Abstract
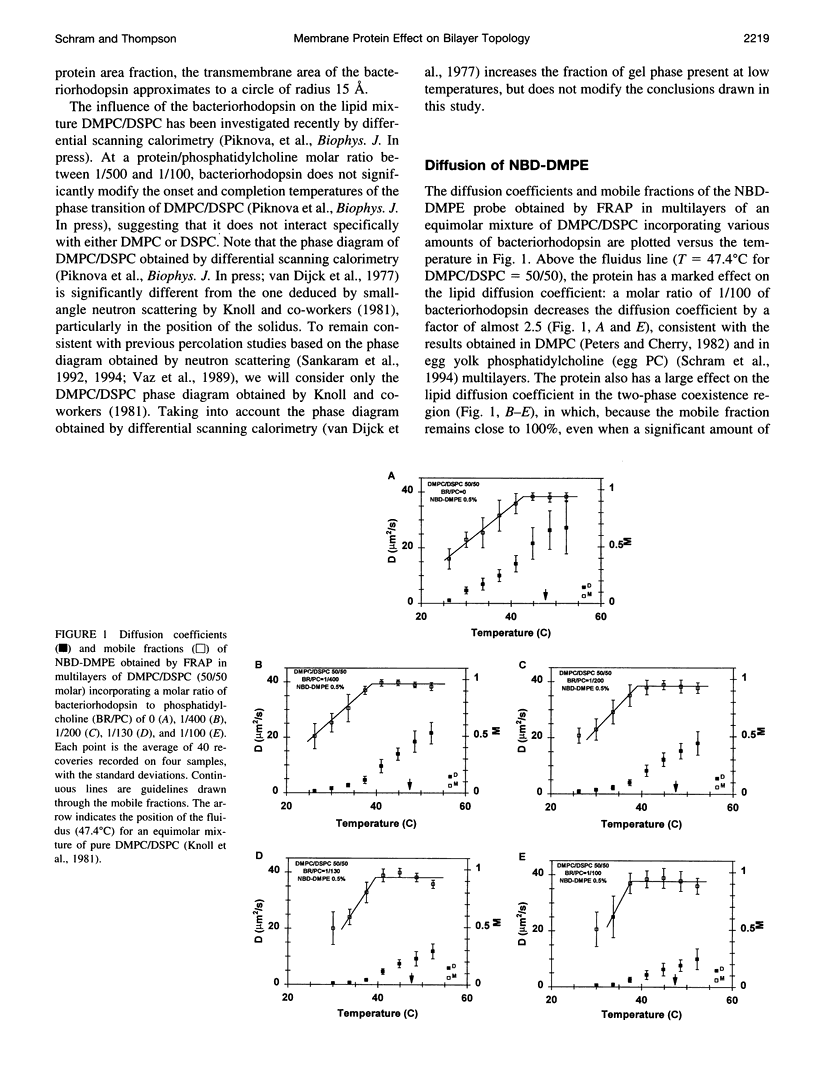
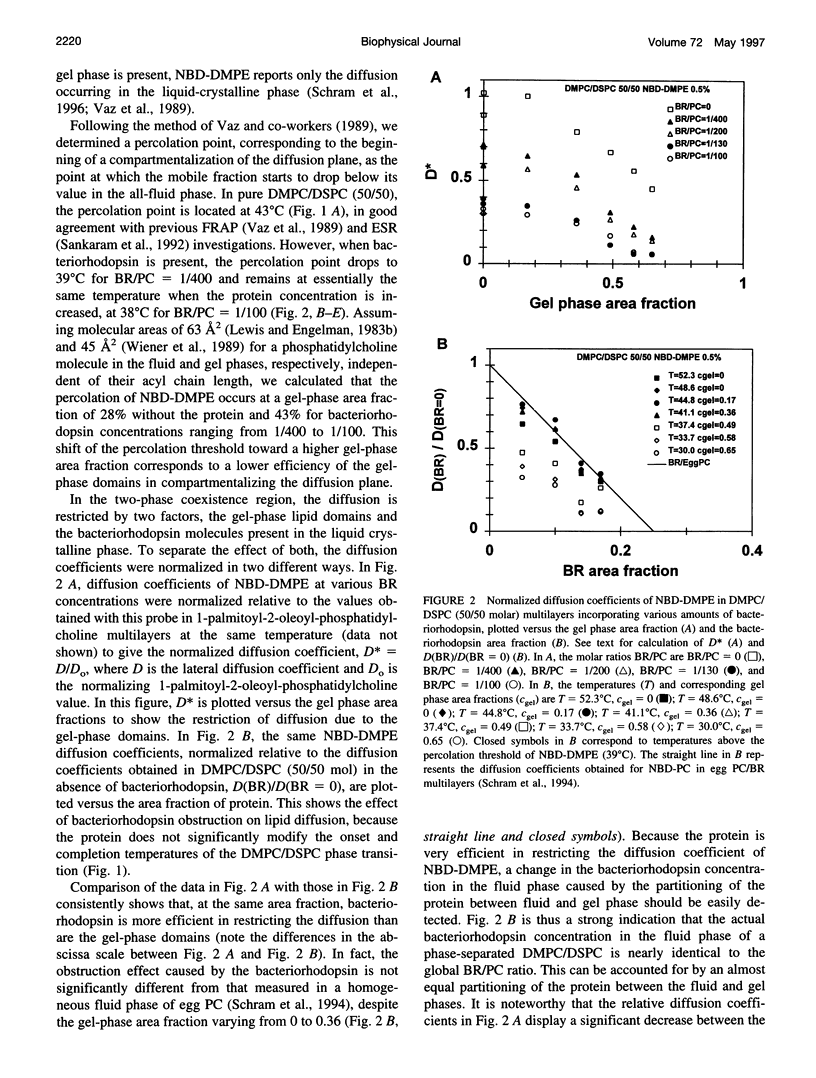
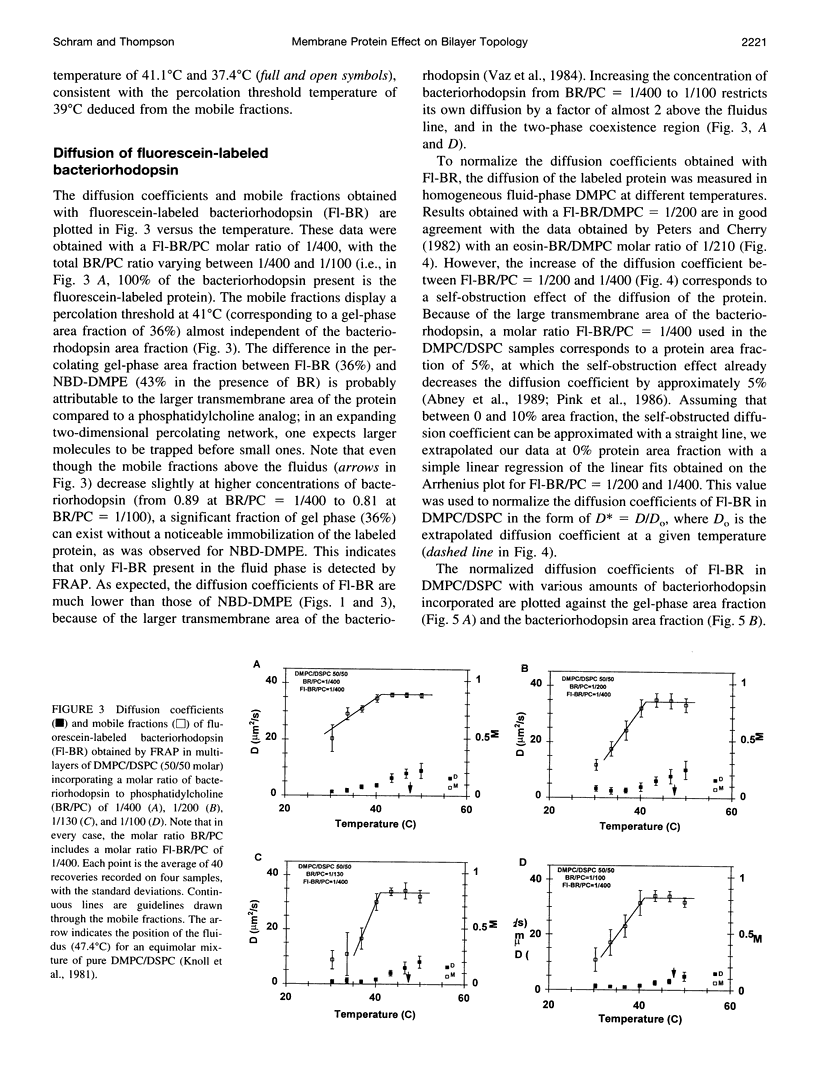
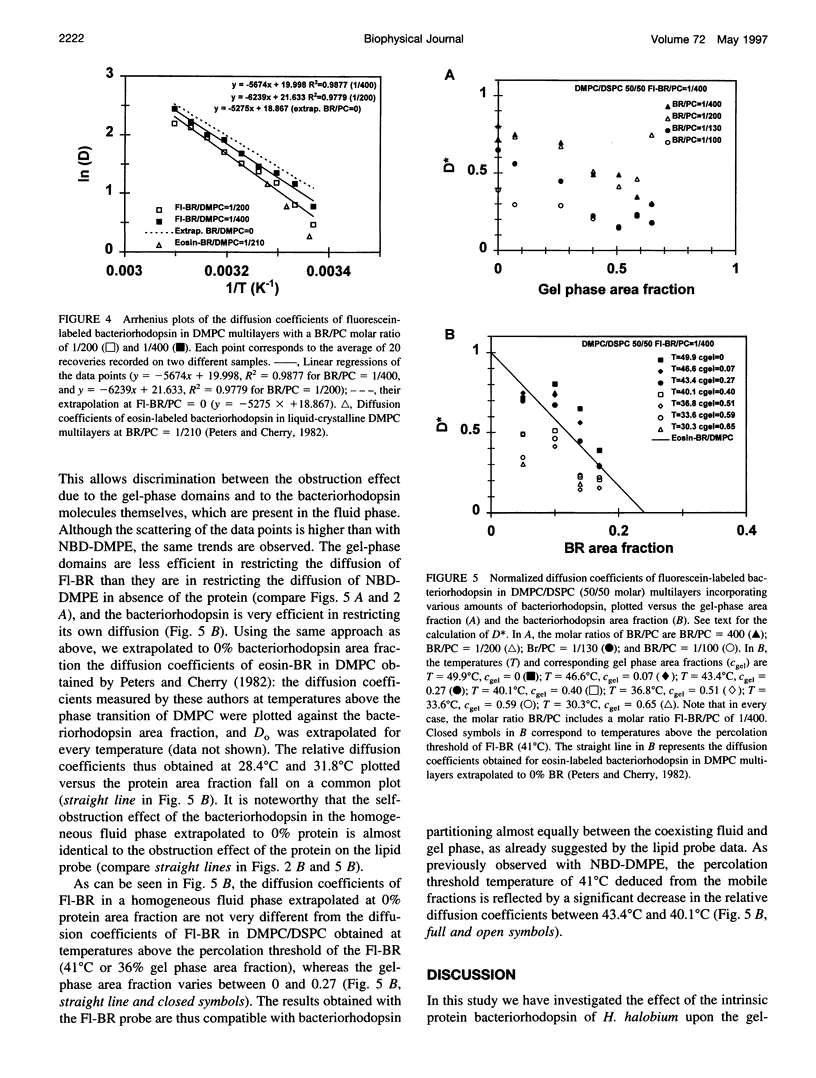
We have investigated the effect of the intrinsic membrane protein bacteriorhodopsin of Halobacterium halobium on the lateral organization of the lipid phase structure in the coexistence region of an equimolar mixture of dimyristoylphos-phatidylcholine and distearoylphosphatidylcholine. The fluorescence recovery after photobleaching (FRAP) technique was used to monitor the diffusion of both a lipid analog (N-(7-nitrobenzoxa-2,3-diazol-4-yl)-dimyristoylphosphatidyle thanolamine, NBD-DMPE) and fluorescein-labeled bacteriorhodopsin (Fl-BR). In the presence of bacteriorhodopsin, the mobile fractions of the two fluorescent probes display a shift of the percolation threshold toward lower temperatures (larger gel-phase fractions), independent of the protein concentration, from 43 degrees C (without bacteriorhodopsin) to 39 degrees C and 41 degrees C for NBD-DMPE and Fl-BR, respectively. Moreover, in the presence of bacteriorhodopsin, the gel-phase domains are much less efficient in restricting the diffusion of both probes than they are in the absence of the protein in the two-phase coexistence region. Bacteriorhodopsin itself, however, obstructs diffusion of NBD-DMPE and Fl-BR to about the same extent in the fluid phase of the two-phase region as it does in the homogeneous fluid phase. These observations suggest that 1) the protein induces the formation of much larger and/or more centrosymmetrical gel-phase domains than those formed in its absence, and 2) bacteriorhodopsin partitions almost equally between the coexisting fluid and gel phases. Although the molecular mechanisms involved are not clear, this phenomenon is fully consistent with the effect of the transmembrane peptide pOmpA of Escherichia coli investigated by electron spin resonance in the same lipid system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Scalettar B. A., Owicki J. C. Self diffusion of interacting membrane proteins. Biophys J. 1989 May;55(5):817–833. doi: 10.1016/S0006-3495(89)82882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion and percolation in two-phase, two-component lipid bilayers. Topology of the solid-phase domains in-plane and across the lipid bilayer. Biochemistry. 1992 Aug 11;31(31):7198–7210. doi: 10.1021/bi00146a024. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Gulik-Krzywicki T., Seigneuret M., Rigaud J. L. Monomer-oligomer equilibrium of bacteriorhodopsin in reconstituted proteoliposomes. A freeze-fracture electron microscope study. J Biol Chem. 1987 Nov 15;262(32):15580–15588. [PubMed] [Google Scholar]
- Heberle J., Dencher N. A. Surface-bound optical probes monitor protein translocation and surface potential changes during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5996–6000. doi: 10.1073/pnas.89.13.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburg T., Biltonen R. L. A Monte Carlo simulation study of protein-induced heat capacity changes and lipid-induced protein clustering. Biophys J. 1996 Jan;70(1):84–96. doi: 10.1016/S0006-3495(96)79551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Blume A., Rehorek M., Dencher N. A. Calorimetric and fluorescence depolarization studies on the lipid phase transition of bacteriorhodopsin--dimyristoylphosphatidylcholine vesicles. Biochemistry. 1981 Dec 8;20(25):7109–7115. doi: 10.1021/bi00528a009. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. I. Theoretical description. Biophys J. 1981 Nov;36(2):329–345. doi: 10.1016/S0006-3495(81)84735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G., Gerber G. E., Herlihy W. C., Gray C. P., Anderegg R. J., Nihei K., Biemann K. Amino acid sequence of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5046–5050. doi: 10.1073/pnas.76.10.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll W., Ibel K., Sackmann E. Small-angle neutron scattering study of lipid phase diagrams by the contrast variation method. Biochemistry. 1981 Oct 27;20(22):6379–6383. doi: 10.1021/bi00525a015. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Bacteriorhodopsin remains dispersed in fluid phospholipid bilayers over a wide range of bilayer thicknesses. J Mol Biol. 1983 May 15;166(2):203–210. doi: 10.1016/s0022-2836(83)80006-0. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Luan P., Yang L., Glaser M. Formation of membrane domains created during the budding of vesicular stomatitis virus. A model for selective lipid and protein sorting in biological membranes. Biochemistry. 1995 Aug 8;34(31):9874–9883. doi: 10.1021/bi00031a008. [DOI] [PubMed] [Google Scholar]
- Morrow M. R., Davis J. H., Sharom F. J., Lamb M. P. Studies on the interaction of human erythrocyte band 3 with membrane lipids using deuterium nuclear magnetic resonance and differential scanning calorimetry. Biochim Biophys Acta. 1986 Jun 13;858(1):13–20. doi: 10.1016/0005-2736(86)90286-5. [DOI] [PubMed] [Google Scholar]
- Peters R., Cherry R. J. Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbrück equations. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4317–4321. doi: 10.1073/pnas.79.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piknová B., Marsh D., Thompson T. E. Fluorescence-quenching study of percolation and compartmentalization in two-phase lipid bilayers. Biophys J. 1996 Aug;71(2):892–897. doi: 10.1016/S0006-3495(96)79291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piknová B., Pérochon E., Tocanne J. F. Hydrophobic mismatch and long-range protein/lipid interactions in bacteriorhodopsin/phosphatidylcholine vesicles. Eur J Biochem. 1993 Dec 1;218(2):385–396. doi: 10.1111/j.1432-1033.1993.tb18388.x. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Distributions of proteins and lipids in the erythrocyte membrane. Biochemistry. 1993 Nov 30;32(47):12591–12598. doi: 10.1021/bi00210a007. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Marsh D., Gierasch L. M., Thompson T. E. Reorganization of lipid domain structure in membranes by a transmembrane peptide: an ESR spin label study on the effect of the Escherichia coli outer membrane protein A signal peptide on the fluid lipid domain connectivity in binary mixtures of dimyristoyl phosphatidylcholine and distearoyl phosphatidylcholine. Biophys J. 1994 Jun;66(6):1959–1968. doi: 10.1016/S0006-3495(94)80989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Marsh D., Thompson T. E. Determination of fluid and gel domain sizes in two-component, two-phase lipid bilayers. An electron spin resonance spin label study. Biophys J. 1992 Aug;63(2):340–349. doi: 10.1016/S0006-3495(92)81619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion and aggregation. A Monte Carlo study. Biophys J. 1992 Jan;61(1):119–128. doi: 10.1016/S0006-3495(92)81821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Dependence on tracer size. Biophys J. 1993 Apr;64(4):1053–1062. doi: 10.1016/S0006-3495(93)81471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram V., Lin H. N., Thompson T. E. Topology of gel-phase domains and lipid mixing properties in phase-separated two-component phosphatidylcholine bilayers. Biophys J. 1996 Oct;71(4):1811–1822. doi: 10.1016/S0006-3495(96)79382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram V., Thompson T. E. Interdigitation does not affect translational diffusion of lipids in liquid crystalline bilayers. Biophys J. 1995 Dec;69(6):2517–2520. doi: 10.1016/S0006-3495(95)80122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram V., Tocanne J. F., Lopez A. Influence of obstacles on lipid lateral diffusion: computer simulation of FRAP experiments and application to proteoliposomes and biomembranes. Eur Biophys J. 1994;23(5):337–348. doi: 10.1007/BF00188657. [DOI] [PubMed] [Google Scholar]
- Scotto A. W., Zakim D. Reconstitution of membrane proteins. Spontaneous incorporation of integral membrane proteins into preformed bilayers of pure phospholipid. J Biol Chem. 1988 Dec 5;263(34):18500–18506. [PubMed] [Google Scholar]
- Sperotto M. M., Mouritsen O. G. Lipid enrichment and selectivity of integral membrane proteins in two-component lipid bilayers. Eur Biophys J. 1993;22(5):323–328. doi: 10.1007/BF00213555. [DOI] [PubMed] [Google Scholar]
- Sperotto M. M., Mouritsen O. G. Monte Carlo simulation studies of lipid order parameter profiles near integral membrane proteins. Biophys J. 1991 Feb;59(2):261–270. doi: 10.1016/S0006-3495(91)82219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Thompson T. E., Sankaram M. B., Biltonen R. L., Marsh D., Vaz W. L. Effects of domain structure on in-plane reactions and interactions. Mol Membr Biol. 1995 Jan-Mar;12(1):157–162. doi: 10.3109/09687689509038512. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Translational diffusion and fluid domain connectivity in a two-component, two-phase phospholipid bilayer. Biophys J. 1989 Nov;56(5):869–876. doi: 10.1016/S0006-3495(89)82733-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., Suter R. M., Nagle J. F. Structure of the fully hydrated gel phase of dipalmitoylphosphatidylcholine. Biophys J. 1989 Feb;55(2):315–325. doi: 10.1016/S0006-3495(89)82807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijck P. W., Kaper A. J., Oonk H. A., de Gier J. Miscibility properties of binary phosphatidylcholine mixtures. A calorimetric study. Biochim Biophys Acta. 1977 Oct 3;470(1):58–69. doi: 10.1016/0005-2736(77)90061-x. [DOI] [PubMed] [Google Scholar]


