proline is not located at position 14. This is confirmed in single-channel experiments. The analogs with proline next to position 14 (i.e., 13, 15, 16) show stable conductance levels, but of reduced number, which follows the order Alam-P14 > Alam-P15 > Alam-P16 > Alam-P13. This reduction in the number of levels is connected with changes in the lifetime of the channels. Analogs with proline at position 11, 12, or 17 produce erratic, extremely short-lived current events that could not be resolved. The changes in functional properties are related to structural properties as probed by circular dichroism. The results indicate that proline at position 14 results in optimal channel activity, whereas channels formed by the analogs bearing proline at different positions are considerably less stable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer S. J., Ellena J. F., Cafiso D. S. Dynamics and aggregation of the peptide ion channel alamethicin. Measurements using spin-labeled peptides. Biophys J. 1991 Aug;60(2):389–398. doi: 10.1016/S0006-3495(91)82064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barranger-Mathys M., Cafiso D. S. Membrane structure of voltage-gated channel forming peptides by site-directed spin-labeling. Biochemistry. 1996 Jan 16;35(2):498–505. doi: 10.1021/bi951985d. [DOI] [PubMed] [Google Scholar]
- Baumann G., Mueller P. A molecular model of membrane excitability. J Supramol Struct. 1974;2(5-6):538–557. doi: 10.1002/jss.400020504. [DOI] [PubMed] [Google Scholar]
- Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J Membr Biol. 1974;19(3):277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- Brachais L., Davoust D., Molle G. Conformational study of a synthetic analogue of alamethicin. Influence of the conformation on ion-channel lifetimes. Int J Pept Protein Res. 1995 Feb;45(2):164–172. doi: 10.1111/j.1399-3011.1995.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Brachais L., Duclohier H., Mayer C., Davoust D., Molle G. Influence of proline-14 substitution on the secondary structure in a synthetic analogue of alamethicin. Biopolymers. 1995 Oct;36(4):547–558. doi: 10.1002/bip.360360416. [DOI] [PubMed] [Google Scholar]
- Cafiso D. S. Alamethicin: a peptide model for voltage gating and protein-membrane interactions. Annu Rev Biophys Biomol Struct. 1994;23:141–165. doi: 10.1146/annurev.bb.23.060194.001041. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Duclohier H., Molle G., Dugast J. Y., Spach G. Prolines are not essential residues in the "barrel-stave" model for ion channels induced by alamethicin analogues. Biophys J. 1992 Sep;63(3):868–873. doi: 10.1016/S0006-3495(92)81637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G., Carver J. A., Boyd J., Campbell I. D. High-resolution 1H NMR study of the solution structure of alamethicin. Biochemistry. 1987 Feb 24;26(4):1043–1050. doi: 10.1021/bi00378a010. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Franklin J. C., Ellena J. F., Jayasinghe S., Kelsh L. P., Cafiso D. S. Structure of micelle-associated alamethicin from 1H NMR. Evidence for conformational heterogeneity in a voltage-gated peptide. Biochemistry. 1994 Apr 5;33(13):4036–4045. doi: 10.1021/bi00179a032. [DOI] [PubMed] [Google Scholar]
- Fraternali F. Restrained and unrestrained molecular dynamics simulations in the NVT ensemble of alamethicin. Biopolymers. 1990;30(11-12):1083–1099. doi: 10.1002/bip.360301109. [DOI] [PubMed] [Google Scholar]
- Gordon L. G., Haydon D. A. Potential-dependent conductances in lipid membranes containing alamethicin. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):433–447. doi: 10.1098/rstb.1975.0021. [DOI] [PubMed] [Google Scholar]
- Gordon L. G., Haydon D. A. The unit conductance channel of alamethicin. Biochim Biophys Acta. 1972 Mar 17;255(3):1014–1018. doi: 10.1016/0005-2736(72)90415-4. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Vodyanoy I., Balasubramanian T. M., Marshall G. R. Alamethicin. A rich model for channel behavior. Biophys J. 1984 Jan;45(1):233–247. doi: 10.1016/S0006-3495(84)84151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke W., Boheim G. The lowest conductance state of the alamethicin pore. Biochim Biophys Acta. 1980 Mar 13;596(3):456–462. doi: 10.1016/0005-2736(80)90134-0. [DOI] [PubMed] [Google Scholar]
- Kelsh L. P., Ellena J. F., Cafiso D. S. Determination of the molecular dynamics of alamethicin using 13C NMR: implications for the mechanism of gating of a voltage-dependent channel. Biochemistry. 1992 Jun 9;31(22):5136–5144. doi: 10.1021/bi00137a007. [DOI] [PubMed] [Google Scholar]
- Meyer C. E., Reusser F. A polypeptide antibacterial agent isolated from Trichoderma viride. Experientia. 1967 Feb 15;23(2):85–86. doi: 10.1007/BF02135929. [DOI] [PubMed] [Google Scholar]
- Molle G., Dugast J. Y., Spach G., Duclohier H. Ion channel stabilization of synthetic alamethicin analogs by rings of inter-helix H-bonds. Biophys J. 1996 Apr;70(4):1669–1675. doi: 10.1016/S0006-3495(96)79729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North C. L., Franklin J. C., Bryant R. G., Cafiso D. S. Molecular flexibility demonstrated by paramagnetic enhancements of nuclear relaxation. Application to alamethicin: a voltage-gated peptide channel. Biophys J. 1994 Nov;67(5):1861–1866. doi: 10.1016/S0006-3495(94)80667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom M. S. Structure and function of channel-forming peptaibols. Q Rev Biophys. 1993 Nov;26(4):365–421. doi: 10.1017/s0033583500002833. [DOI] [PubMed] [Google Scholar]
- Sansom M. S. The biophysics of peptide models of ion channels. Prog Biophys Mol Biol. 1991;55(3):139–235. doi: 10.1016/0079-6107(91)90004-c. [DOI] [PubMed] [Google Scholar]
- Vanhoof G., Goossens F., De Meester I., Hendriks D., Scharpé S. Proline motifs in peptides and their biological processing. FASEB J. 1995 Jun;9(9):736–744. [PubMed] [Google Scholar]
- Vogel H. Comparison of the conformation and orientation of alamethicin and melittin in lipid membranes. Biochemistry. 1987 Jul 14;26(14):4562–4572. doi: 10.1021/bi00388a060. [DOI] [PubMed] [Google Scholar]
- Vogel H., Nilsson L., Rigler R., Meder S., Boheim G., Beck W., Kurth H. H., Jung G. Structural fluctuations between two conformational states of a transmembrane helical peptide are related to its channel-forming properties in planar lipid membranes. Eur J Biochem. 1993 Mar 1;212(2):305–313. doi: 10.1111/j.1432-1033.1993.tb17663.x. [DOI] [PubMed] [Google Scholar]
- Woolfson D. N., Mortishire-Smith R. J., Williams D. H. Conserved positioning of proline residues in membrane-spanning helices of ion-channel proteins. Biochem Biophys Res Commun. 1991 Mar 29;175(3):733–737. doi: 10.1016/0006-291x(91)91627-o. [DOI] [PubMed] [Google Scholar]
- Woolley G. A., Wallace B. A. Model ion channels: gramicidin and alamethicin. J Membr Biol. 1992 Aug;129(2):109–136. doi: 10.1007/BF00219508. [DOI] [PubMed] [Google Scholar]
- Yee A. A., O'Neil J. D. Uniform 15N labeling of a fungal peptide: the structure and dynamics of an alamethicin by 15N and 1H NMR spectroscopy. Biochemistry. 1992 Mar 31;31(12):3135–3143. doi: 10.1021/bi00127a014. [DOI] [PubMed] [Google Scholar]



