Abstract
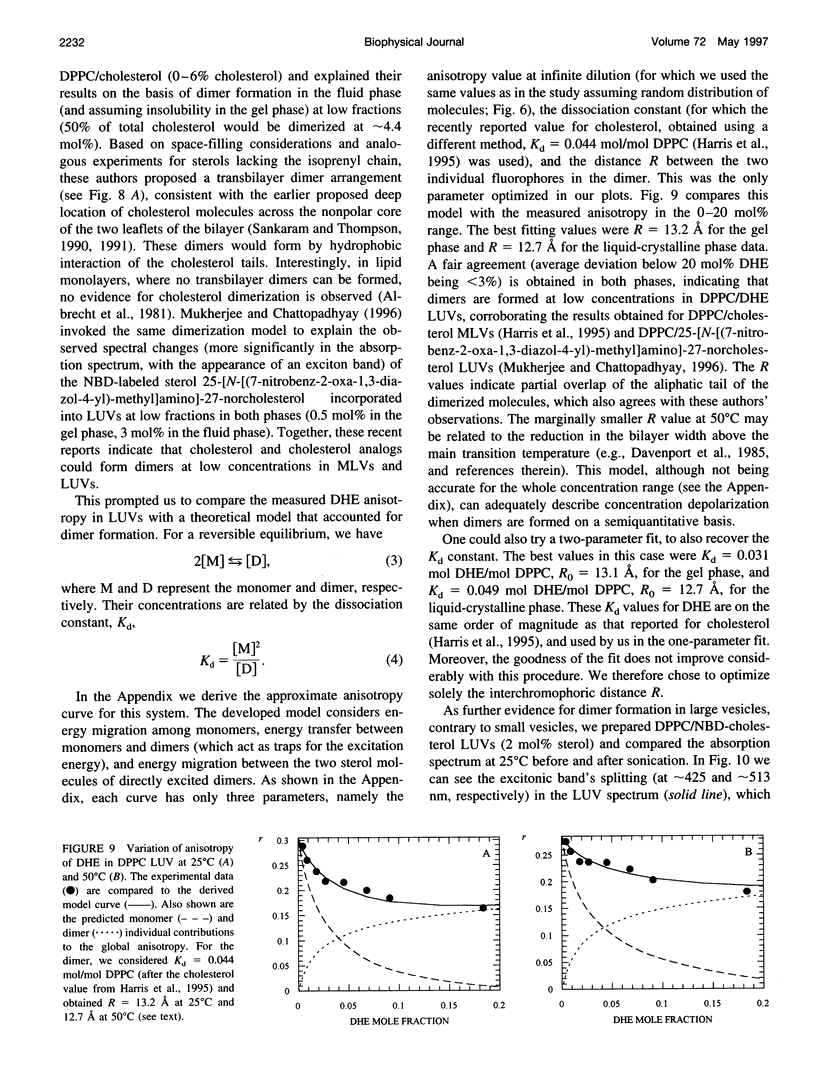
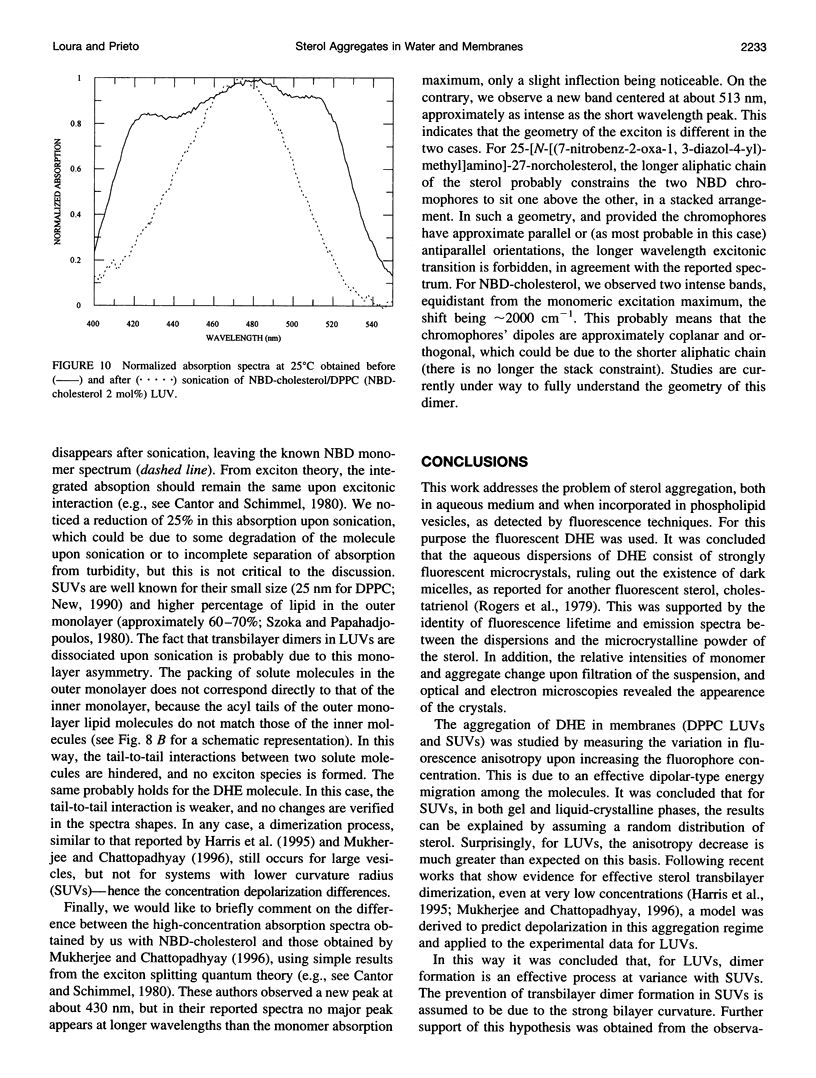
The aggregation of delta 5,7,9(11),22-ergostatetraen-3 beta-ol (dehydroergosterol or DHE), a fluorescent analog of cholesterol, was studied by photophysical techniques. It was concluded that the aqueous dispersions of DHE consist of strongly fluorescent microcrystals, and no evidence for self-quenching in micellar-type aggregates was found. The organization of DHE in model systems of membranes (phospholipid vesicles) is strongly dependent on the vesicle type. In small unilamellar vesicles, no evidence for aggregation is obtained, and the fluorescence anisotropy is rationalized on the basis of a random distribution of fluorophores. On the contrary, in large unilamellar vesicles (LUVs), a steeper concentration depolarization was observed. To explain this, a model that takes into account transbilayer dimer formation was derived. This was further confirmed from observation of excitonic absorption bands of 22-(N-7-nitrobenz-2-oxa-1,3-diazol-4-yl-amino)-23,24-bisnor- 5-cholen-3 beta-ol (NBD-cholesterol) in LUV, which disappear upon sonication. It is concluded that, in agreement with recent works, sterol aggregation is a very efficient process in large vesicles (and probably in natural membranes), even at very low concentrations (approximately 5 mol%).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butko P., Hapala I., Nemecz G., Schroeder F. Sterol domains in phospholipid membranes: dehydroergosterol polarization measures molecular sterol transfer. J Biochem Biophys Methods. 1992 Mar;24(1-2):15–37. doi: 10.1016/0165-022x(92)90043-a. [DOI] [PubMed] [Google Scholar]
- Castanho M. A., Brown W., Prieto M. J. Rod-like cholesterol micelles in aqueous solution studied using polarized and depolarized dynamic light scattering. Biophys J. 1992 Dec;63(6):1455–1461. doi: 10.1016/S0006-3495(92)81733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D. S., Benedek G. B., Konikoff F. M., Donovan J. M. Elastic free energy of anisotropic helical ribbons as metastable intermediates in the crystallization of cholesterol. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11341–11345. doi: 10.1073/pnas.90.23.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport L., Dale R. E., Bisby R. H., Cundall R. B. Transverse location of the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene in model lipid bilayer membrane systems by resonance excitation energy transfer. Biochemistry. 1985 Jul 16;24(15):4097–4108. doi: 10.1021/bi00336a044. [DOI] [PubMed] [Google Scholar]
- Eaton D. F. International Union of Pure and Applied Chemistry Organic Chemistry Division Commission on Photochemistry. Reference materials for fluorescence measurement. J Photochem Photobiol B. 1988 Dec;2(4):523–531. doi: 10.1016/1011-1344(88)85081-4. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Harris J. S., Epps D. E., Davio S. R., Kézdy F. J. Evidence for transbilayer, tail-to-tail cholesterol dimers in dipalmitoylglycerophosphocholine liposomes. Biochemistry. 1995 Mar 21;34(11):3851–3857. doi: 10.1021/bi00011a043. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., Morel B., Sauerheber R. D. Organization and interaction of cholesterol and phosphatidylcholine in model bilayer membranes. Biochemistry. 1990 Jan 30;29(4):1025–1038. doi: 10.1021/bi00456a027. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Moore B. M., Barrow D. A. Light-scattering effects in the measurement of membrane microviscosity with diphenylhexatriene. Biophys J. 1979 Mar;25(3):489–494. doi: 10.1016/S0006-3495(79)85318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClare C. W. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971 Feb;39(2):527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- McMullen T. P., Lewis R. N., McElhaney R. N. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 1993 Jan 19;32(2):516–522. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Chattopadhyay A. Membrane organization at low cholesterol concentrations: a study using 7-nitrobenz-2-oxa-1,3-diazol-4-yl-labeled cholesterol. Biochemistry. 1996 Jan 30;35(4):1311–1322. doi: 10.1021/bi951953q. [DOI] [PubMed] [Google Scholar]
- Prieto M. J., Castanho M., Coutinho A., Ortiz A., Aranda F. J., Gómez-Fernández J. C. Fluorescence study of a derivatized diacylglycerol incorporated in model membranes. Chem Phys Lipids. 1994 Jan;69(1):75–85. doi: 10.1016/0009-3084(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Renshaw P. F., Janoff A. S., Miller K. W. On the nature of dilute aqueous cholesterol suspensions. J Lipid Res. 1983 Jan;24(1):47–51. [PubMed] [Google Scholar]
- Rogers J., Lee A. G., Wilton D. C. The organisation of cholesterol and ergosterol in lipid bilayers based on studies using non-perturbing fluorescent sterol probes. Biochim Biophys Acta. 1979 Mar 23;552(1):23–37. doi: 10.1016/0005-2736(79)90243-8. [DOI] [PubMed] [Google Scholar]
- Runnels L. W., Scarlata S. F. Theory and application of fluorescence homotransfer to melittin oligomerization. Biophys J. 1995 Oct;69(4):1569–1583. doi: 10.1016/S0006-3495(95)80030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Cholesterol-induced fluid-phase immiscibility in membranes. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8686–8690. doi: 10.1073/pnas.88.19.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry. 1990 Nov 27;29(47):10676–10684. doi: 10.1021/bi00499a015. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Barenholz Y., Gratton E., Thompson T. E. A fluorescence study of dehydroergosterol in phosphatidylcholine bilayer vesicles. Biochemistry. 1987 May 5;26(9):2441–2448. doi: 10.1021/bi00383a007. [DOI] [PubMed] [Google Scholar]
- Schroeder F. Fluorescent sterols: probe molecules of membrane structure and function. Prog Lipid Res. 1984;23(2):97–113. doi: 10.1016/0163-7827(84)90009-2. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Jefferson J. R., Kier A. B., Knittel J., Scallen T. J., Wood W. G., Hapala I. Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proc Soc Exp Biol Med. 1991 Mar;196(3):235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- Smutzer G. A fluorescent sterol probe study of cholesterol/phospholipid membranes. Biochim Biophys Acta. 1988 Dec 22;946(2):270–280. doi: 10.1016/0005-2736(88)90402-6. [DOI] [PubMed] [Google Scholar]
- Smutzer G., Crawford B. F., Yeagle P. L. Physical properties of the fluorescent sterol probe dehydroergosterol. Biochim Biophys Acta. 1986 Nov 17;862(2):361–371. doi: 10.1016/0005-2736(86)90239-7. [DOI] [PubMed] [Google Scholar]
- Snyder B., Freire E. Fluorescence energy transfer in two dimensions. A numeric solution for random and nonrandom distributions. Biophys J. 1982 Nov;40(2):137–148. doi: 10.1016/S0006-3495(82)84468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- Vist M. R., Davis J. H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990 Jan 16;29(2):451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Bensen J., Greco M., Arena C. Cholesterol behavior in human serum lipoproteins. Biochemistry. 1982 Mar 16;21(6):1249–1254. doi: 10.1021/bi00535a022. [DOI] [PubMed] [Google Scholar]



