Abstract
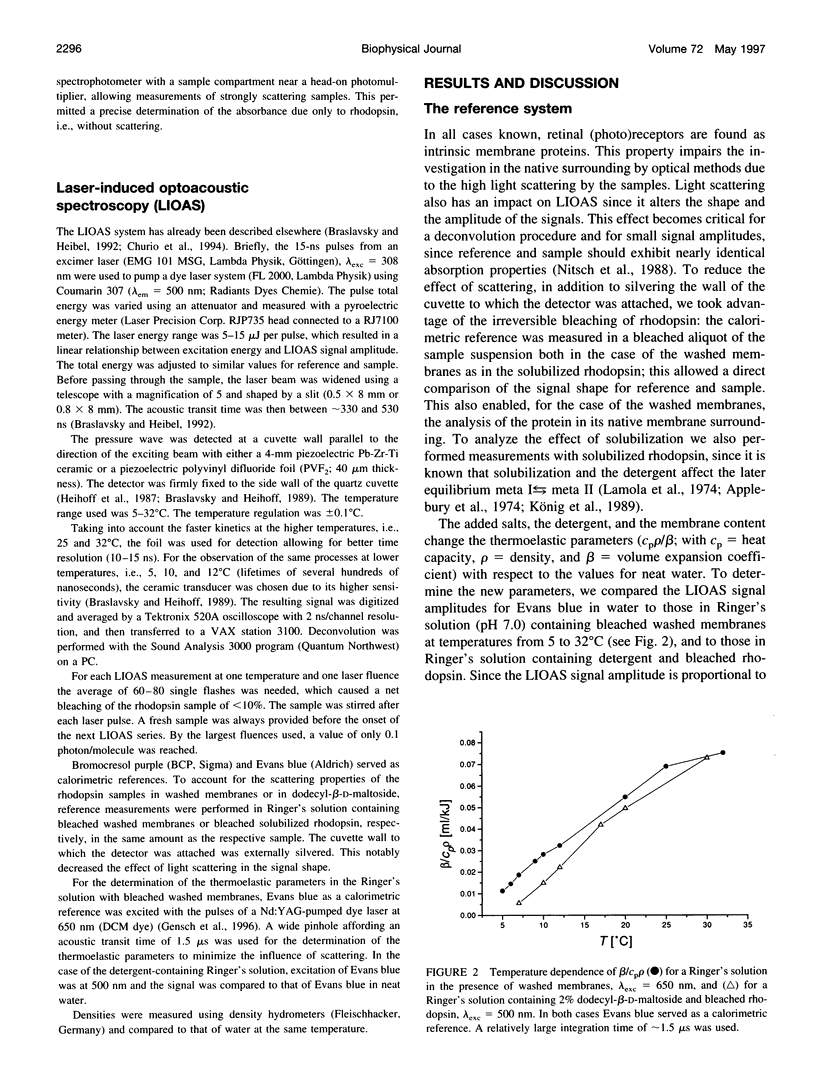
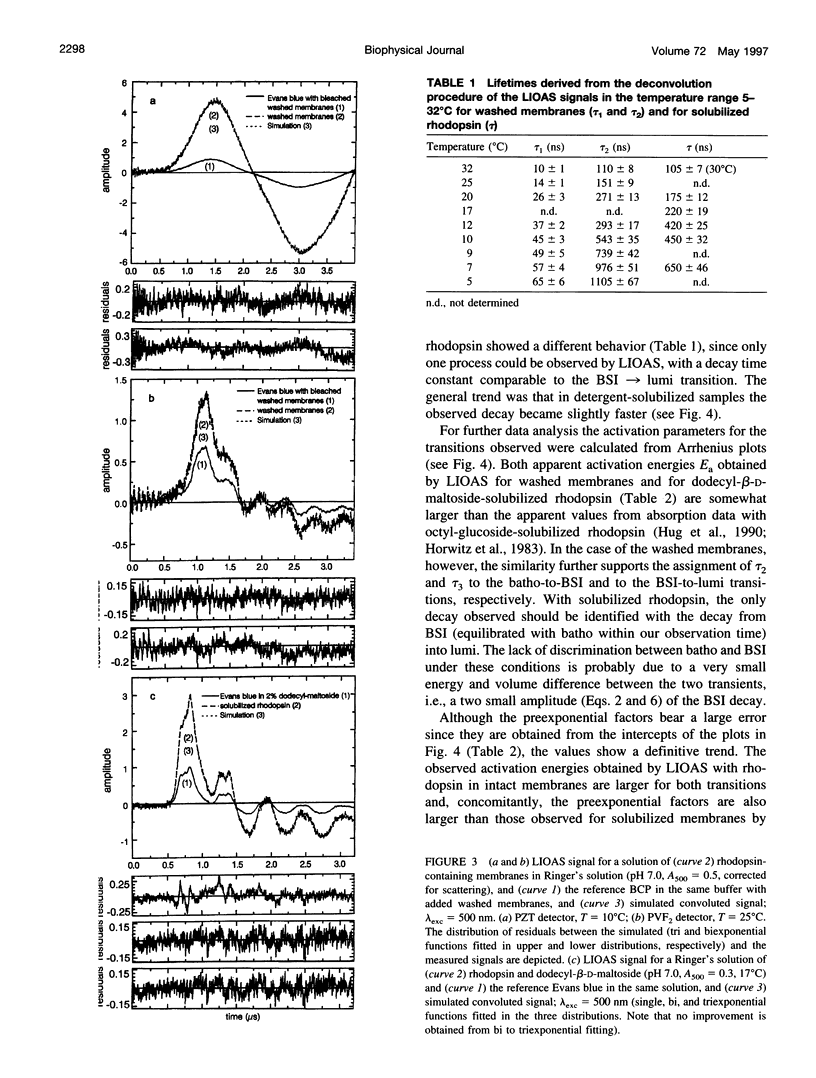
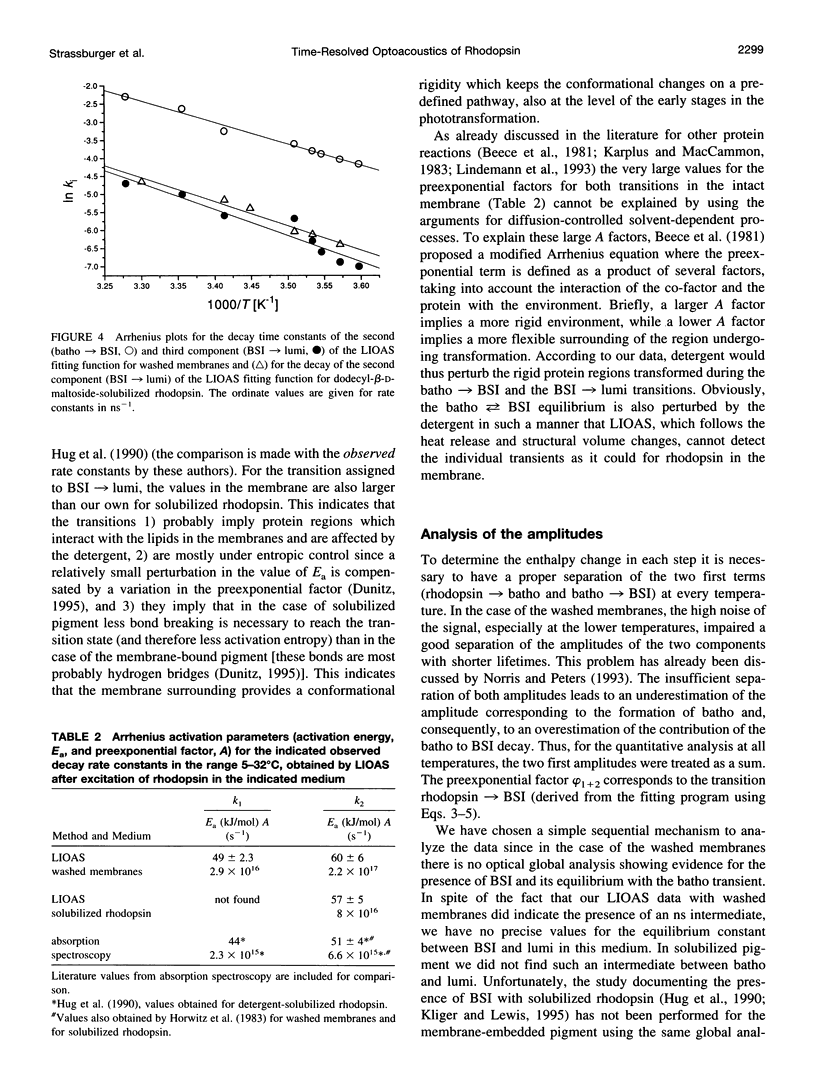
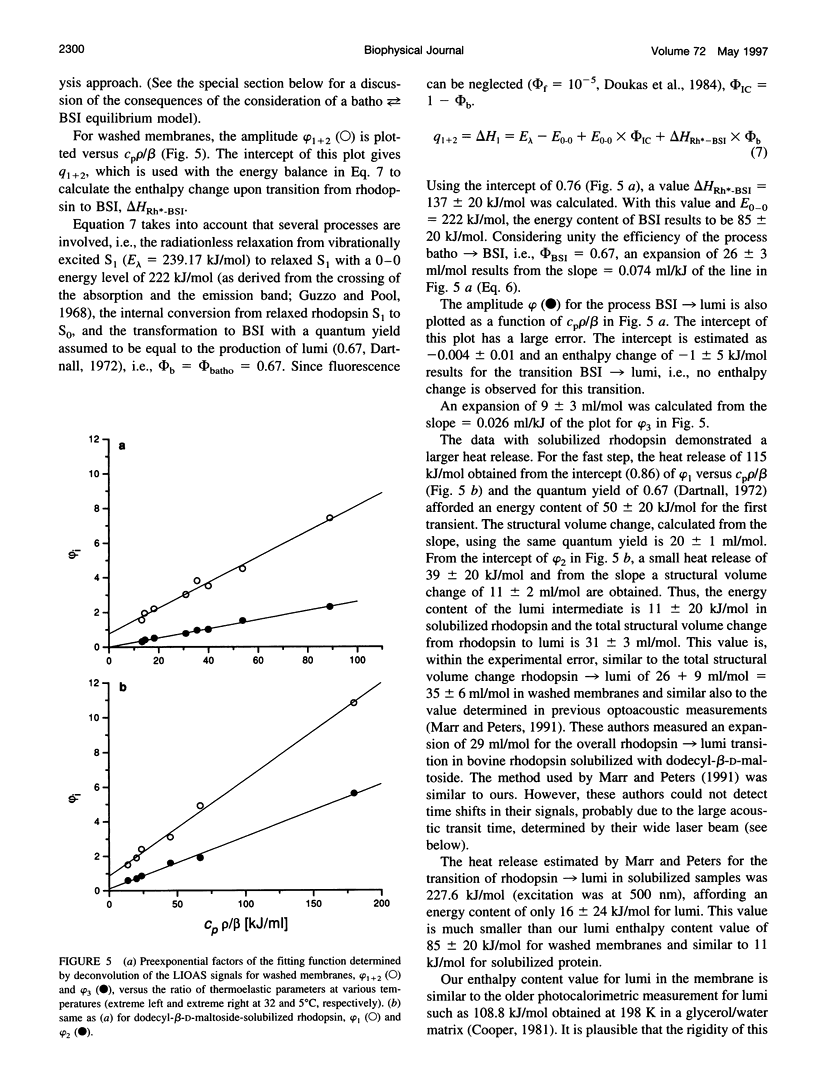
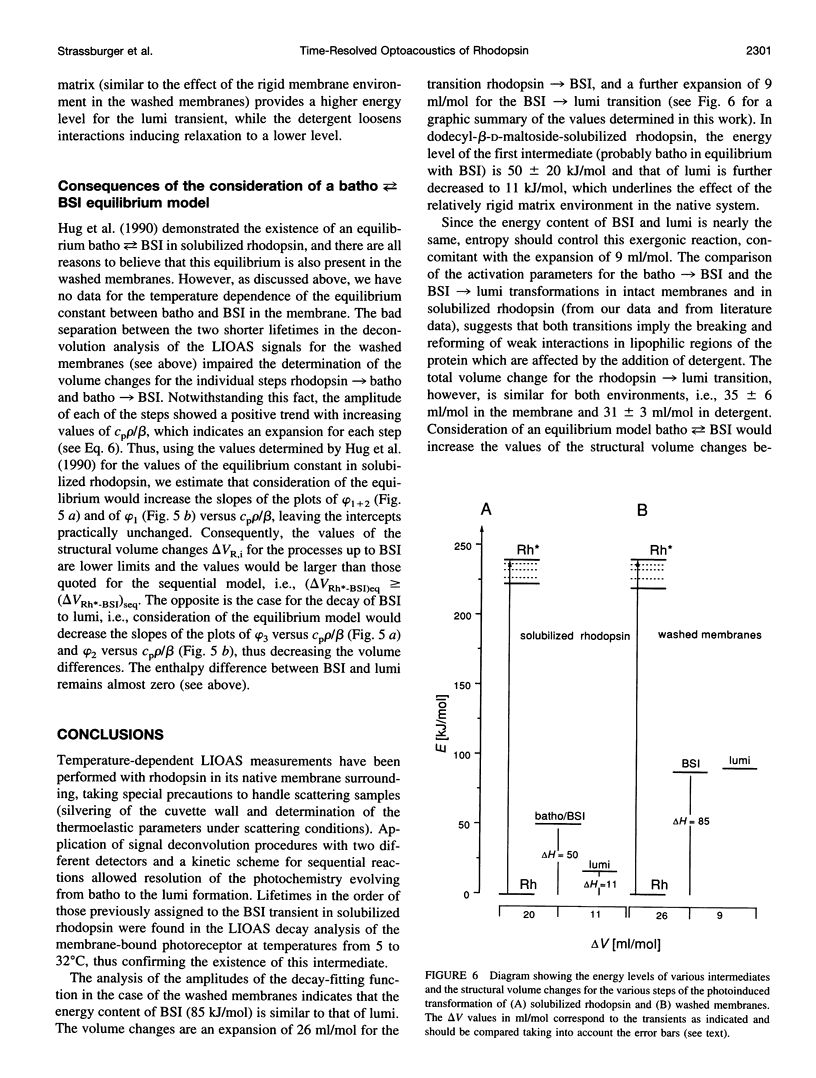
Laser-induced optoacoustic measurements were performed with bovine rhodopsin in the temperature range 5-32 degrees C in its natural environment (i.e., in washed membranes) as well as solubilized in dodecyl-beta-D-maltoside. A signal deconvolution procedure using a simple sequential kinetic scheme for the photobaric time evolution revealed, in the case of the washed membranes, the presence of an intermediate with a 14-ns lifetime at 25 degrees C, of the same order as that reported for the BSI intermediate in solubilized rhodopsin (Hug, S. J., W. J. Lewis, C. M. Einterz, T. E. Thorgeirsson, and D. S. Kliger. 1990. Nanosecond photolysis of rhodopsin: evidence for a new, blue-shifted intermediate. Biochemistry. 29:1475-1485), with an energy content of (85 +/- 20) kJ/mol, and accompanied by an expansion of 26 +/- 3 ml/mol. The difference in energy content between BSI and the next transient lumi was estimated in only -1 +/- 5 kJ/mol, concomitant with an expansion of 9 +/- 3 ml/mol. Thus, this transition, which according to literature involves an equilibrium, should be controlled by an entropic change, rather than by an enthalpic difference. This is supported by the fact that both activation parameters for the decay of batho and BSI decrease upon solubilization. For detergent-solubilized rhodopsin, two time constants were enough to fit the sample signal. A short lifetime ascribable to BSI was not detected in this case. For the first intermediate (probably batho in equilibrium with BSI), an energy content of 50 +/- 20 kJ/mol and an expansion of 20 +/- 1 ml/mol, and for lumi an energy content of 11 +/- 20 kJ/mol and a further expansion of 11 +/- 2 ml/mol were determined. Thus, the intermediates of the membrane-embedded form of rhodopsin (in contrast to solubilized samples) are kept in a higher energy level, although the total expansion from rhodopsin to lumi is similar for both conditions (35 +/- 6 and 31 +/- 3 ml/mol). The expansions are interpreted as protein reorganization processes as a consequence of the photoisomerization of the chromophore. As a result, weak interactions are probably perturbed and the protein gains conformational flexibility.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Zuckerman D. M., Lamola A. A., Jovin T. M. Rhodopsin. Purification and recombination with phospholipids assayed by the metarhodopsin I leads to metarhodopsin II transition. Biochemistry. 1974 Aug 13;13(17):3448–3458. doi: 10.1021/bi00714a005. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993 Apr;12(4):1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim Biophys Acta. 1990 Apr 26;1016(3):293–327. doi: 10.1016/0005-2728(90)90163-x. [DOI] [PubMed] [Google Scholar]
- Boucher F., Leblanc R. M. Energy storage in the primary photoreaction of bovine rhodopsin. A photoacoustic study. Photochem Photobiol. 1985 Apr;41(4):459–465. doi: 10.1111/j.1751-1097.1985.tb03512.x. [DOI] [PubMed] [Google Scholar]
- Callis J. B., Parson W. W., Gouterman M. Fast changes of enthalpy and volume on flash excitation of Chromatium chromatophores. Biochim Biophys Acta. 1972 May 25;267(2):348–362. doi: 10.1016/0005-2728(72)90122-3. [DOI] [PubMed] [Google Scholar]
- Cooper A. Rhodopsin photoenergetics: lumirhodopsin and the complete energy profile. FEBS Lett. 1981 Jan 26;123(2):324–326. doi: 10.1016/0014-5793(81)80319-5. [DOI] [PubMed] [Google Scholar]
- De Grip W. J., Daemen F. J., Bonting S. L. Isolation and purification of bovine rhodopsin. Methods Enzymol. 1980;67:301–320. doi: 10.1016/s0076-6879(80)67038-4. [DOI] [PubMed] [Google Scholar]
- Doukas A. G., Junnarkar M. R., Alfano R. R., Callender R. H., Kakitani T., Honig B. Fluorescence quantum yield of visual pigments: evidence for subpicosecond isomerization rates. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4790–4794. doi: 10.1073/pnas.81.15.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunitz J. D. Win some, lose some: enthalpy-entropy compensation in weak intermolecular interactions. Chem Biol. 1995 Nov;2(11):709–712. doi: 10.1016/1074-5521(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Quintana D., Francesch A., Garriga P., de Lera A. R., Padrós E., Manyosa J. Fourier transform infrared spectroscopy indicates a major conformational rearrangement in the activation of rhodopsin. Biophys J. 1995 Sep;69(3):1077–1082. doi: 10.1016/S0006-3495(95)79981-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo A. V., Pool G. L. Visual pigment fluorescence. Science. 1968 Jan 19;159(3812):312–314. doi: 10.1126/science.159.3812.312. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., Hamm H. E., Hofmann K. P. Interaction of rhodopsin with the G-protein, transducin. Bioessays. 1993 Jan;15(1):43–50. doi: 10.1002/bies.950150107. [DOI] [PubMed] [Google Scholar]
- Horwitz J. S., Lewis J. W., Powers M. A., Kliger D. S. Nanosecond laser photolysis of rhodopsin and isorhodopsin. Photochem Photobiol. 1983 Feb;37(2):181–188. doi: 10.1111/j.1751-1097.1983.tb04456.x. [DOI] [PubMed] [Google Scholar]
- Hug S. J., Lewis J. W., Einterz C. M., Thorgeirsson T. E., Kliger D. S. Nanosecond photolysis of rhodopsin: evidence for a new, blue-shifted intermediate. Biochemistry. 1990 Feb 13;29(6):1475–1485. doi: 10.1021/bi00458a019. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. Dynamics of proteins: elements and function. Annu Rev Biochem. 1983;52:263–300. doi: 10.1146/annurev.bi.52.070183.001403. [DOI] [PubMed] [Google Scholar]
- König B., Welte W., Hofmann K. P. Photoactivation of rhodopsin and interaction with transducin in detergent micelles. Effect of 'doping' with steroid molecules. FEBS Lett. 1989 Oct 23;257(1):163–166. doi: 10.1016/0014-5793(89)81811-3. [DOI] [PubMed] [Google Scholar]
- Lamola A. A., Yamane T., Zipp A. Effects of detergents and high pressures upon the metarhodopsin I--metarhodopsin II equilibrium. Biochemistry. 1974 Feb 12;13(4):738–745. doi: 10.1021/bi00701a016. [DOI] [PubMed] [Google Scholar]
- Lindemann P., Braslavsky S. E., Cordonnier M. M., Pratt L. H., Schaffner K. Effects of bound monoclonal antibodies on the decay of the phototransformation intermediates I700(1,2) from native Avena phytochrome. Photochem Photobiol. 1993 Sep;58(3):417–424. doi: 10.1111/j.1751-1097.1993.tb09584.x. [DOI] [PubMed] [Google Scholar]
- Marr K., Peters K. S. Photoacoustic calorimetric study of the conversion of rhodopsin and isorhodopsin to lumirhodopsin. Biochemistry. 1991 Feb 5;30(5):1254–1258. doi: 10.1021/bi00219a013. [DOI] [PubMed] [Google Scholar]
- Mizukami T., Kandori H., Shichida Y., Chen A. H., Derguini F., Caldwell C. G., Biffe C. F., Nakanishi K., Yoshizawa T. Photoisomerization mechanism of the rhodopsin chromophore: picosecond photolysis of pigment containing 11-cis-locked eight-membered ring retinal. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4072–4076. doi: 10.1073/pnas.90.9.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris C. L., Peters K. S. A photoacoustic calorimetry study of horse carboxymyoglobin on the 10-nanosecond time scale. Biophys J. 1993 Oct;65(4):1660–1665. doi: 10.1016/S0006-3495(93)81223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA Rhodopsin and bacteriorhodopsin: structure-function relationships. FEBS Lett. 1982 Nov 8;148(2):179–191. doi: 10.1016/0014-5793(82)80805-3. [DOI] [PubMed] [Google Scholar]
- Palings I., Pardoen J. A., van den Berg E., Winkel C., Lugtenburg J., Mathies R. A. Assignment of fingerprint vibrations in the resonance Raman spectra of rhodopsin, isorhodopsin, and bathorhodopsin: implications for chromophore structure and environment. Biochemistry. 1987 May 5;26(9):2544–2556. doi: 10.1021/bi00383a021. [DOI] [PubMed] [Google Scholar]
- Peteanu L. A., Schoenlein R. W., Wang Q., Mathies R. A., Shank C. V. The first step in vision occurs in femtoseconds: complete blue and red spectral studies. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11762–11766. doi: 10.1073/pnas.90.24.11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K. S., Snyder G. J. Time-resolved photoacoustic calorimetry: probing the energetics and dynamics of fast chemical and biochemical reactions. Science. 1988 Aug 26;241(4869):1053–1057. doi: 10.1126/science.3045967. [DOI] [PubMed] [Google Scholar]
- Schertler G. F., Villa C., Henderson R. Projection structure of rhodopsin. Nature. 1993 Apr 22;362(6422):770–772. doi: 10.1038/362770a0. [DOI] [PubMed] [Google Scholar]
- Schoenlein R. W., Peteanu L. A., Mathies R. A., Shank C. V. The first step in vision: femtosecond isomerization of rhodopsin. Science. 1991 Oct 18;254(5030):412–415. doi: 10.1126/science.1925597. [DOI] [PubMed] [Google Scholar]
- Small J. R. Deconvolution analysis for pulsed-laser photoacoustics. Methods Enzymol. 1992;210:505–521. doi: 10.1016/0076-6879(92)10026-a. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Fong T. M., Tota M. R., Underwood D., Dixon R. A. Structure and function of G protein-coupled receptors. Annu Rev Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- van Brederode M. E., Gensch T., Hoff W. D., Hellingwerf K. J., Braslavsky S. E. Photoinduced volume change and energy storage associated with the early transformations of the photoactive yellow protein from Ectothiorhodospira halophila. Biophys J. 1995 Mar;68(3):1101–1109. doi: 10.1016/S0006-3495(95)80284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


