Abstract
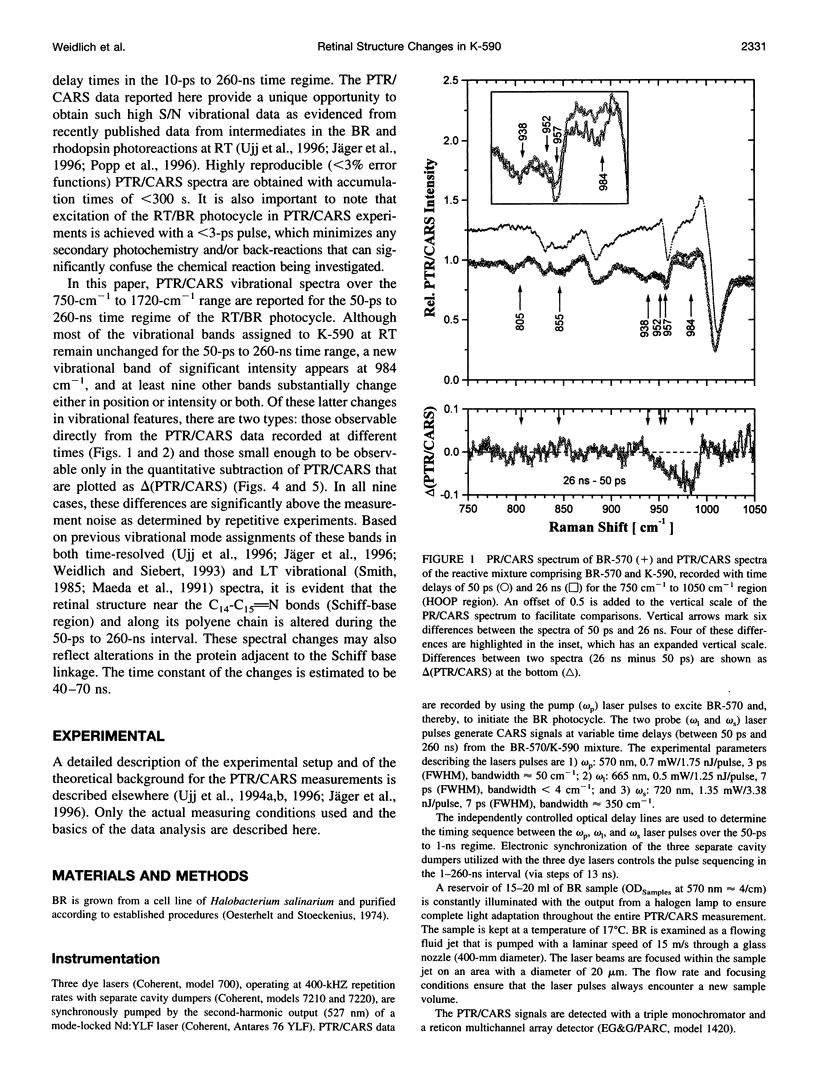
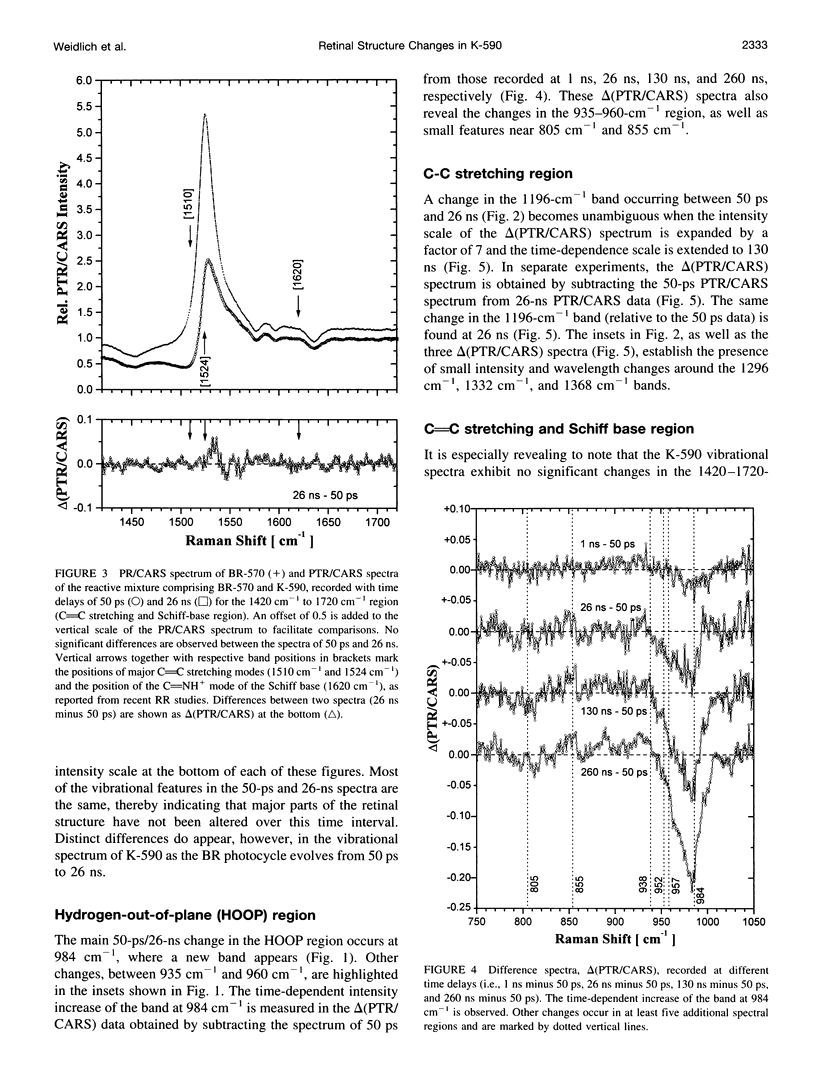
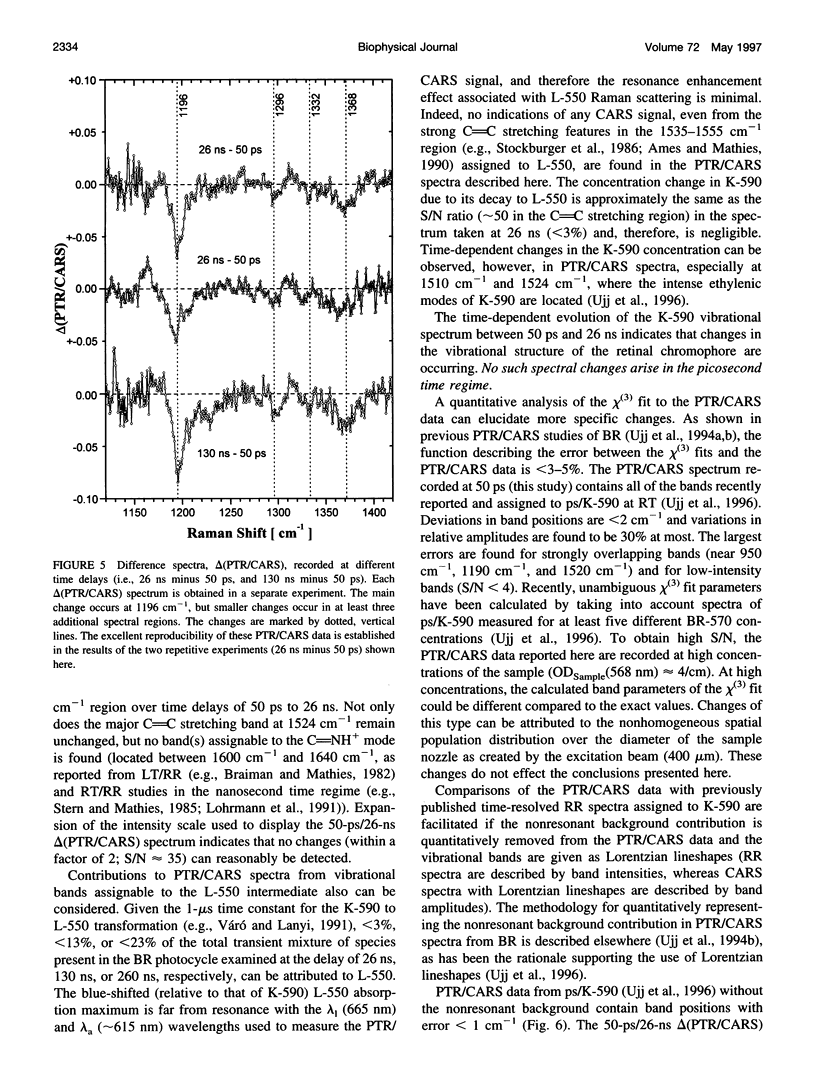
Time-resolved vibrational spectra are used to elucidate the structural changes in the retinal chromophore within the K-590 intermediate that precedes the formation of the L-550 intermediate in the room-temperature (RT) bacteriorhodopsin (BR) photocycle. Measured by picosecond time-resolved coherent anti-Stokes Raman scattering (PTR/CARS), these vibrational data are recorded within the 750 cm-1 to 1720 cm-1 spectral region and with time delays of 50-260 ns after the RT/BR photocycle is optically initiated by pulsed (< 3 ps, 1.75 nJ) excitation. Although K-590 remains structurally unchanged throughout the 50-ps to 1-ns time interval, distinct structural changes do appear over the 1-ns to 260-ns period. Specifically, comparisons of the 50-ps PTR/CARS spectra with those recorded with time delays of 1 ns to 260 ns reveal 1) three types of changes in the hydrogen-out-of-plane (HOOP) region: the appearance of a strong, new feature at 984 cm-1; intensity decreases for the bands at 957 cm-1, 952 cm-1, and 939 cm-1; and small changes intensity and/or frequency of bands at 855 cm-1 and 805 cm-1; and 2) two types of changes in the C-C stretching region: the intensity increase in the band at 1196 cm-1 and small intensity changes and/or frequency shifts for bands at 1300 cm-1 and 1362 cm-1. No changes are observed in the C = C stretching region, and no bands assignable to the Schiff base stretching mode (C = NH+) mode are found in any of the PTR/CARS spectra assignable to K-590. These PTR/CARS data are used, together with vibrational mode assignments derived from previous work, to characterize the retinal structural changes in K-590 as it evolves from its 3.5-ps formation (ps/K-590) through the nanosecond time regime (ns/K-590) that precedes the formation of L-550. The PTR/CARS data suggest that changes in the torsional modes near the C14-C15 = N bonds are directly associated with the appearance of ns/K-590, and perhaps with the KL intermediate proposed in earlier studies. These vibrational data can be primarily interpreted in terms of the degree of twisting of the C14-C15 retinal bond. Such twisting may be accompanied by changes in the adjacent protein. Other smaller, but nonetheless clear, spectral changes indicate that alterations along the retinal polyene chain also occur. The changes in the retinal structure are preliminary to the deprotonation of the Schiff base nitrogen during the formation of M-412. The time constant for the ps/ns K-590 transformation is estimated from the amplitude change of four vibrational bands in the HOOP region to be 40-70 ns.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Mathies R. A. The role of back-reactions and proton uptake during the N----O transition in bacteriorhodopsin's photocycle: a kinetic resonance Raman study. Biochemistry. 1990 Aug 7;29(31):7181–7190. doi: 10.1021/bi00483a005. [DOI] [PubMed] [Google Scholar]
- Birge R. R. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim Biophys Acta. 1990 Apr 26;1016(3):293–327. doi: 10.1016/0005-2728(90)90163-x. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Bousché O., Rothschild K. J. Protein dynamics in the bacteriorhodopsin photocycle: submillisecond Fourier transform infrared spectra of the L, M, and N photointermediates. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2388–2392. doi: 10.1073/pnas.88.6.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancsházy Z., Govindjee R., Ebrey T. G. Independent photocycles of the spectrally distinct forms of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6358–6361. doi: 10.1073/pnas.85.17.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney J. K., Brack T. L., Atkinson G. H. Time-resolved absorption and fluorescence from the bacteriorhodopsin photocycle in the nanosecond time regime. Biophys J. 1993 May;64(5):1512–1519. doi: 10.1016/S0006-3495(93)81520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller R., Iannone M., Bogomolni R., Hochstrasser R. M. Ultrafast infrared spectroscopy of bacteriorhodopsin. Biophys J. 1991 Jul;60(1):286–289. doi: 10.1016/S0006-3495(91)82050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller R., Iannone M., Cowen B. R., Maiti S., Bogomolni R. A., Hochstrasser R. M. Picosecond dynamics of bacteriorhodopsin, probed by time-resolved infrared spectroscopy. Biochemistry. 1992 Jun 23;31(24):5567–5572. doi: 10.1021/bi00139a020. [DOI] [PubMed] [Google Scholar]
- Eisfeld W., Pusch C., Diller R., Lohrmann R., Stockburger M. Resonance Raman and optical transient studies on the light-induced proton pump of bacteriorhodopsin reveal parallel photocycles. Biochemistry. 1993 Jul 20;32(28):7196–7215. doi: 10.1021/bi00079a017. [DOI] [PubMed] [Google Scholar]
- Fahmy K., Siebert F., Tavan P. Structural investigation of bacteriorhodopsin and some of its photoproducts by polarized Fourier transform infrared spectroscopic methods-difference spectroscopy and photoselection. Biophys J. 1991 Nov;60(5):989–1001. doi: 10.1016/S0006-3495(91)82136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwert K., Siebert F. Evidence for light-induced 13-cis, 14-s-cis isomerization in bacteriorhodopsin obtained by FTIR difference spectroscopy using isotopically labelled retinals. EMBO J. 1986 Apr;5(4):805–811. doi: 10.1002/j.1460-2075.1986.tb04285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessling B., Souvignier G., Gerwert K. A model-independent approach to assigning bacteriorhodopsin's intramolecular reactions to photocycle intermediates. Biophys J. 1993 Nov;65(5):1929–1941. doi: 10.1016/S0006-3495(93)81264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M. P., Khorana H. G. Mechanism of light-dependent proton translocation by bacteriorhodopsin. J Bacteriol. 1993 Mar;175(6):1555–1560. doi: 10.1128/jb.175.6.1555-1560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Xie A., Hofrichter J., Clore G. M. Reversible steps in the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3610–3614. doi: 10.1073/pnas.89.8.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R. A., Brito Cruz C. H., Pollard W. T., Shank C. V. Direct observation of the femtosecond excited-state cis-trans isomerization in bacteriorhodopsin. Science. 1988 May 6;240(4853):777–779. doi: 10.1126/science.3363359. [DOI] [PubMed] [Google Scholar]
- Mathies R. A., Lin S. W., Ames J. B., Pollard W. T. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- Milder S. J., Kliger D. S. A time-resolved spectral study of the K and KL intermediates of bacteriorhodopsin. Biophys J. 1988 Mar;53(3):465–468. doi: 10.1016/S0006-3495(88)83124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J., Bamberg E. A unifying concept for ion translocation by retinal proteins. J Bioenerg Biomembr. 1992 Apr;24(2):181–191. doi: 10.1007/BF00762676. [DOI] [PubMed] [Google Scholar]
- Polland H. J., Franz M. A., Zinth W., Kaiser W., Kölling E., Oesterhelt D. Early picosecond events in the photocycle of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):651–662. doi: 10.1016/S0006-3495(86)83692-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp A., Ujj L., Atkinson G. H. Bathorhodopsin structure in the room-temperature rhodopsin photosequence: picosecond time-resolved coherent anti-Stokes Raman scattering. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):372–376. doi: 10.1073/pnas.93.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J. FTIR difference spectroscopy of bacteriorhodopsin: toward a molecular model. J Bioenerg Biomembr. 1992 Apr;24(2):147–167. doi: 10.1007/BF00762674. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Marrero H. Infrared evidence that the Schiff base of bacteriorhodopsin is protonated: bR570 and K intermediates. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4045–4049. doi: 10.1073/pnas.79.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Roepe P., Gillespie J. Fourier transform infrared spectroscopic evidence for the existence of two conformations of the bacteriorhodopsin primary photoproduct at low temperature. Biochim Biophys Acta. 1985 Jun 26;808(1):140–148. doi: 10.1016/0005-2728(85)90036-2. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Maeda A., Kato C., Hamaguchi H. Time-resolved infrared spectral analysis of the KL-to-L conversion in the photocycle of bacteriorhodopsin. Biochemistry. 1993 Jan 26;32(3):867–871. doi: 10.1021/bi00054a018. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Yuzawa T., Kandori H., Maeda A., Hamaguchi H. Nanosecond time-resolved infrared spectroscopy distinguishes two K species in the bacteriorhodopsin photocycle. Biophys J. 1995 May;68(5):2073–2080. doi: 10.1016/S0006-3495(95)80386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulten K., Tavan P. A mechanism for the light-driven proton pump of Halobacterium halobium. Nature. 1978 Mar 2;272(5648):85–86. doi: 10.1038/272085a0. [DOI] [PubMed] [Google Scholar]
- Siebert F., Mäntele W. Investigation of the primary photochemistry of bacteriorhodopsin by low-temperature Fourier-transform infrared spectroscopy. Eur J Biochem. 1983 Feb 15;130(3):565–573. doi: 10.1111/j.1432-1033.1983.tb07187.x. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Hornung I., van der Steen R., Pardoen J. A., Braiman M. S., Lugtenburg J., Mathies R. A. Are C14-C15 single bond isomerizations of the retinal chromophore involved in the proton-pumping mechanism of bacteriorhodopsin? Proc Natl Acad Sci U S A. 1986 Feb;83(4):967–971. doi: 10.1073/pnas.83.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner J., Hsieh C. L., Burns A. R., El-Sayed M. A. Time-resolved resonance Raman spectroscopy of intermediates of bacteriorhodopsin: The bK(590) intermediate. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3046–3050. doi: 10.1073/pnas.76.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Kinetic and spectroscopic evidence for an irreversible step between deprotonation and reprotonation of the Schiff base in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5008–5015. doi: 10.1021/bi00234a024. [DOI] [PubMed] [Google Scholar]
- Xu D., Martin C., Schulten K. Molecular dynamics study of early picosecond events in the bacteriorhodopsin photocycle: dielectric response, vibrational cooling and the J, K intermediates. Biophys J. 1996 Jan;70(1):453–460. doi: 10.1016/S0006-3495(96)79588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg R., Du-Jeon-Jang, Bitting H. C., El-Sayed M. A. Subpicosecond resonance Raman spectra of the early intermediates in the photocycle of bacteriorhodopsin. Biophys J. 1990 Jul;58(1):135–141. doi: 10.1016/S0006-3495(90)82359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


