Abstract
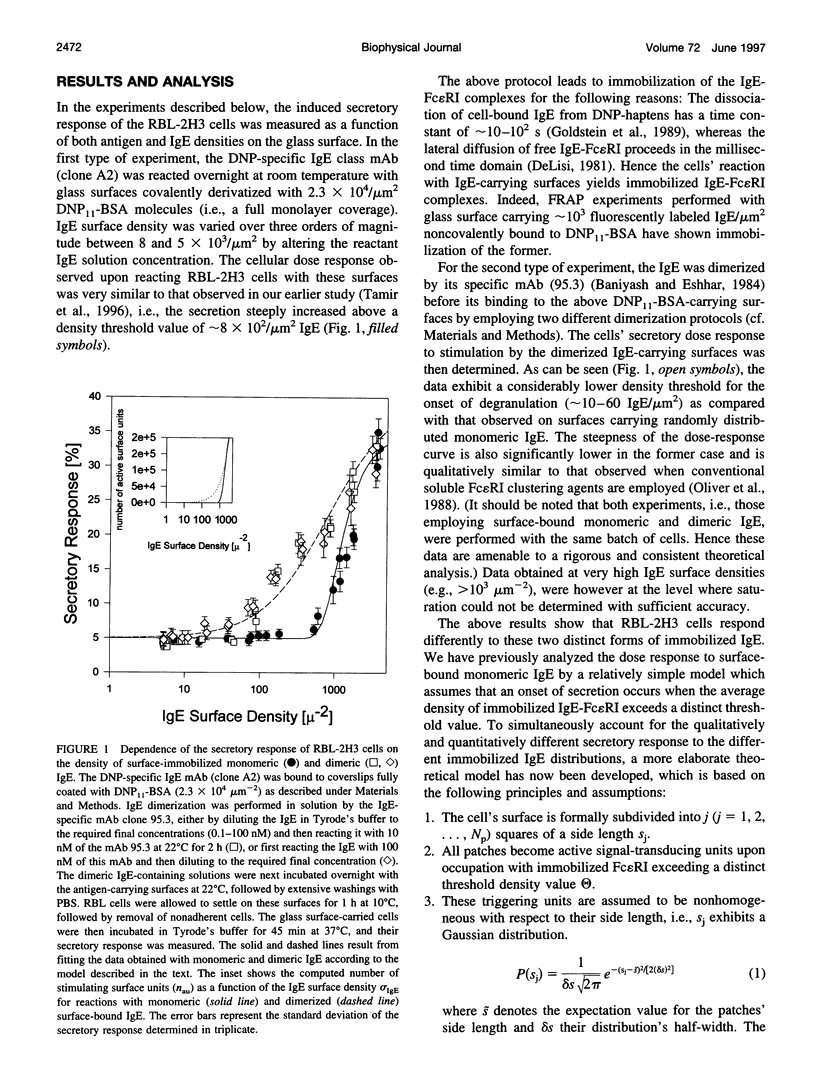
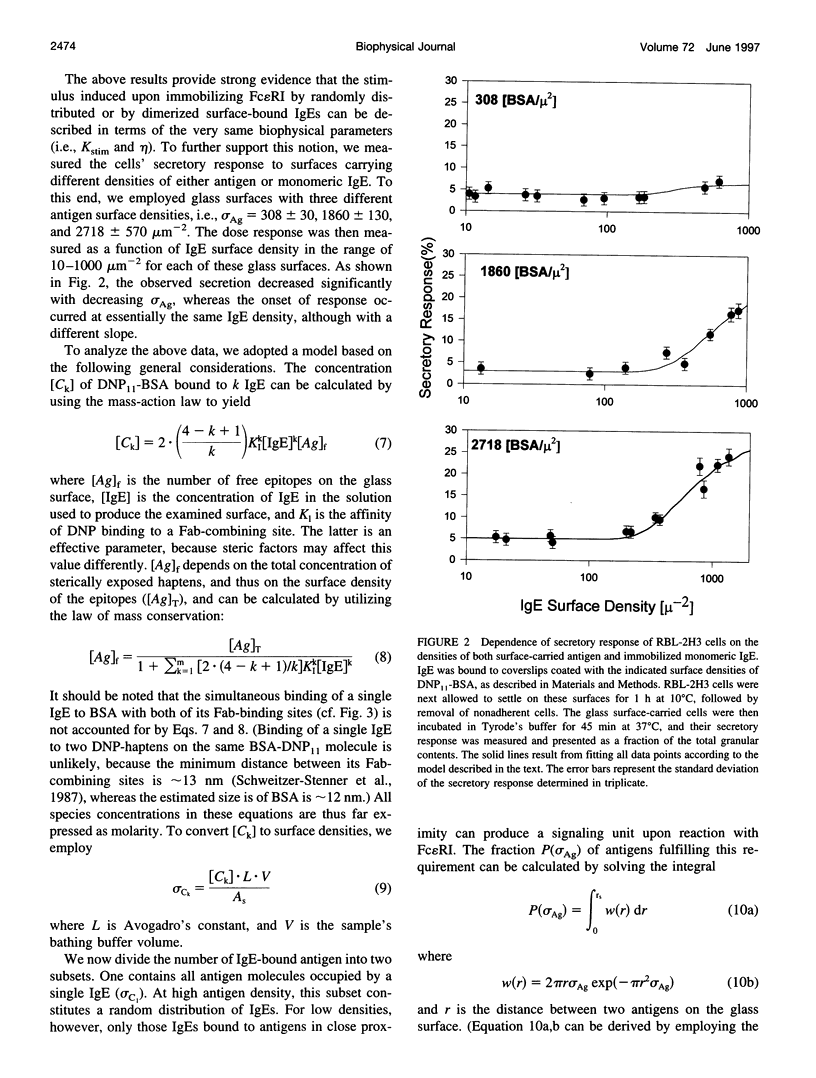
Clustering of the type I receptor for IgE (Fc[epsilon]RI) on mast cells initiates a cascade of biochemical processes that result in secretion of inflammatory mediators. To determine the Fc(epsilon)RI proximity, cluster size, and mobility requirements for initiating the Fc(epsilon)RI cascade, a novel experimental protocol has been developed in which mast cells are reacted with glass surfaces carrying different densities of both antigen and bound IgE, and the cell's secretory response to these stimuli is measured. The results have been analyzed in terms of a model based on the following assumptions: 1) the glass surface antigen distribution and consequently that of the bound IgE are random; 2) Fc(epsilon)RI binding to these surface-bound IgEs immobilizes the former and saturates the latter; 3) the cell surface is formally divided into small elements, which function as a secretory stimulus unit when occupied by two or more immobilized IgE-Fc(epsilon)RI complexes; 4) alternatively, similar stimulatory units can be formed by binding of surface-carried IgE dimers to two Fc(epsilon)RI. This model yielded a satisfactory and self-consistent fitting of all of the different experimental data sets. Hence the present results establish the essential role of Fc(epsilon)RI immobilization for initiating its signaling cascade. Moreover, it provides independent support for the notion that as few as two Fc(epsilon)RIs immobilized at van der Waals contact constitute an "elementary stimulatory unit" leading to mast cell (RBL-2H3 line) secretory response.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamczewski M., Kinet J. P. The high-affinity receptor for immunoglobulin E. Chem Immunol. 1994;59:173–190. [PubMed] [Google Scholar]
- Adamczewski M., Paolini R., Kinet J. P. Evidence for two distinct phosphorylation pathways activated by high affinity immunoglobulin E receptors. J Biol Chem. 1992 Sep 5;267(25):18126–18132. [PubMed] [Google Scholar]
- Balakrishnan K., Hsu F. J., Cooper A. D., McConnell H. M. Lipid hapten containing membrane targets can trigger specific immunoglobulin E-dependent degranulation of rat basophil leukemia cells. J Biol Chem. 1982 Jun 10;257(11):6427–6433. [PubMed] [Google Scholar]
- Baniyash M., Eshhar Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur J Immunol. 1984 Sep;14(9):799–807. doi: 10.1002/eji.1830140907. [DOI] [PubMed] [Google Scholar]
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Basciano L. K., Berenstein E. H., Kmak L., Siraganian R. P. Monoclonal antibodies that inhibit IgE binding. J Biol Chem. 1986 Sep 5;261(25):11823–11831. [PubMed] [Google Scholar]
- Bolen J. B. Protein tyrosine kinases in the initiation of antigen receptor signaling. Curr Opin Immunol. 1995 Jun;7(3):306–311. doi: 10.1016/0952-7915(95)80103-0. [DOI] [PubMed] [Google Scholar]
- Cooper A. D., Balakrishnan K., McConnell H. M. Mobile haptens in liposomes stimulate serotonin release by rat basophil leukemia cells in the presence of specific immunoglobulin E. J Biol Chem. 1981 Sep 25;256(18):9379–9381. [PubMed] [Google Scholar]
- DeLisi C. The biophysics of ligand-receptor interactions. Q Rev Biophys. 1980 May;13(2):201–230. doi: 10.1017/s0033583500001657. [DOI] [PubMed] [Google Scholar]
- DeLisi C. The magnitude of signal amplification by ligand-induced receptor clustering. Nature. 1981 Jan 22;289(5795):322–323. doi: 10.1038/289322a0. [DOI] [PubMed] [Google Scholar]
- Dembo M., Goldstein B., Sobotka A. K., Lichtenstein L. M. Degranulation of human basophils: quantitative analysis of histamine release and desensitization, due to a bivalent penicilloyl hapten. J Immunol. 1979 Oct;123(4):1864–1872. [PubMed] [Google Scholar]
- EISEN H. N., KERN M., NEWTON W. T., HELMREICH E. A study of the distribution of 2,4-dinitrobenzene sensitizers between isolated lymph node cells and extracellular medium in relation to induction of contact skin sensitivity. J Exp Med. 1959 Aug 1;110(2):187–206. doi: 10.1084/jem.110.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992 Jan 2;355(6355):78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Posner R. G., Goldstein B., Holowka D., Baird B. Bivalent ligand dissociation kinetics from receptor-bound immunoglobulin E: evidence for a time-dependent increase in ligand rebinding at the cell surface. Biochemistry. 1991 Mar 5;30(9):2357–2363. doi: 10.1021/bi00223a009. [DOI] [PubMed] [Google Scholar]
- Fewtrell C., Metzger H. Larger oligomers of IgE are more effective than dimers in stimulating rat basophilic leukemia cells. J Immunol. 1980 Aug;125(2):701–710. [PubMed] [Google Scholar]
- Goldstein B., Posner R. G., Torney D. C., Erickson J., Holowka D., Baird B. Competition between solution and cell surface receptors for ligand. Dissociation of hapten bound to surface antibody in the presence of solution antibody. Biophys J. 1989 Nov;56(5):955–966. doi: 10.1016/S0006-3495(89)82741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Steinberg I. Z. On the analysis of fluorescence decay kinetics by the method of least-squares. Anal Biochem. 1974 Jun;59(2):583–598. doi: 10.1016/0003-2697(74)90312-1. [DOI] [PubMed] [Google Scholar]
- Holowka D., Baird B. Antigen-mediated IGE receptor aggregation and signaling: a window on cell surface structure and dynamics. Annu Rev Biophys Biomol Struct. 1996;25:79–112. doi: 10.1146/annurev.bb.25.060196.000455. [DOI] [PubMed] [Google Scholar]
- Holowka D., Conrad D. H., Baird B. Structural mapping of membrane-bound immunoglobulin E-receptor complexes: use of monoclonal anti-IgE antibodies to probe the conformation of receptor-bound IgE. Biochemistry. 1985 Oct 22;24(22):6260–6267. doi: 10.1021/bi00343a033. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Reay P. A., Ehrich E. W., Davis M. M. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Kane P. M., Holowka D., Baird B. Cross-linking of IgE-receptor complexes by rigid bivalent antigens greater than 200 A in length triggers cellular degranulation. J Cell Biol. 1988 Sep;107(3):969–980. doi: 10.1083/jcb.107.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitscheck U., Schweitzer-Stenner R., Arndt-Jovin D. J., Jovin T. M., Pecht I. Distribution of type I Fc epsilon-receptors on the surface of mast cells probed by fluorescence resonance energy transfer. Biophys J. 1993 Jan;64(1):110–120. doi: 10.1016/S0006-3495(93)81345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Cicala C., Scharenberg A. M., Kinet J. P. The Fc(epsilon)RIbeta subunit functions as an amplifier of Fc(epsilon)RIgamma-mediated cell activation signals. Cell. 1996 Jun 28;85(7):985–995. doi: 10.1016/s0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- McCloskey M. A., Poo M. M. Contact-induced redistribution of specific membrane components: local accumulation and development of adhesion. J Cell Biol. 1986 Jun;102(6):2185–2196. doi: 10.1083/jcb.102.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeithan T. W. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K., Holowka D., Baird B. Small oligomers of immunoglobulin E (IgE) cause large-scale clustering of IgE receptors on the surface of rat basophilic leukemia cells. J Cell Biol. 1984 Feb;98(2):577–583. doi: 10.1083/jcb.98.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K., Holowka D., Webb W. W., Baird B. Clustering, mobility, and triggering activity of small oligomers of immunoglobulin E on rat basophilic leukemia cells. J Cell Biol. 1986 Feb;102(2):534–540. doi: 10.1083/jcb.102.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K., Holowka D., Webb W. W., Baird B. Cross-linking of receptor-bound IgE to aggregates larger than dimers leads to rapid immobilization. J Cell Biol. 1986 Feb;102(2):541–550. doi: 10.1083/jcb.102.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P. Lateral diffusion of membrane proteins in protein-rich membranes. A simple hard particle model for concentration dependence of the two-dimensional diffusion coefficient. Biophys J. 1989 Apr;55(4):805–808. doi: 10.1016/S0006-3495(89)82880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. N., Holowka D., Baird B. Rotational motion of monomeric and dimeric immunoglobulin E-receptor complexes. Biochemistry. 1992 Jan 21;31(2):567–575. doi: 10.1021/bi00117a038. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Seagrave J., Stump R. F., Pfeiffer J. R., Deanin G. G. Signal transduction and cellular response in RBL-2H3 mast cells. Prog Allergy. 1988;42:185–245. [PubMed] [Google Scholar]
- Ortega Soto E., Pecht I. A monoclonal antibody that inhibits secretion from rat basophilic leukemia cells and binds to a novel membrane component. J Immunol. 1988 Dec 15;141(12):4324–4332. [PubMed] [Google Scholar]
- Ortega E., Schweitzer-Stenner R., Pecht I. Kinetics of ligand binding to the type 1 Fc epsilon receptor on mast cells. Biochemistry. 1991 Apr 9;30(14):3473–3483. doi: 10.1021/bi00228a018. [DOI] [PubMed] [Google Scholar]
- Ortega E., Schweitzer-Stenner R., Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. EMBO J. 1988 Dec 20;7(13):4101–4109. doi: 10.1002/j.1460-2075.1988.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecht I., Ortega E., Jovin T. M. Rotational dynamics of the Fc epsilon receptor on mast cells monitored by specific monoclonal antibodies and IgE. Biochemistry. 1991 Apr 9;30(14):3450–3458. doi: 10.1021/bi00228a015. [DOI] [PubMed] [Google Scholar]
- Posner R. G., Erickson J. W., Holowka D., Baird B., Goldstein B. Dissociation kinetics of bivalent ligand-immunoglobulin E aggregates in solution. Biochemistry. 1991 Mar 5;30(9):2348–2356. doi: 10.1021/bi00223a008. [DOI] [PubMed] [Google Scholar]
- Posner R. G., Lee B., Conrad D. H., Holowka D., Baird B., Goldstein B. Aggregation of IgE-receptor complexes on rat basophilic leukemia cells does not change the intrinsic affinity but can alter the kinetics of the ligand-IgE interaction. Biochemistry. 1992 Jun 16;31(23):5350–5356. doi: 10.1021/bi00138a015. [DOI] [PubMed] [Google Scholar]
- Posner R. G., Subramanian K., Goldstein B., Thomas J., Feder T., Holowka D., Baird B. Simultaneous cross-linking by two nontriggering bivalent ligands causes synergistic signaling of IgE Fc epsilon RI complexes. J Immunol. 1995 Oct 1;155(7):3601–3609. [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Rudolph A. K., Burrows P. D., Wabl M. R. Thirteen hybridomas secreting hapten-specific immunoglobulin E from mice with Iga or Igb heavy chain haplotype. Eur J Immunol. 1981 Jun;11(6):527–529. doi: 10.1002/eji.1830110617. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion and aggregation. A Monte Carlo study. Biophys J. 1992 Jan;61(1):119–128. doi: 10.1016/S0006-3495(92)81821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Single-particle diffusion. Biophys J. 1993 Jun;64(6):1766–1780. doi: 10.1016/S0006-3495(93)81548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. The spectrin network as a barrier to lateral diffusion in erythrocytes. A percolation analysis. Biophys J. 1989 Jan;55(1):21–28. doi: 10.1016/S0006-3495(89)82776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg A. M., Lin S., Cuenod B., Yamamura H., Kinet J. P. Reconstitution of interactions between tyrosine kinases and the high affinity IgE receptor which are controlled by receptor clustering. EMBO J. 1995 Jul 17;14(14):3385–3394. doi: 10.1002/j.1460-2075.1995.tb07344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Schlissel M. S. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Licht A., Lüscher I., Pecht I. Oligomerization and ring closure of immunoglobulin E class antibodies by divalent haptens. Biochemistry. 1987 Jun 16;26(12):3602–3612. doi: 10.1021/bi00386a053. [DOI] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Licht A., Pecht I. Dimerization kinetics of the IgE-class antibodies by divalent haptens. II. The interactions between intact IgE and haptens. Biophys J. 1992 Aug;63(2):563–568. doi: 10.1016/S0006-3495(92)81610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Ortega E., Pecht I. Kinetics of Fc epsilon RI dimer formation by specific monoclonal antibodies on mast cells. Biochemistry. 1994 Jul 26;33(29):8813–8825. doi: 10.1021/bi00195a025. [DOI] [PubMed] [Google Scholar]
- Subramanian K., Holowka D., Baird B., Goldstein B. The Fc segment of IgE influences the kinetics of dissociation of a symmetrical bivalent ligand from cyclic dimeric complexes. Biochemistry. 1996 Apr 30;35(17):5518–5527. doi: 10.1021/bi9523522. [DOI] [PubMed] [Google Scholar]
- Tamir I., Schweitzer-Stenner R., Pecht I. Immobilization of the type I receptor for IgE initiates signal transduction in mast cells. Biochemistry. 1996 May 28;35(21):6872–6883. doi: 10.1021/bi952556i. [DOI] [PubMed] [Google Scholar]
- Weetall M., Holowka D., Baird B. Heterologous desensitization of the high affinity receptor for IgE (Fc epsilon R1) on RBL cells. J Immunol. 1993 May 1;150(9):4072–4083. [PubMed] [Google Scholar]
- Zheng Y., Shopes B., Holowka D., Baird B. Dynamic conformations compared for IgE and IgG1 in solution and bound to receptors. Biochemistry. 1992 Aug 25;31(33):7446–7456. doi: 10.1021/bi00148a004. [DOI] [PubMed] [Google Scholar]


