Abstract
Objective
The objective of this study is to examine the therapeutic efficacy of miR-133a-transfected bone marrow mesenchymal stem cells (BM-MSCs) in restoring damaged myocardium, reducing myocardial fibrosis, and improving cardiac function following myocardial infarction (MI).
Methods
Bone marrow mesenchymal stem cells (BM-MSCs) were transfected with miR-133a using lentivirus vectors, and the in vitro transfection efficiency was assessed. A rat MI animal model was established to examine the survival rate of miR-133a-transfected BM-MSCs in ischemic myocardium. The effects of miR-133a transfection on rat primary cardiac fibroblasts were evaluated both in vitro and in vivo.
Results
The experimental group had a significantly higher concentration of double-stranded DNA (dsDNA) compared to the control group. Fluorescent staining revealed an enhanced survival rate of MSCs in the miR-133a transfection group compared to controls. Additionally, the protein and gene expression of apoptosis-related indicators in the infarcted myocardium were lower in the experimental group compared to the control group. Following co-culture with rat primary cardiac fibroblasts, the miR-133a-transfected MSCs exhibited a significantly lower expression of myofibroblast-specific proteins and mRNA compared to controls. The levels of collagen I, connective tissue growth factor (CTGF) protein, and messenger RNA (mRNA) in the infarcted myocardium of rats transplanted with BM-MSCs transfected with miR-133a were significantly lower than those in the control group, and their left ventricular ejection fraction (LVEF) was significantly increased compared with the group that received unmodified BM-MSCs.
Conclusion
Our results demonstrate that miR-133a transfection following MI improves the survival rate of transplanted MSCs in ischemia-hypoxic myocardium, inhibits the transformation of cardiac fibroblasts into myofibroblasts, reduces myocardial fibrosis, and improves cardiac function following MI. This approach holds promise as a novel therapeutic strategy for myocardial repair.
Keywords: Cardiac dysfunction, miR-133a transfected stem cells, Myocardial fibrosis, Myocardial infarction, Stem cell survival
Introduction
Myocardial infarction (MI) remains the leading cause of morbidity and mortality worldwide, imposing a substantial economic burden on healthcare systems [1]. Myocardial fibrosis, a common sequela of MI, can further exacerbate cardiac dysfunction and contribute to poor clinical outcomes. In recent years, stem cell transplantation, which leverages the regenerative potential of differentiated, self-renewing stem cells to replace necrotic cardiomyocytes, has emerged as a promising treatment for MI. However, studies in China and other countries have revealed a low survival rate of transplanted stem cells in the myocardium [2, 3]. Hypoxia and nutrient-deficient conditions in the ischemic microenvironment induce apoptosis in the majority of transplanted stem cells, thereby limiting the efficacy of this therapeutic strategy. Consequently, stem cell therapy remains largely restricted to preclinical and early-phase clinical trials, with limited application in routine clinical practice. Hence, improving the survival rate of transplanted stem cells is particularly crucial in advancing stem cell-based therapies for MI.
Although stem cells have the capacity to differentiate into cardiomyocytes and vascular endothelial cells to replace dead cells, their impact on myocardial fibrosis post-MI remains unclear. Acute MI results in extensive myocardial cell injury, characterized by the proliferation of fibroblasts, excessive collagen synthesis, and localized deposition of fibrotic tissue in the infarcted area. These pathological processes drive myocardial fibrosis, which reduces myocardial compliance, impairs cardiac perfusion, and lowers perfusion reserve. In addition, the accumulation of fibrous tissue disrupts myocardial electrical conduction, triggering ventricular arrhythmias such as ventricular tachycardia and ventricular fibrillation [4]. Over time, these complications lead to progressive left ventricular dysfunction and the development of heart failure. It has been estimated that, within five years of acute MI, 8–20% of men and 18–23% of women experience heart failure [5], underscoring the urgent need for effective therapeutic interventions.
Current management of MI focuses on revascularization to minimize further myocardial damage [6]. However, while effective in mitigating acute injury, revascularization strategies fail to restore the functionality of the already injured myocardium. The treatment of congestive heart failure secondary to MI mainly targets hemodynamic parameters, focusing on regulating preload and afterload, as well as increasing the contractile performance of surviving cardiomyocytes [7]. Unfortunately, these measures do not directly address the underlying myocardial fibrosis, nor do they inhibit the progression of heart failure after MI.
Despite significant advances in preventive measures and comprehensive treatment options such as pharmacotherapy, interventional techniques, and left ventricular assist device implantation, the prognosis for patients with MI-induced left ventricular dysfunction remains poor. Notably, the one-year survival rate for such patients remains only 13% [8]. Given these limitations, there is an urgent need to develop new therapeutic strategies that can effectively restore damaged myocardium, prevent the development of myocardial fibrosis, and ultimately improve cardiac function post-MI.
miR-133a was selected as the focus of this study due to its well-documented role in cardiac development and function [9]. As one of the most abundant microRNAs in the heart, miR-133a is a key regulator of cardiomyocyte differentiation, proliferation, and other processes essential for heart development [10].
In this study, the survival rate of miR-133a-transfected bone marrow mesenchymal stem cells (BM-MSCs) was assessed in a rat model of acute MI. Additionally, the degree of myocardial fibrosis following the injection of miR-133a-transfected stem cells and their therapeutic impact on cardiac function were evaluated to provide a new target for enhancing the efficacy of stem cell-based therapy for acute MI.
Materials and methods
Main reagents
The primary reagents used in this study were as follows: bone marrow mesenchymal stem cells (BM-MSCs) (obtained from Shanghai Bihe Biochemical Technology Co., Ltd., Catalog no.: PCLRa0113-RT); fetal bovine serum (FBS) (Thermo Fisher Scientific, Catalog no.: A5670701); Dulbecco’s Modified Eagle Medium (DMEM) (Bio Excellent International Tech Co., Ltd, Catalog no.: 112-013-101CS); SYBR Green Pro Taq HS premixed quantitative real-time polymerase chain reaction (qRT-PCR) kit II (Accurate Biotechnology (Hunan) Co., Ltd., Catalog no.: AG11736); SYBR Green Pro Taq HS Premix II (Accurate Biotechnology (Hunan) Co., Ltd, Catalog no.: AG11741); real-time fluorescence polymerase chain reaction (qRT-PCR) primers (synthesized by Shanghai Gene Licheng Biotechnology Co., Ltd.); sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Biosharp Life Sciences, Catalog no.: BL502A); polyvinylidene fluoride (PVDF) membranes (Biosharp Life Sciences, Catalog no.: 5–37). Primary antibodies: Caspase-3 (Proteintech, Catalog no.: 66470-2-Ig), Caspase-9 (Proteintech, Catalog no.: 10380-1-AP), CTGF (Atlas Antibodies, Catalog no.: AMAb91366), α-smooth muscle actin (α-SMA) (Fuzhou Yilisa Biological Technology Co., Ltd., Catalog no.: YLS-K1532), Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Proteintech, Catalog no.: 60004-1-Ig). Secondary antibodies for immunoblotting: anti-caspase-3 (Shanghai Boke Biotechnology Co., Ltd. Catalog no.: BK-KT9164), anti-caspase-9 (Shanghai Kanglang Biotechnology Co., Ltd., Catalog no.: KL200984), anti-CTGF (Shanghai Zeye Biotechnology Co., Ltd. Catalog no.: ZY-6802-69R), anti-α-SMA (Shanghai Boke Biotechnology Co., Ltd. Catalog no.: BK-KT11236), anti-GAPDH (Atlas Antibodies, Catalog no.: HPA061280) antibodies for second antibody (2nd Ab) incubations.
Methods
Lentiviral infection of MSCs
Lentiviral plasmids engineered for high expression of miR-133a, along with green fluorescent protein (GFP) as a marker, were purchased from Shanghai Gene Licheng Biotechnology Co., Ltd. BM-MSCs were cultured to reach 60–80% confluence, subjected to trypsin digestion to prepare cell suspensions, and seeded into 12-well plates at a density of 30,000 cells per well. Transfection was performed by incubating the cells with miR-133a lentivirus with a viral titer of 1 × 109 TU/mL for 24 h at 37℃ in an incubator. The miR-133a-transfected BM-MSCs were used for subsequent experiments [1].
Cell culture under hypoxic conditions
Both BM-MSCs transfected with the miR-133a lentivirus and untransfected BM-MSCs were cultured under hypoxic conditions of 1% O2 and 5% CO2 at 37℃ in an incubator. To simulate hypoxia, the cell culture medium was pre-equilibrated in a 1% O2 environment and replaced every other day.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells and tissues in each group using an RNA extraction kit (TransZol Up Plus RNA Kit TransGen Biotech, ER501-01-V2), and RNA concentrations were quantified. Complementary DNA (cDNA) was synthesized through reverse transcription using a reverse transcription kit. The SYBR Green Pro Taq HS Premixed qPCR Kit II was used for detection. The PCR reaction system (20 µL) comprised 10 µL of 2X SYBR Green Pro Taq HS Premix II, 0.5 µL each of upstream and downstream primers, 0.5 µL of ROX Reference Dye, 2 µL of total RNA, and 7 µL of RNase-Free dH2O. PCR reaction conditions included an initial denaturation at 95℃ for 5 s, annealing at 60℃ for 30 s, repeated for 40 cycles. Subsequently, the 2-ΔΔCT method was used to calculate the relative expression levels of mRNA. Primer sequences used in this study are detailed in Table 1.
Table 1.
Primer sequences for RT-qPCR in rats
| Primer | Forward | Reverse |
|---|---|---|
| mi-133a | TACAGACCCTGAAGTTACCC | CCTTGGAATGAGGATGTTT |
| caspase-3 | GGACCTGTGGACCTGAAAAAA | GCATGCCATATCATCGTCAG |
| caspase-9 | AAGACCATGGCTTTGAGGTG | CAGGAACCGCTCTTCTTGTC |
| α-SMA | AGGATGCAGAAGGAGATCACAG | CTGGAAGGTAGATAGAGAAGCC |
| CTGF | GACCCAACTATGATGCGAGC | CAGGCACAGGTCTTGATGAA |
| Collagen I | ATCAGCCCAAACCCCAAGGAGA | CGCAGGAAGGTCAGCTGGATAG |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
Western blot
A volume of 100 µL of potent lysate was added to 10 mg of myocardial tissue, ground with an ultrasonic homogenizer on ice, and centrifuged for 10 min at 4℃ (12,000 r/min, r = 8 cm). The supernatant was collected, and the protein concentration was quantified using the bicinchoninic acid (BCA) method. The loading volume was adjusted, and the remaining samples were mixed in a 4:1 ratio of loading buffer, boiled in a metal bath at 100℃ for 10 min, cooled, and stored in a freezer at -80℃.
For electrophoresis, 50 µg of protein per sample was loaded onto an SDS-PAGE gel separated based on molecular weight. Proteins were then transferred onto a PVDF membrane as per standard protocols. Membranes were subjected to rapid blocking with a blocking solution for 15 min at room temperature and then incubated overnight at 4 °C with primary antibodies against Caspase-3 (1:1,000), Caspase-9 (1:1,000), CTGF (1:1,000), α-SMA (1:1,500), and GAPDH (1:1,000) as a loading control.
The membranes were then incubated overnight on a shaker at 4℃. The corresponding secondary antibody (1:10,000) was added and incubated for 1.5 h on a rocker at room temperature. After washing, the PVDF membranes were scanned using an imaging system. The developer was prepared in the dark and used to cover the PVDF membrane, which was then exposed and developed. The developed images were processed and analyzed with the ImageJ analysis system. The relative expression of the target protein was represented by the ratio of the gray value of the target band to that of the internal reference band. Enhanced chemiluminescence (ECL) was used for staining.
Determining the concentration of double-stranded DNA (dsDNA)
BM-MSCs transfected with miR-133a and exhibiting significantly increased MSCs and miR-133a expression levels were cultured under hypoxic and low-nutrient conditions (0.4% O2, Iscove’s Modified Dulbecco’s Medium [IMEM] without fetal bovine serum [FBS]). The culture medium was replaced every other day, and cells were collected after 1, 7, 14, and 21 days of incubation. Samples were taken on days 1, 7, and 14 of cell treatment for quantitation of double-stranded DNA (dsDNA).
Preparation of reagents
To prepare reagents for DNA quantification, 1 ml of 20× TE buffer was diluted with 19 ml of DNA enzyme-free water to achieve a 1× working concentration of Tris-EDTA (TE) buffer. Next, the Quant-iT™ PicoGreen working solution was prepared by adding 100 µl of Quant-iT™ PicoGreen DMSO stock solution to 19.9 ml of 1× TE buffer. This was prepared just before use and stored protected from light. For the preparation of the DNA standard curve, a 2 µg/ml DNA stock solution was prepared by diluting 30 µl of DNA standard solution with 1.47 ml TE buffer. This stock solution was then diluted to five different concentrations as detailed in Table 2.
Table 2.
Concentrations of DNA stock solution
| TE Buffer Volume (ìl) | Volume of DNA Storage Solution 2ìg/ml (ìl) | Volume of Diluted Quant-iT™ PicoGreen Reaction Solution (ìl) | Final concentration of the processed DNA |
|---|---|---|---|
| 0 | 1,000 | 1,000 | 1 µg/ml |
| 900 | 100 | 1,000 | 100 µg/ml |
| 990 | 10 | 1,000 | 10 µg/ml |
| 999 | 1 | 1,000 | 1 µg/ml |
| 1,000 | 0 | 1,000 | Blank Control |
DNA quantification protocol
To generate the standard curve, DNA solutions of various specified concentrations were pipetted into individual wells of a 96-well plate, with three replicate wells for each concentration. One ml of the Quant-iT™ PicoGreen working solution was added to each well, mixed thoroughly, and incubated for five minutes at room temperature in the dark. Fluorescence intensity was measured at an excitation wavelength of 480 nm using a multiplate reader, and the resulting fluorescence intensities and their corresponding DNA concentrations were plotted to generate a standard curve using Excel.
Sample analysis
For sample preparation, the DNA samples to be tested were diluted 50-fold in TE buffer, obtaining a final volume of 1 ml in 96-well plates. One ml of the Quant-iT™ PicoGreen working solution was then added to each well and incubated for five minutes at room temperature in the dark. Fluorescence intensity was measured using a multifunctional microplate reader. The DNA concentration in each well was calculated using the standard curve. Finally, the original DNA concentration of each sample was determined by adjusting for the 50-fold dilution factor.
Establishment of the MI rat model and triphenyltetrazolium chloride (TTC) staining
Sprague-Dawley (SD) rats (procured from the Chengdu Dossy Laboratory Animal Co., Ltd.) were housed in a standard animal facility, with two animals per cage. The environmental conditions included air changes within the facility every 8 to 10 h, an ammonia concentration of < 20 ppm, relative humidity of 40–80%, and a temperature range of 20–23℃. The basal diet contained 55% carbohydrates, 4% fat, 16% protein, and 25% cellulose, trace elements, and moisture.
Preparation of rats for creating the acute MI model
-
A.
Anesthesia and Tracheal Intubation: In the animal room, the rats were placed in the supine position on a sterile operating table and anesthetized using 2% isoflurane inhalation. The neck and left chest regions were shaved and disinfected. A cervical incision was made along the direction of the trachea. The neck muscles were bluntly dissected to expose the trachea. Tracheotomy was performed, followed by tracheal intubation. The ventilator was connected with the respiratory rate set at 60–70 breaths/minute and a tidal volume of 1.0–2.0 ml.
-
B.
Surgical Access to the Heart: The rat was placed in the right lateral recumbent position. An incision approximately 3 cm long was made along the fourth or fifth intercostal space on the left side of the sternum, and the subcutaneous tissue was bluntly dissected to expose the heart.
-
C.
Coronary Artery Ligation and Injection: The pericardium was carefully dissected under a microscope to expose the heart, and the left anterior branch of the coronary artery was ligated using a 6 − 0 polypropylene suture. In both the control and experimental groups, four injection points (arranged in a square pattern) were identified around the periphery of the ligated area. Each point was injected with either normal saline (control group) or the experimental reagent (lentivirus with high expression miR-133a). The muscle and skin layers were sutured in sequence using a 4 − 0 silk suture.
-
D.
Postoperative Care: After surgery, the animals were placed in an incubator at 37℃. The animals, initially lethargic post-surgery, were placed supine until they recovered from anesthesia to prevent suffocation due to relaxation of the tongue and pharyngeal muscles. Once the animals regained consciousness, they were transferred back to their home cages with unrestricted access to water and food. Buprenorphine (0.1 mg/kg, q12h) was administered subcutaneously for three days to manage postoperative pain.
TTC staining
Model Treatment and Sample Collection: After the rat model of MI was successfully established, the rats were divided into two groups: rats in the control group were injected at the infarct site with untreated BM-MSCs, while rats in the miR-133a transfection group were injected with BM-MSCs transfected with miR-133a.
Sample Preparation: On postoperative day 28, the rats were euthanized using cervical dislocation. The hearts were immediately removed (within 10 min), transferred into a 0–4℃ PBS solution, and frozen in a -20℃ freezer for 30 min. Heart sections of 2 mm thickness were then cut and placed in a light-resistant water bath for 30 min at 37℃ in a 2% 2, 3, 5-TTC solution.
Staining procedure
The container with heart sections was gently shaken every five minutes to ensure adequate staining. After staining, the heart sections were removed, washed with PBS solution for three to five minutes, and immediately photographed.
Fluorescent staining of viable cells
For both BM-MSC groups, viable cells were fluorescently stained as per the following method before being injected into the body:
Preparation of the CM-Dil staining solution
A total of 2 mg of CM-Dil dye was weighed and dissolved in 1 ml of dimethyl sulfoxide (DMSO) to prepare a stock solution with a concentration of 2 mg/ml. The stock solution was stored at -20℃. Prior to cell staining, the stock solution was diluted 1000-fold with Dulbecco’s phosphate-buffered saline (D-PBS) to achieve a final working concentration of 2 µg/ml.
Cell staining protocol
BM-MSCs were digested using a papain solution for five minutes and neutralized with fetal bovine serum (FBS). The resulting cell suspension was transferred to a 50-ml centrifuge tube and centrifuged at 800 rpm for 5 min. The supernatant was discarded.
Staining process
The diluted dye solution was added to the centrifuge tube containing the cells. The cells were gently mixed by pipetting and incubated in a 37℃ water bath for 30 min. Later, the tube was transferred to a 4℃ refrigerator for an additional 15 min of incubation.
Washing steps: Following incubation, the cells were centrifuged again at 800 rpm for 5 min, and the supernatant was discarded. D-PBS was then added to the tube, and the cells were mixed by gentle pipetting, and the washing step was repeated to remove excess dye.
Injection into Rat Myocardium: Finally, the fluorescently stained BM-MSCs were injected into the myocardial tissue of rats as described in the experimental protocol.
Immunofluorescent staining of cells (immunohistochemistry, IHC)
Preparation of reagents
Both BM-MSCs were cultured as described above and sampled one week later. PBS, 10% formalin, and 3% bovine serum albumin (BSA) were pre-warmed before the experiment.
Fixation and blocking
The cultured BM-MSCs were rinsed with 1 ml of pre-warmed (37℃) PBS. After aspirating PBS, 1 ml of 10% formalin solution was added and incubated at 37℃ for a 40-minute fixation period. Post-fixation, the formalin was aspirated, and the cells were washed three times with 1 ml of pre-warmed (37℃) PBS. Following this, 500 µl of 37℃ 3% bovine serum albumin (BSA) was added to block non-specific binding sites, and the mixture was incubated in a 37℃ water bath for one hour.
Primary antibody incubation
The cells were rinsed three times with pre-warmed (37 °C) PBS, after which 300 µl of the primary antibody was added. The cells were then incubated with the primary antibody in a 37 °C water bath overnight.
Secondary antibody and DAPI staining
After overnight incubation, the cells were rinsed 20 times with 37 °C PBS to remove any unbound primary antibody. Subsequently, 300 µl of the secondary antibody was added in the dark and incubated for one hour in a 37 °C water bath. After a single rinse with 37 °C PBS, 300 µl of DAPI solution was added, and the cells were incubated in the dark at 37 °C for 10 min to stain the nuclei.
Mounting and Slide Preparation: Cells were rinsed 20 times with 37 °C PBS. The samples were carefully removed from their container and placed onto glass slides. The slides were fixed with neutral gum and prepared for microscopic examination.
Tissue immunofluorescence
Preparation of frozen tissue sections
Frozen sections were prepared prior to the staining process.
Fixation and washing
Sections were immersed in pre-warmed 37℃ 10% formalin for 30 s. This was followed by a series of washing steps, each involving immersion in 50 ml of 37℃ PBS for 10 insertions per wash. Fresh PBS was used in each iteration, and the process was repeated four times in total.
Blocking non-specific binding
Blocking non-specific binding sites was achieved by adding 500 µl of 37℃ 3% BSA to the sections and incubating them in a 37℃ water bath for one hour.
Primary antibody incubation
This was done after the washing steps as described above to remove any residual BSA. A total of 300 µl of the primary antibody was added to the sections and left to incubate overnight in a 37℃ water bath.
Secondary antibody and DAPI staining
A total of 300 µl of the secondary antibody was added to the sections and incubated for one hour in the dark at 37℃. For nuclear staining, the sections were rinsed once with 37℃ PBS, and 300 µl of DAPI solution was added in the dark at 37℃ for 10 min.
Final washing and mounting
The process concluded with final washing steps, repeated three times, and the sections were fixed with neutral gum before mounting the slides for microscopic examination.
Hematoxylin and eosin (H&E) staining
Preparation and staining of paraffin sections
For paraffin removal, the sections were placed in a 65℃ incubator for 20 min to melt the paraffin. The slides were then immersed in xylene twice for 10 min each to deparaffinize them. Following this, the slides underwent rehydration by immersion in 100% ethanol twice for five minutes each. The slides were allowed to air dry in a fume hood.
Staining procedure
For hematoxylin staining, filtered 0.1% hematoxylin was applied to the slides for 10 min, after which the slides were rinsed with cold deionized water for five minutes. Eosin staining was performed by immersing the sections in eosin solution repeatedly for 12 dips, followed by three rinses with deionized water.
Dehydration
For dehydration, the slides were immersed in 50% ethanol for 10 min, then in 75% ethanol for 10 min, equilibrating them for 30 s in 95% ethanol, and finally immersed in 100% ethanol for one minute.
Clearing and mounting
The sections were cleared by immersing them in xylene several times. The slides were fixed with neutral gum and mounted with coverslips for microscopic examination.
Picrosirius red staining
Slide preparation
The slides underwent a series of treatments beginning with deparaffinization, where they were sequentially immersed in xylene three times for 5 min each, followed by a 1-minute treatment in absolute ethanol, a 1-minute treatment in 95% ethanol, a 1-minute treatment in 75% ethanol, and a final 5-minute immersion in distilled water to complete the rehydration process.
Hematoxylin staining
The slides were then stained with hematoxylin solution applied dropwise for 5 to 10 min, after which any excess stain was removed by washing with distilled water for 10 to 20 s. Following this, the slides were washed with tap water for five minutes to remove residual water-soluble components.
Sirius red staining
For staining, the slides were immersed in Sirius Red solution for a duration of 15 to 30 min. The slides were then gently rinsed with running water to ensure the removal of excess stain from the surface.
Dehydration and clearing
For dehydration, the slides were treated sequentially in 75% ethanol for 1 min, followed by 95% ethanol for 1 min, and absolute ethanol for 1 min. For clearing, the slides were then immersed in xylene three times for 1 to 2 min each.
Mounting
Finally, the slides were sealed with neutral gum and mounted with coverslips to prepare them for microscopic analysis and observation of the tissue sections for accurate histopathological evaluation.
Assessment of cardiac function in rats
Cardiac ultrasound: The rats were placed in a supine position and anesthetized with inhalation of 2% isoflurane. The chest area was shaved, and the limbs were fixed onto an electrocardiographic probe for synchronized cardiac monitoring. The left ventricular ejection fraction (LVEF) and fractional shortening (FS) were recorded using a small animal ultrasound probe.
Statistical analysis
SPSS 29.0 was used to create the database and for analysis. Normally distributed measurement data were expressed as the mean ± standard error. The least significant difference (LSD) t-test was used for pairwise comparisons between groups. A P value of < 0.05 was considered statistically significant.
Results
Successful infection of MSCs using lentiviral technology
MSCs were successfully infected with a lentivirus, and flow cytometry sorting was used to isolate GFP-positive cells, confirming successful transfection. Molecular analysis results from qRT-PCR and Western blot assays indicated that the expression levels of miR-133a at both the mRNA and protein levels were significantly increased in the transfected group compared to the control group (P < 0.05), as shown in Fig. 1. These findings confirm the efficient upregulation of miR-133a following lentiviral transfection.
Fig. 1.
miR-133a and gene expression levels in the control group and the miR-133a transfection group
In vitro observation of the survival rate of MSCs transfected with miR-133a
MSCs from the control group and the miR-133a transfection group were cultured under hypoxic conditions. Samples were collected on days 1, 7, and 14 for determining the concentration of dsDNA. The dsDNA concentration serves as a reliable indicator of live cell count, as supported by previous studies [11]. As shown in Fig. 2, the dsDNA concentration in the control group was significantly lower than that in the experimental group and decreased progressively over time, indicating reduced cell survival. In contrast, the concentration of viable dsDNA in the miR-133a transfection group exhibited a significant increase over time, indicating active cell proliferation under hypoxic and low-nutrient conditions.
Fig. 2.
Determination of dsDNA concentration in the two groups
The experiment was independently repeated three times, consistently showing that miR-133a-transfected MSCs had a higher survival rate compared to the control group. The statistical results of dsDNA concentration (comparison of sampling results at each time point between the two groups) confirmed these observations, with significant differences between the two groups (P < 0.05).
The results confirm that miR-133a overexpression enhances the survival and proliferation of MSCs under hypoxic and nutrient-deficient conditions, supporting the hypothesis that miR-133a improves MSC resilience in adverse microenvironments.
Establishment of the experimental animal model of MI
SD rats used for the establishment of the experimental animal model of MI were housed in pairs within standard laboratory cages under controlled environmental conditions: air changes within the facility every 8–10 h, an ammonia concentration of < 20 ppm, relative humidity of 40–80%, and a temperature range of 20–23℃. Their basal diet consisted of 55% carbohydrates, 4% fat, 16% protein, and 25% constituted by cellulose, trace elements, and moisture.
Induction of acute MI in the rats
In the animal room, the rats were anesthetized on a sterile operating table by inhalation of 2% isoflurane in the supine position. The neck and left chest were shaved and disinfected. The neck skin was incised along the direction of the trachea, and a blunt dissection of the neck muscles was performed to expose the trachea. Tracheotomy with tracheal intubation was performed, and the ventilator was connected with the respiratory rate set at 60–70 breaths/minute and a tidal volume of 1.0–2.0 ml.
The rat was placed in the right lateral recumbent position, and an incision of approximately 3 cm was made along the fourth or fifth intercostal space on the left side of the sternum, and the subcutaneous tissue was bluntly dissected to expose the heart. The pericardium was carefully torn under a dissecting microscope, and the left anterior branch of the coronary artery was ligated with a 6 − 0 polypropylene suture (Fig. 3A). In both the control and experimental groups, four injection points (arranged in a square pattern) were identified around the ligated area. Each point was injected with either normal saline (control group) or the experimental reagent (lentivirus with high expression of miR-133a). The muscle and skin were then sutured layer by layer with 4 − 0 silk thread (Fig. 3B). Subsequently, the wound was sutured (Fig. 3C). Rats were euthanized through cervical dislocation 28 days after surgery, and the hearts were harvested for subsequent analysis (Fig. 3D).
Fig. 3.
Procedures for establishing the MI animal model in rats. A: exposed heart, ligation of anterior descending artery; B: injected MSCs or miR-133a transfected MSCs; C: muscle and skin sutured layer by layer; D: heart removed for further examination after the end of the experiment
Validation of the experimental animal model of MI
TTC staining in the heart of rats with MI (Fig. 4) indicated that the rat MI model was successfully established, with the infarcted area being white, indicating necrotic tissue, while the normal tissue retained a red color, confirming the presence of viable tissue.
Fig. 4.

TTC staining in the rat heart with MI (white area is the infarcted area and red is normal tissue)
In vivo observation of the survival rate of MSCs transfected with miR-133a in the ischemic myocardium
After 28 days post-surgery Rats in the MI animal model were euthanized, and the infarcted myocardium was harvested for viable cell fluorescence staining. It was found that BM-MSCs from the miR-133a transfected group had a significantly higher survival rate in the ischemic myocardium compared to the control group (Fig. 5), and the difference was statistically significant (P < 0.05).
Fig. 5.
Imaging of the infarcted myocardium in both groups (MSCs in miR-133a transfected group show a significantly higher survival rate in the ischemic myocardium than those in control group)
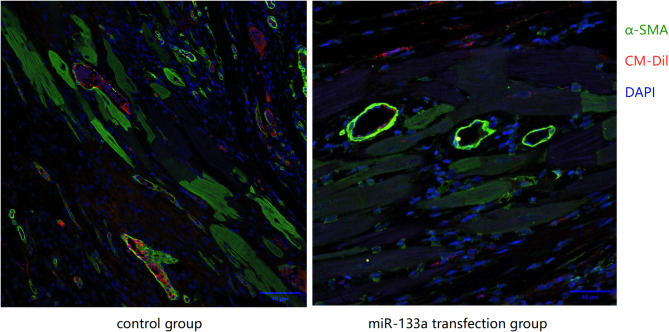
IHC imaging results of the infarcted myocardium in the two groups (Fig. 6) revealed that the transplanted MSCs differentiated into cardiomyocytes and vascular endothelial cells in both groups.
Fig. 6.
IHC imaging of the infarcted myocardium in both groups (CM-Dil is denoted in red, α-SMA is denoted in green, and the nucleus is denoted in blue)
These results suggest that the miR-133a-transfected MSCs have a greater potential for tissue regeneration and differentiation into myocardial and vascular cells.
In vivo observation of the expression of apoptosis-related proteins and genes in the infarcted myocardium of rats in the two groups
Rats in the control group received untreated MSCs, while those in the miR-133a transfected group were transplanted with miR-133a-transfected MSCs. At 28 days post-surgery, the rats were euthanized, and the infarcted myocardium samples were subjected to qRT-PCR and Western blot analyses to detect apoptosis-related markers such as Caspase-3 and Caspase-9. The results showed that the protein and gene expressions of Caspase-3 and Caspase-9 in the infarcted myocardium of rats in the miR-133a transfected group were significantly lower compared to the control group (P < 0.05) (Fig. 7).
Fig. 7.
Protein and gene expression of apoptosis-related markers in the infarcted myocardium of rats in both groups
The miR-133a-transfected MSCs demonstrated a protective effect against apoptosis in the ischemic myocardium, as evidenced by the reduced expression of Caspase-3 and Caspase-9. These findings suggest that miR-133a may promote cell survival and contribute to cardiac repair by inhibiting apoptosis in the infarcted tissue.
Effect of rat primary cardiac fibroblasts in the control group and miR-133a transfection group in vitro
In the control group, rat primary cardiac fibroblasts were cultured under hypoxic and low-nutrient conditions, and samples were collected seven days later for IHC staining. The miR-133a transfected group consisted of lentivirus-transfected MSCs with high expression of miR-133a co-cultured with rat primary cardiac fibroblasts under similar conditions for seven days and then sampled for IHC staining.
In vitro Evaluation: α-SMA positivity was used as a marker to identify myofibroblast transformation. The number of α-SMA-positive cells (red fluorescence) in the miR-133a-transfected group was significantly lower than that in the control group (Fig. 8). Additionally, the cell morphology in the miR-133a transfected group appeared more rounded, indicating that miR-133a treatment inhibited the transformation of cardiac fibroblasts into myofibroblasts.
Fig. 8.
IHC staining in the control group and the miR-133a transfection group, with red fluorescence indicating α-SMA and blue fluorescence indicating the nucleus (the miR-133a transfection group has significantly lesser α-SMA-positive cells than the control group)
Protein and Gene Expression Analysis: Using the same culture method, the two groups of rat primary cardiac fibroblasts were sampled, and qRT-PCR and western blot were performed to detect the protein and gene expression levels of specific proteins of myofibroblasts, such as α-SMA and CTGF. The results revealed that the expression of myofibroblast-specific proteins such as α-SMA, CTGF protein, and mRNA in rat primary fibroblasts co-cultured with MSCs transfected with miR-133a was significantly lower compared to the control group (Fig. 9). This indicated that MSCs transfected with miR-133a could inhibit the transformation of cardiac fibroblasts into myofibroblasts.
Fig. 9.
α-SMA and CTGF protein and gene expression in the control group and the miR-133a transfection group
The findings suggest that miR-133a transfection in MSCs plays a role in preventing the activation of cardiac fibroblasts into myofibroblasts by downregulating α-SMA and CTGF expression. This effect may contribute to the suppression of fibrosis and the preservation of normal cardiac function in an ischemic environment.
Effect of MSC transplantation on rat cardiac fibroblasts in the blank control and miR-133a transfected groups under in vivo conditions
Following the successful establishment of the MI rat models, the rats were divided into two groups: rats in the control group were injected with untreated MSCs directly at the infarct site, while those in the miR-133a transfected group were injected with miR-133a transfected MSCs at the infarct site.
In Vivo Evaluation: qRT-PCR and Western blot tests were used to measure the protein and mRNA expression levels of collagen I and CTGF, which are key markers for myocardial fibrosis, to observe their effects on cardiac fibroblasts in vivo. The infarcted myocardium of rats transplanted with MSCs transfected with miR-133a had significantly lower levels of collagen I, CTGF protein, and mRNA compared to the control group (Fig. 10), indicating that MSCs transfected with miR-133a could inhibit post-infarction myocardial fibrosis.
Fig. 10.
Collagen I and CTGF protein and gene expression in the control group and the miR-133a transfection group
With respect to histological observation of myocardial fibrosis, myocardial fibrosis at the infarct site was reduced in the miR-133a transfected group when compared to the control group (Fig. 11).
Fig. 11.
Degree of fibrosis in the control group and the miR-133a transfection group
Transplantation of miR-133a-transfected MSCs effectively suppressed myocardial fibrosis post-infarction by reducing the expression of fibrosis markers, such as collagen I and CTGF. This suggests that miR-133a could be a potential therapeutic target for preventing cardiac fibrosis and promoting better heart function after MI.
Effect of MSC transplantation on cardiac function in rats in the control and miR-133a transfection groups under in vivo conditions
With respect to cardiac function assessment in the MI rat models, LVEF was used as a key indicator of cardiac function. Samples from MI rats transplanted with BM-MSCs transfected with miR-133a showed increased LVEF compared with those from the transplanted BM-MSCs group (Fig. 12), and the difference was statistically significant (P < 0.05), suggesting that miR-133a-transfected BM-MSCs improved cardiac function post-infarction.
Fig. 12.
Cardiac function in the control group and the miR-133a transfection group
Transplantation of miR-133a-transfected BM-MSCs enhanced cardiac function as evidenced by the increase in LVEF compared to the control group. This finding supports the potential therapeutic role of miR-133a in improving heart function following myocardial infarction.
Discussion
In recent years, stem cell transplantation has emerged as a promising treatment for MI, offering potential benefits through the replacement of dead cardiomyocytes with differentiated, self-renewing stem cells. Clinical trials and preclinical studies have demonstrated the efficacy of stem cell therapy in improving cardiac function and promoting myocardial repair [12, 13]. Despite these advancements, significant challenges remain in the clinical translation of this therapy.
In our study, BM-MSCs were successfully injected into the myocardium of rats with acute MI, and the results showed that these MSCs could successfully differentiate into cardiomyocytes and vascular endothelial cells, contributing to myocardial regeneration. Notably, the therapeutic efficacy of stem cell transplantation depends considerably on the survival rate of stem cells transplanted into the ischemic myocardium [14–16].
Results of studies done in China and other countries reveal a low survival rate of transplanted stem cells in the myocardium [1, 2], and extensive apoptosis of the transplanted stem cells due to hypoxia and the nutrient-deficient environment of the infarcted myocardium. As a result, stem cell therapy is still restricted to animal experiments and clinical trials, with these limitations hindering its application in clinical practice. Improving the survival rate of transplanted stem cells, therefore, remains a critical scientific challenge to be addressed in the field of stem cell transplantation therapy at present. In addition, although stem cells can differentiate into cardiomyocytes and vascular endothelial cells to replace damaged tissues, their impact on mitigating myocardial fibrosis post-MI is minimal, with no effective solutions to this currently available in China or globally.
MicroRNAs (miRNAs) are single-stranded non-coding RNAs, approximately 21–22 nucleotides in length, that regulate gene expression by blocking the transcription or accelerating the degradation of their cognate messenger RNAs through RNA interference. In recent years, several studies have found that miRNAs, such as miR-1, miR-21, and miR-133, can regulate the expression of cardiovascular disease-related genes that are involved in the pathophysiological process of cardiovascular diseases such as myocardial fibrosis, myocardial hypertrophy, and arrhythmia [17–19]. Among these, miR-133a, a key member of the miR-133 family, is highly expressed in cardiac tissue and exerts an important regulatory role in cardiomyocyte differentiation, proliferation, and other processes related to heart development [16–19]. Research has shown that rats deficient in the miR-133a gene exhibit abnormal cardiac structural development such as ventricular septal defects, enlarged cardiac chambers, and thinned ventricular walls, accompanied by severe myocardial fibrosis and eventual heart failure [20–23]. Additionally, miR-133a has been reported to regulate apoptosis-related genes, further underscoring its importance in cardiovascular health. Emerging evidence highlights the potential of miR-133a in improving the survival rate of transplanted stem cells [24]. Consistent with these findings, in our study, we found that miR-133a could improve stem cell survival by modulating apoptosis-related genes and reducing stem cell apoptosis in infarcted myocardium transplanted into a hypoxic and nutrient-deficient environment.
Recent studies have found that MI triggers the transformation of cardiac fibroblasts (CFs) into myofibroblasts under the influence of several humoral factors and mechanical stimuli. This transformation causes collagen remodeling (characterized by increased total collagen and increased ratio of type I collagen to type III collagen), increased synthesis of fibronectin and extracellular matrix proteins, ultimately leading to myocardial fibrosis, impairing cardiac function, and increasing the risk of heart failure.
In our study, miR-133a was found to play a pivotal role in mitigating myocardial fibrosis in rats with acute MI. Specifically, miR-133a effectively inhibited the transformation of cardiac fibroblasts into myofibroblasts under both in vitro and in vivo conditions. The impact of miR-133a-transfected BM-MSCs on MI, both in vitro and in vivo, was comprehensively assessed through a series of meticulously designed experiments. In vitro, it was observed that miR-133a markedly inhibited the transformation of cardiac fibroblasts into myofibroblasts and reduced collagen deposition. Complementing these findings, in vivo studies using a rat model of MI confirmed that miR-133a-transfected BM-MSCs improved cell survival in the ischemic myocardium and enhanced cardiac function. This systematic study, encompassing investigations at the molecular and cellular levels as well as in whole animal models, provides deep insights into the mechanisms by which miR-133a facilitates cardiac repair. By simultaneously addressing stem cell survival and myocardial fibrosis, miR-133a offers a dual mechanism of action, highlighting its potential as a therapeutic agent in cardiac regenerative medicine.
In summary, stem cell therapy for MI has emerged as a promising treatment with significant prospects for clinical application. Our study demonstrated that MSCs injected into the myocardium successfully differentiated into cardiomyocytes and vascular endothelial cells. Furthermore, the local injection of a lentivirus expressing high levels of miR-133a into the infarcted myocardium significantly reduced the severity of myocardial fibrosis. Complementary in vitro experiments confirmed that miR-133a could inhibit the transformation of cardiac fibroblasts into myofibroblasts, further reducing fibrosis, improving cardiac function, and preventing post-MI heart failure. These findings highlight the prospects of miR-133a being a new and promising target for improving stem cell therapy in treating acute MI.
Conclusion
The use of stem cells in the treatment of MI has not yielded satisfactory outcomes, and the low survival rate of stem cells post-transplantation remains a critical challenge. The results of our study suggest that MSCs transfected with miR-133a have a higher survival rate in vitro and in vivo when compared to untreated controls. The hypoxic and nutrient-deficient conditions of infarcted myocardium exacerbate stem cell apoptosis, posing a key concern that persists in stem cell transplantation for MI. In the current study, it was demonstrated that miR-133a transfection could effectively improve the survival of transplanted MSCs in ischemia-hypoxic myocardium. In addition, there is no effective solution for cardiac dysfunction caused by myocardial fibrosis post-MI, and myocardial fibrosis is one of the important causes contributing to poor outcomes. Our findings in rats with MI revealed that miR-133a-transfected MSCs could inhibit the transformation of cardiac fibroblasts into myofibroblasts both in vitro and in vivo, reduce myocardial fibrosis following MI, and thus improve cardiac function. These findings provide evidence supporting the therapeutic potential of miR-133a in addressing key limitations of current stem cell therapies and offer a promising strategy for enhancing the treatment of acute MI.
Acknowledgements
Not applicable.
Author contributions
Conception and design of the research: Minghuan Fu, Yanglanduo Cao. Acquisition of data: Xiaohan Chen,Yanglanduo Cao. Analysis and interpretation of the data: Biao Cheng,Xiaohan Chen,Jie Gao. Statistical analysis: Jie Gao,Wei Zhang, Yong Shi. Xiaohan Chen Obtaining financing: Minghuan Fu,Biao Cheng,Xuefei Tao. Writing of the manuscript: Minghuan Fu. Critical revision of the manuscript for intellectual content: Wei Zhang, Yong Shi, Xuefei Tao. All authors read and approved the final draft.
Funding
This study was supported by Sichuan Science and Technology Program.
(2022YFS0154)
Data availability
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author (Minghuan Fu )on reasonable request.
Declarations
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Care and Use Committee at the Sichuan Provincial People’s Hospital(2021 − 109), and were conducted in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanglanduo Cao and Xiaohan Chen contributed equally to this work.
Contributor Information
Jie Gao, Email: gaojie9@21cn.com.
Minghuan Fu, Email: fuminghuanfmh@126.com.
References
- 1.Chen J, Li FJ, Fang K, et al. Effects of Lentivirus-mediated GFP transfection on MSSC proliferation, cell phenotype and differentiation capacity after CSF induction in rat. Progress Mod Biomed. 2021;21(19):3617–21. [Google Scholar]
- 2.Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11(5):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu J, Liu XJ, You BA, Ren N, Liu H. Application of nanomaterials in Stem Cell-based therapeutics for Cardiac Repair and Regeneration. Small. 2023;19(11):e2206487. [DOI] [PubMed] [Google Scholar]
- 4.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Investig. 2005;115(9):2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virk HUH, Lo KB, Krittanawong C, et al. The effects of Geography on outcomes of Routine Early Versus Selective Late Revascularization Strategy in the treatment of unstable angina and Non-ST-Segment elevation myocardial infarction: a Meta-analysis of Transatlantic Randomized controlled trials. J Clin Med Res. 2018;10(12):868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roura S, Galvez-Monton C, Mirabel C, et al. Mesenchymal stem cells for cardiac repair: are the actors ready for the clinical scenario? Stem Cell Res Ther. 2017;8(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollmann H, Nef H, Elsasser A, et al. Stem cells in myocardial infarction: from bench to bedside. Heart. 2009;95(6):508–14. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Padilla C, Garcia-Lopez V, Aranega A, Franco D, Garcia-Martinez V, Lopez-Sanchez C. Inhibition of RhoA and Cdc42 by miR-133a modulates retinoic acid signalling during early development of posterior cardiac tube segment. Int J Mol Sci. 2022;23(8):4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao S, Zhang Y, Gong M et al. Ivabradine Ameliorates Cardiac Function in Heart Failure with Preserved and Reduced Ejection Fraction via Upregulation of miR-133a. Oxid Med Cell Longev. 2021. 2021: 1257283. [DOI] [PMC free article] [PubMed]
- 11.Patil PS, Mansouri M, Leipzig ND. Fluorinated Chitosan Microgels to Overcome Internal Oxygen Transport Deficiencies in Microtissue Culture Systems. Adv Biosyst. 2020;4(8):e1900250. doi: 10.1002/adbi.201900250. Epub 2020 Jul 19. PMID: 32686345; PMCID: PMC10286855. [DOI] [PMC free article] [PubMed]
- 12.Roura S, Galvez-Monton C, Lupon J, et al. Biotherapies and biomarkers for cardiovascular diseases: a 15-year journey at the ICREC (heart failure and Cardiac Regeneration) Research Laboratory in Barcelona, Spain. Eur Heart J. 2017;38(23):1784–6. [DOI] [PubMed] [Google Scholar]
- 13.Ebrahimi B. Cardiac progenitor reprogramming for heart regeneration. Cell Regeneration. 2018;7(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12(6):689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radisic M, Christman KL. Materials science and tissue engineering: repairing the heart. Mayo Clinic proceedings. 2013,88(8):884– 98. [DOI] [PMC free article] [PubMed]
- 17.Eryilmaz U, Akgullu C, Beser N, et al. Circulating microRNAs in patients with ST-elevation myocardial infarction. Anatol J Cardiol. 2016;16(6):392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, Liu F, Xie H, et al. Diagnostic performance of microRNA-133a in acute myocardial infarction: a meta-analysis. Cardiol J. 2018;25(2):260–7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Weng S, Yin J, et al. Vitamin K2 promotes mesenchymal stem cell differentiation by inhibiting miR133a expression. Mol Med Rep. 2017;15(5):2473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorr O, Liebetrau C, Mollmann H et al. Effect of renal sympathetic denervation on specific MicroRNAs as an Indicator of Reverse remodeling processes in Hypertensive Heart Disease. J Clin Hypertens. 2016. [DOI] [PMC free article] [PubMed]
- 21.Huang L, Xi Z, Wang C et al. Phenanthrene exposure induces cardiac hypertrophy via reducing miR-133a expression by DNA methylation. Sci Rep. 2016,620105. [DOI] [PMC free article] [PubMed]
- 22.Nicolini G, Forini F, Kusmic C et al. Early and short-term triiodothyronine supplementation prevents adverse post-ischemic cardiac remodeling: role of transforming growth factor-beta1 and anti-fibrotic miRNA signaling. Mol Med. 2015. [DOI] [PMC free article] [PubMed]
- 23.Angelini A, Li Z, Mericskay M, et al. Regulation of connective tissue growth factor and Cardiac Fibrosis by an SRF/MicroRNA-133a Axis. PLoS ONE. 2015;10(10):e0139858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dakhlallah D, Zhang J, Yu L, et al. MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. J Cardiovasc Pharmacol. 2015;65(3):241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author (Minghuan Fu )on reasonable request.