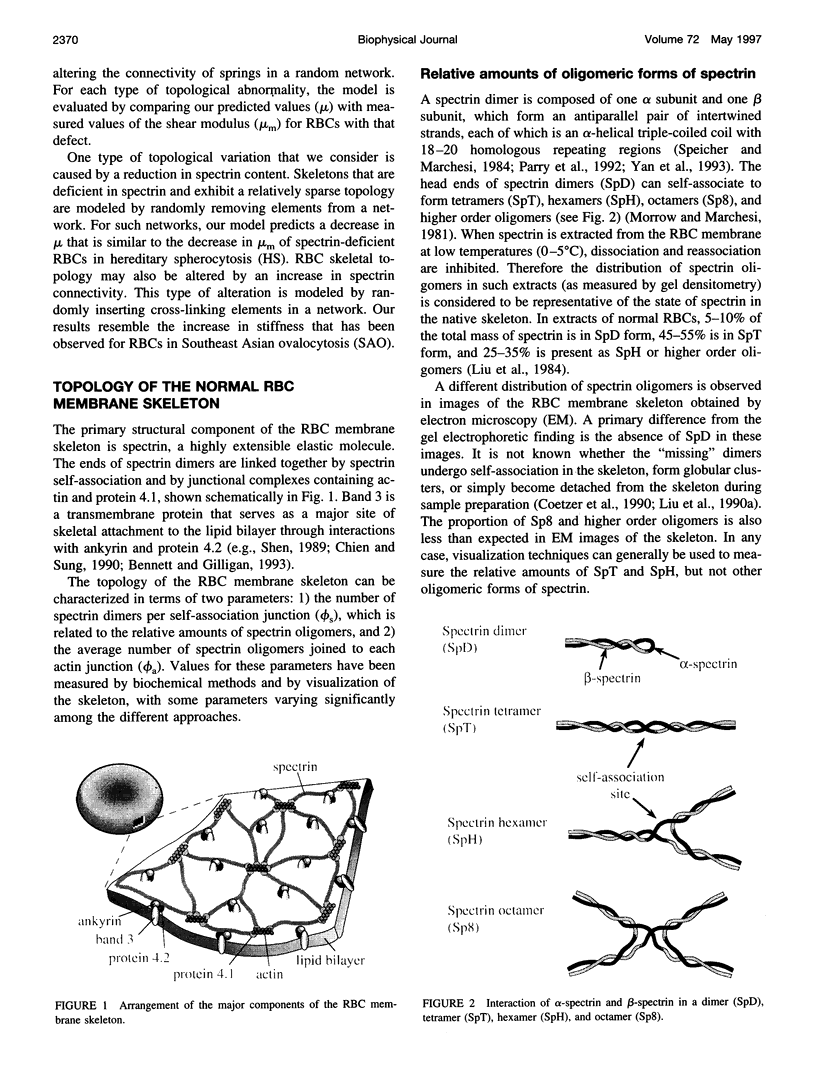
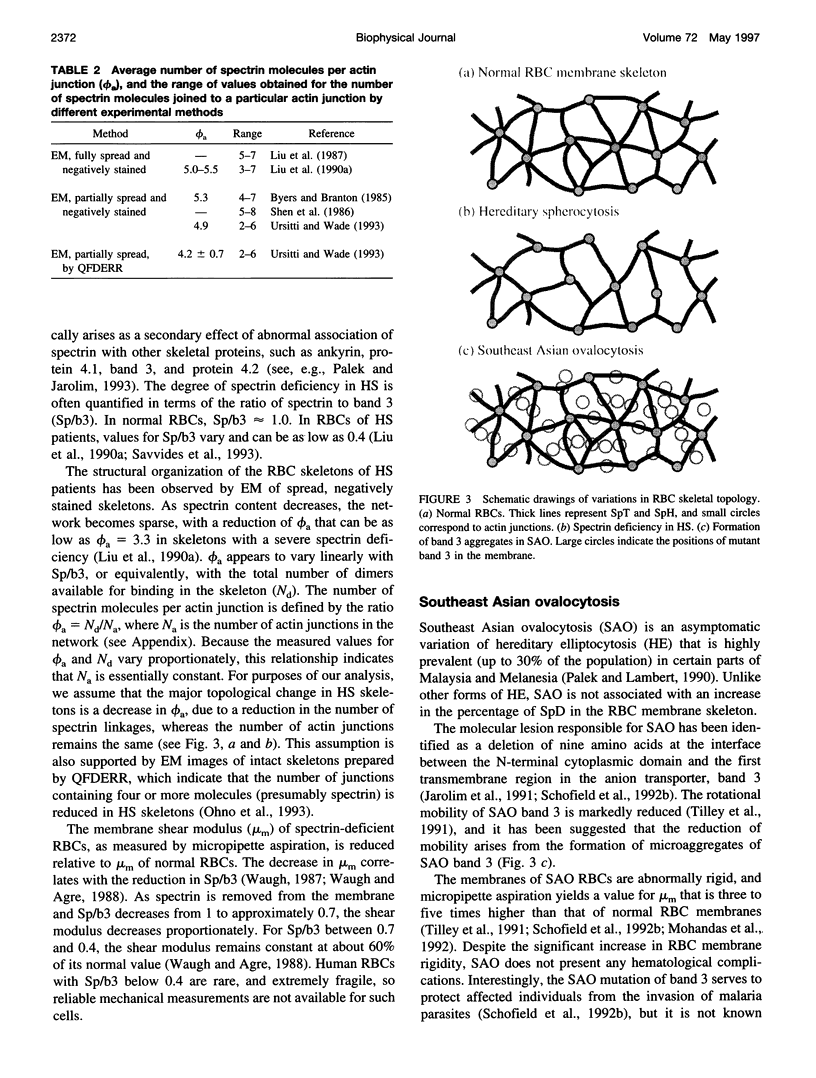
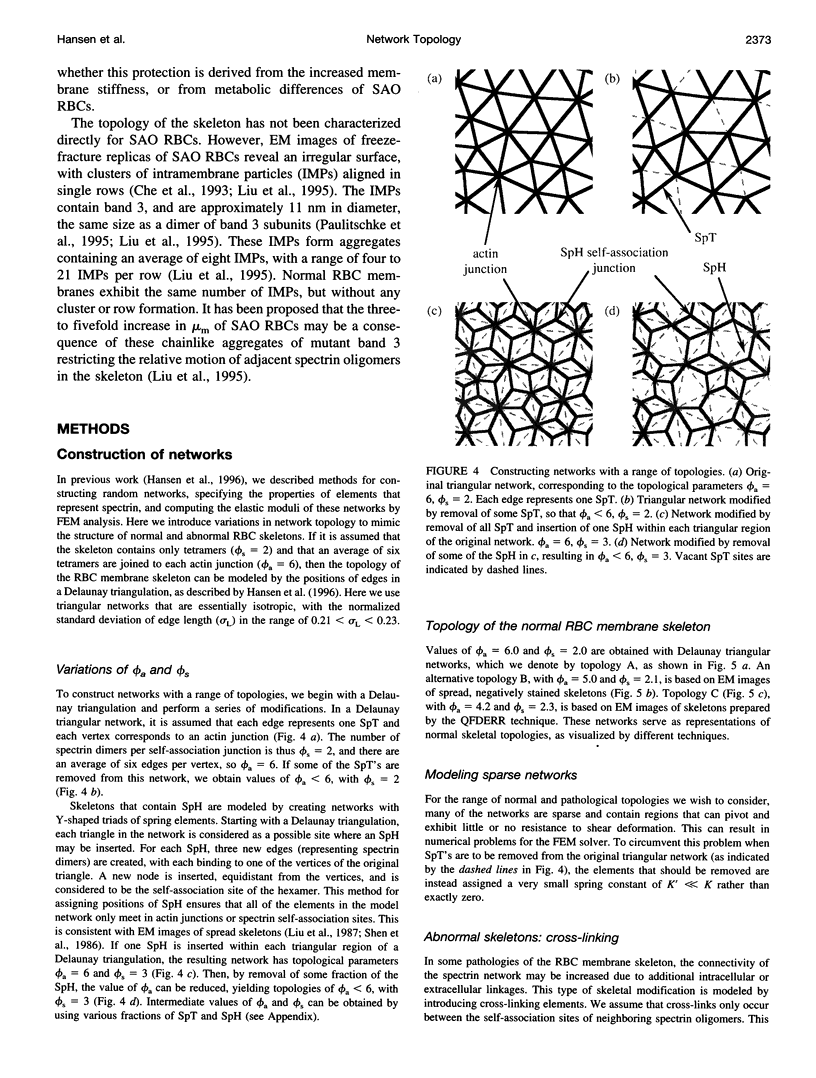
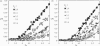Abstract
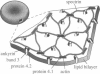
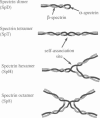
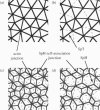
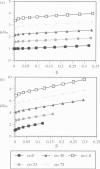
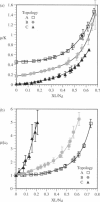
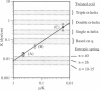
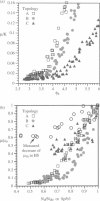
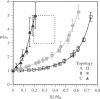
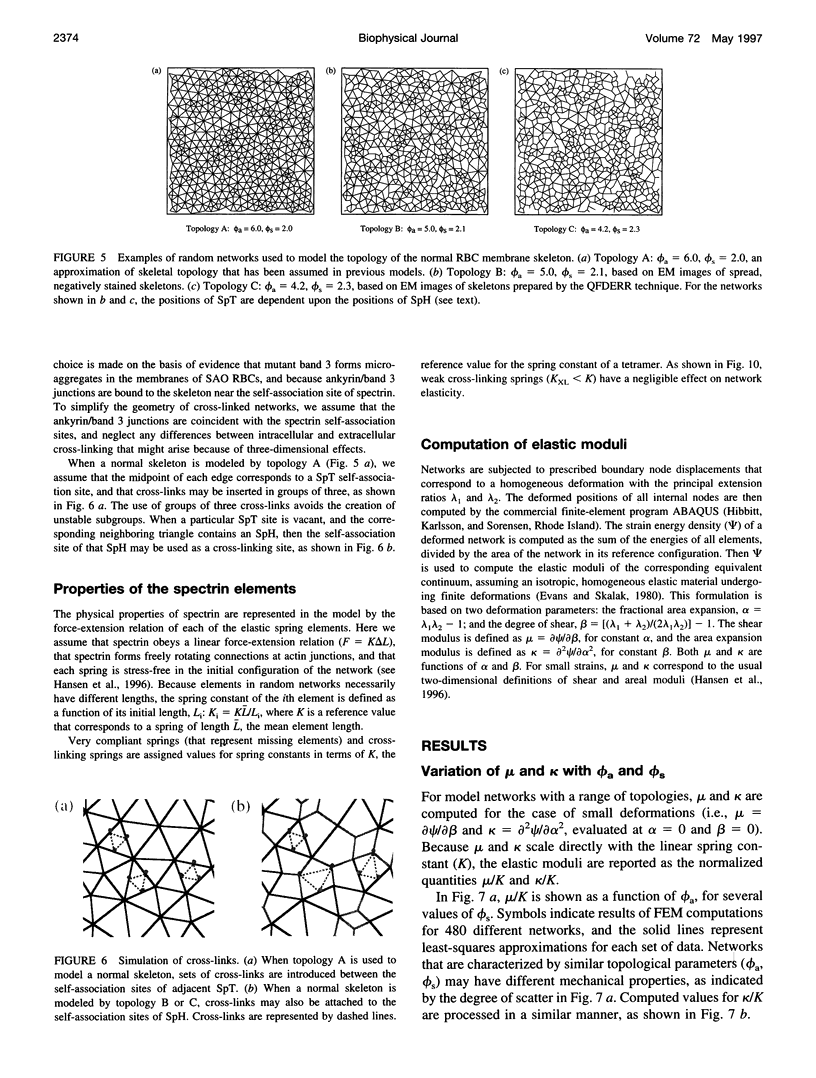
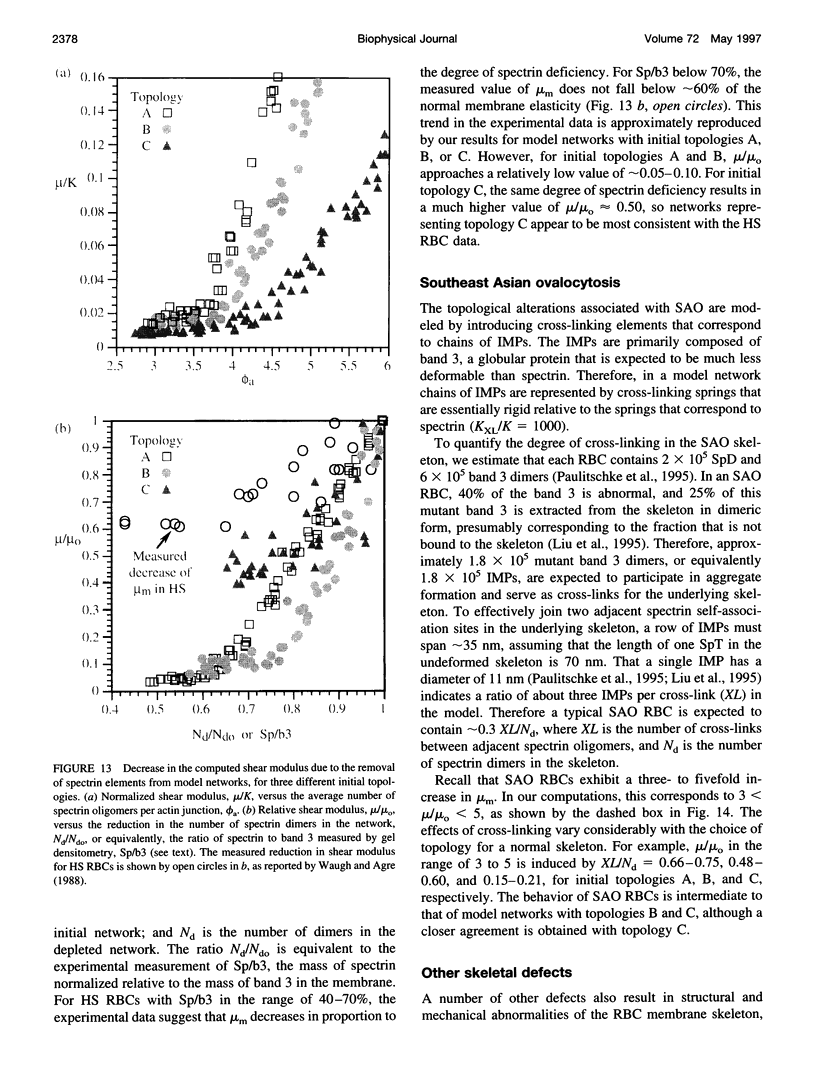
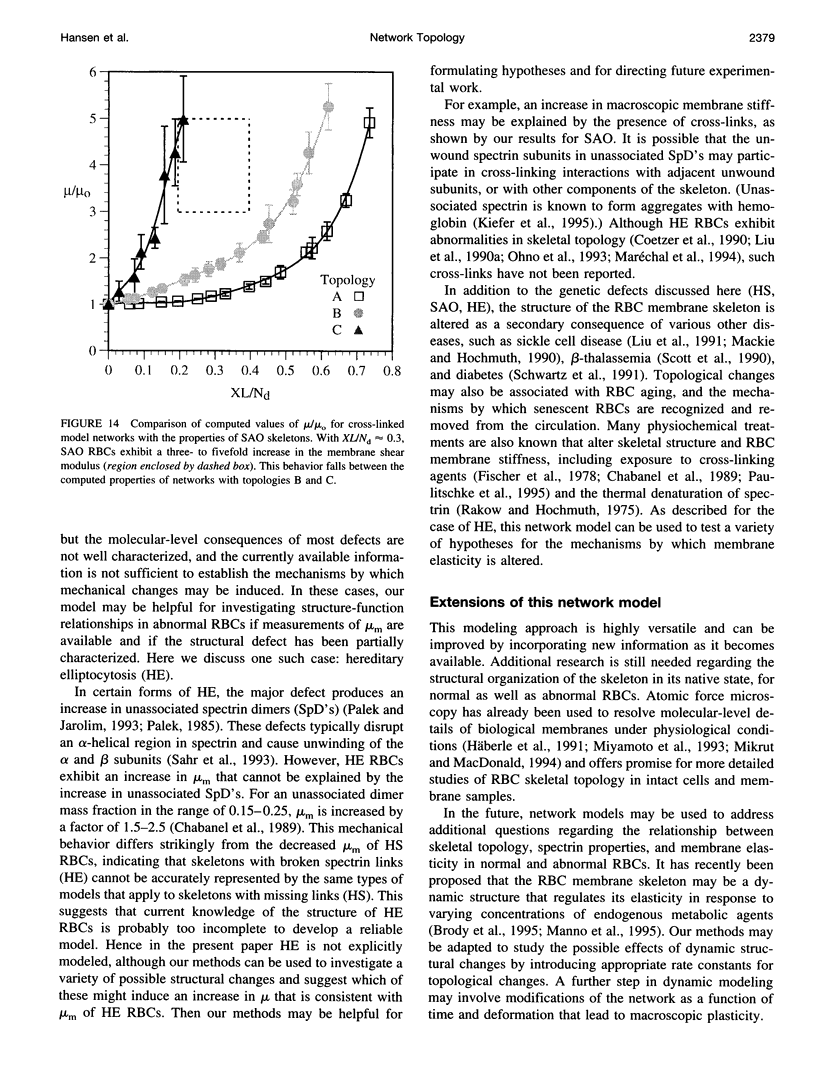
A finite-element network model is used to investigate the influence of the topology of the red blood cell membrane skeleton on its macroscopic mechanical properties. Network topology is characterized by the number of spectrin oligomers per actin junction (phi a) and the number of spectrin dimers per self-association junction (phi s). If it is assumed that all associated spectrin is in tetrameric form, with six tetramers per actin junction (i.e., phi a = 6.0 and phi s = 2.0), then the topology of the skeleton may be modeled by a random Delaunay triangular network. Recent images of the RBC membrane skeleton suggest that the values for these topological parameters are in the range of 4.2 < phi a < 5.5 and 2.1 < phi s < 2.3. Model networks that simulate these realistic topologies exhibit values of the shear modulus that vary by more than an order of magnitude relative to triangular networks. This indicates that networks with relatively sparse nontriangular topologies may be needed to model the RBC membrane skeleton accurately. The model is also used to simulate skeletal alterations associated with hereditary spherocytosis and Southeast Asian ovalocytosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker P. S., Lux S. E. Hereditary spherocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):15–43. [PubMed] [Google Scholar]
- Bennett V., Gilligan D. M. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Boal D. H. Computer simulation of a model network for the erythrocyte cytoskeleton. Biophys J. 1994 Aug;67(2):521–529. doi: 10.1016/S0006-3495(94)80511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody J. P., Han Y., Austin R. H., Bitensky M. Deformation and flow of red blood cells in a synthetic lattice: evidence for an active cytoskeleton. Biophys J. 1995 Jun;68(6):2224–2232. doi: 10.1016/S0006-3495(95)80443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. J., Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabanel A., Sung K. L., Rapiejko J., Prchal J. T., Palek J., Liu S. C., Chien S. Viscoelastic properties of red cell membrane in hereditary elliptocytosis. Blood. 1989 Feb;73(2):592–595. [PubMed] [Google Scholar]
- Che A., Cherry R. J., Bannister L. H., Dluzewski A. R. Aggregation of band 3 in hereditary ovalocytic red blood cell membranes. Electron microscopy and protein rotational diffusion studies. J Cell Sci. 1993 Jul;105(Pt 3):655–660. doi: 10.1242/jcs.105.3.655. [DOI] [PubMed] [Google Scholar]
- Chien S., Sung K. L., Skalak R., Usami S., Tözeren A. Theoretical and experimental studies on viscoelastic properties of erythrocyte membrane. Biophys J. 1978 Nov;24(2):463–487. doi: 10.1016/S0006-3495(78)85395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S., Sung L. P. Molecular basis of red cell membrane rheology. Part 1. Biorheology. 1990;27(3-4):327–344. doi: 10.3233/bir-1990-273-410. [DOI] [PubMed] [Google Scholar]
- Coetzer T., Palek J., Lawler J., Liu S. C., Jarolim P., Lahav M., Prchal J. T., Wang W., Alter B. P., Schewitz G. Structural and functional heterogeneity of alpha spectrin mutations involving the spectrin heterodimer self-association site: relationships to hematologic expression of homozygous hereditary elliptocytosis and hereditary pyropoikilocytosis. Blood. 1990 Jun 1;75(11):2235–2244. [PubMed] [Google Scholar]
- Derick L. H., Liu S. C., Chishti A. H., Palek J. Protein immunolocalization in the spread erythrocyte membrane skeleton. Eur J Cell Biol. 1992 Apr;57(2):317–320. [PubMed] [Google Scholar]
- Fischer T. M., Haest C. W., Stöhr M., Kamp D., Deuticke B. Selective alteration of erythrocyte deformabiliby by SH-reagents: evidence for an involvement of spectrin in membrane shear elasticity. Biochim Biophys Acta. 1978 Jul 4;510(2):270–282. doi: 10.1016/0005-2736(78)90027-5. [DOI] [PubMed] [Google Scholar]
- Gilligan D. M., Bennett V. The junctional complex of the membrane skeleton. Semin Hematol. 1993 Jan;30(1):74–83. [PubMed] [Google Scholar]
- Hansen J. C., Skalak R., Chien S., Hoger A. An elastic network model based on the structure of the red blood cell membrane skeleton. Biophys J. 1996 Jan;70(1):146–166. doi: 10.1016/S0006-3495(96)79556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Waugh R. E. Erythrocyte membrane elasticity and viscosity. Annu Rev Physiol. 1987;49:209–219. doi: 10.1146/annurev.ph.49.030187.001233. [DOI] [PubMed] [Google Scholar]
- Jarolim P., Palek J., Amato D., Hassan K., Sapak P., Nurse G. T., Rubin H. L., Zhai S., Sahr K. E., Liu S. C. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11022–11026. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer C. R., Trainor J. F., McKenney J. B., Valeri C. R., Snyder L. M. Hemoglobin-spectrin complexes: interference with spectrin tetramer assembly as a mechanism for compartmentalization of band 1 and band 2 complexes. Blood. 1995 Jul 1;86(1):366–371. [PubMed] [Google Scholar]
- Kozlov M. M., Markin V. S. Model of red blood cell membrane skeleton: electrical and mechanical properties. J Theor Biol. 1987 Dec 21;129(4):439–452. doi: 10.1016/s0022-5193(87)80023-1. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Derick L. H., Agre P., Palek J. Alteration of the erythrocyte membrane skeletal ultrastructure in hereditary spherocytosis, hereditary elliptocytosis, and pyropoikilocytosis. Blood. 1990 Jul 1;76(1):198–205. [PubMed] [Google Scholar]
- Liu S. C., Derick L. H., Palek J. Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J Cell Biol. 1987 Mar;104(3):527–536. doi: 10.1083/jcb.104.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Derick L. H., Zhai S., Palek J. Uncoupling of the spectrin-based skeleton from the lipid bilayer in sickled red cells. Science. 1991 Apr 26;252(5005):574–576. doi: 10.1126/science.2020854. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Yi S. J., Nichols P. E., Derick L. H., Chiou S. S., Amato D., Corbett J. D., Cho M. R., Golan D. E. Molecular basis of altered red blood cell membrane properties in Southeast Asian ovalocytosis: role of the mutant band 3 protein in band 3 oligomerization and retention by the membrane skeleton. Blood. 1995 Jul 1;86(1):349–358. [PubMed] [Google Scholar]
- Liu S. C., Windisch P., Kim S., Palek J. Oligomeric states of spectrin in normal erythrocyte membranes: biochemical and electron microscopic studies. Cell. 1984 Jun;37(2):587–594. doi: 10.1016/0092-8674(84)90389-1. [DOI] [PubMed] [Google Scholar]
- Manno S., Takakuwa Y., Nagao K., Mohandas N. Modulation of erythrocyte membrane mechanical function by beta-spectrin phosphorylation and dephosphorylation. J Biol Chem. 1995 Mar 10;270(10):5659–5665. doi: 10.1074/jbc.270.10.5659. [DOI] [PubMed] [Google Scholar]
- Maréchal J., Wada H., Koffa T., Kanzaki A., Wilmotte R., Ikoma K., Yawata A., Inoue T., Takanashi K., Miura A. Hereditary elliptocytosis associated with spectrin Le Puy in a Japanese family: ultrastructural aspect of the red cell skeleton. Eur J Haematol. 1994 Feb;52(2):92–98. doi: 10.1111/j.1600-0609.1994.tb01292.x. [DOI] [PubMed] [Google Scholar]
- McGough A. M., Josephs R. On the structure of erythrocyte spectrin in partially expanded membrane skeletons. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5208–5212. doi: 10.1073/pnas.87.13.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikrut J. M., Mac Donald R. C. Analysis of red blood cell cytoskeleton using an atomic force microscope. Am Biotechnol Lab. 1994 Apr;12(5):26–26. [PubMed] [Google Scholar]
- Mohandas N., Winardi R., Knowles D., Leung A., Parra M., George E., Conboy J., Chasis J. Molecular basis for membrane rigidity of hereditary ovalocytosis. A novel mechanism involving the cytoplasmic domain of band 3. J Clin Invest. 1992 Feb;89(2):686–692. doi: 10.1172/JCI115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Terada N., Fujii Y., Ueda H., Kuramoto H., Kamisawa N. Immunocytochemical study of membrane skeletons in abnormally shaped erythrocytes as revealed by a quick-freezing and deep-etching method. Virchows Arch A Pathol Anat Histopathol. 1993;422(1):73–80. doi: 10.1007/BF01605136. [DOI] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):45–87. [PubMed] [Google Scholar]
- Palek J., Jarolim P. Clinical expression and laboratory detection of red blood cell membrane protein mutations. Semin Hematol. 1993 Oct;30(4):249–283. [PubMed] [Google Scholar]
- Palek J., Lambert S. Genetics of the red cell membrane skeleton. Semin Hematol. 1990 Oct;27(4):290–332. [PubMed] [Google Scholar]
- Parry D. A., Dixon T. W., Cohen C. Analysis of the three-alpha-helix motif in the spectrin superfamily of proteins. Biophys J. 1992 Apr;61(4):858–867. doi: 10.1016/S0006-3495(92)81893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulitschke M., Nash G. B., Anstee D. J., Tanner M. J., Gratzer W. B. Perturbation of red blood cell membrane rigidity by extracellular ligands. Blood. 1995 Jul 1;86(1):342–348. [PubMed] [Google Scholar]
- Rakow A. L., Hochmuth R. M. Effect of heat treatment on the elasticity of human erythrocyte membrane. Biophys J. 1975 Nov;15(11):1095–1100. doi: 10.1016/S0006-3495(75)85885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahr K. E., Coetzer T. L., Moy L. S., Derick L. H., Chishti A. H., Jarolim P., Lorenzo F., Miraglia del Giudice E., Iolascon A., Gallanello R. Spectrin cagliari. an Ala-->Gly substitution in helix 1 of beta spectrin repeat 17 that severely disrupts the structure and self-association of the erythrocyte spectrin heterodimer. J Biol Chem. 1993 Oct 25;268(30):22656–22662. [PubMed] [Google Scholar]
- Savvides P., Shalev O., John K. M., Lux S. E. Combined spectrin and ankyrin deficiency is common in autosomal dominant hereditary spherocytosis. Blood. 1993 Nov 15;82(10):2953–2960. [PubMed] [Google Scholar]
- Schofield A. E., Reardon D. M., Tanner M. J. Defective anion transport activity of the abnormal band 3 in hereditary ovalocytic red blood cells. Nature. 1992 Feb 27;355(6363):836–838. doi: 10.1038/355836a0. [DOI] [PubMed] [Google Scholar]
- Schofield A. E., Tanner M. J., Pinder J. C., Clough B., Bayley P. M., Nash G. B., Dluzewski A. R., Reardon D. M., Cox T. M., Wilson R. J. Basis of unique red cell membrane properties in hereditary ovalocytosis. J Mol Biol. 1992 Feb 20;223(4):949–958. doi: 10.1016/0022-2836(92)90254-h. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Madsen J. W., Rybicki A. C., Nagel R. L. Oxidation of spectrin and deformability defects in diabetic erythrocytes. Diabetes. 1991 Jun;40(6):701–708. doi: 10.2337/diab.40.6.701. [DOI] [PubMed] [Google Scholar]
- Scott M. D., Rouyer-Fessard P., Lubin B. H., Beuzard Y. Entrapment of purified alpha-hemoglobin chains in normal erythrocytes. A model for beta thalassemia. J Biol Chem. 1990 Oct 15;265(29):17953–17959. [PubMed] [Google Scholar]
- Shen B. W., Josephs R., Steck T. L. Ultrastructure of the intact skeleton of the human erythrocyte membrane. J Cell Biol. 1986 Mar;102(3):997–1006. doi: 10.1083/jcb.102.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Stokke B. T., Mikkelsen A., Elgsaeter A. The human erythrocyte membrane skeleton may be an ionic gel. I. Membrane mechanochemical properties. Eur Biophys J. 1986;13(4):203–218. doi: 10.1007/BF00260368. [DOI] [PubMed] [Google Scholar]
- Tilley L., Nash G. B., Jones G. L., Sawyer W. H. Decreased rotational diffusion of band 3 in Melanesian ovalocytes from Papua, New Guinea. J Membr Biol. 1991 Apr;121(1):59–66. doi: 10.1007/BF01870651. [DOI] [PubMed] [Google Scholar]
- Ursitti J. A., Pumplin D. W., Wade J. B., Bloch R. J. Ultrastructure of the human erythrocyte cytoskeleton and its attachment to the membrane. Cell Motil Cytoskeleton. 1991;19(4):227–243. doi: 10.1002/cm.970190402. [DOI] [PubMed] [Google Scholar]
- Ursitti J. A., Wade J. B. Ultrastructure and immunocytochemistry of the isolated human erythrocyte membrane skeleton. Cell Motil Cytoskeleton. 1993;25(1):30–42. doi: 10.1002/cm.970250105. [DOI] [PubMed] [Google Scholar]
- Waugh R. E., Agre P. Reductions of erythrocyte membrane viscoelastic coefficients reflect spectrin deficiencies in hereditary spherocytosis. J Clin Invest. 1988 Jan;81(1):133–141. doi: 10.1172/JCI113284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E. Effects of abnormal cytoskeletal structure on erythrocyte membrane mechanical properties. Cell Motil. 1983;3(5-6):609–622. doi: 10.1002/cm.970030526. [DOI] [PubMed] [Google Scholar]
- Waugh R. E. Effects of inherited membrane abnormalities on the viscoelastic properties of erythrocyte membrane. Biophys J. 1987 Mar;51(3):363–369. doi: 10.1016/S0006-3495(87)83358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R., Evans E. A. Thermoelasticity of red blood cell membrane. Biophys J. 1979 Apr;26(1):115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Winograd E., Viel A., Cronin T., Harrison S. C., Branton D. Crystal structure of the repetitive segments of spectrin. Science. 1993 Dec 24;262(5142):2027–2030. doi: 10.1126/science.8266097. [DOI] [PubMed] [Google Scholar]