Abstract
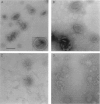
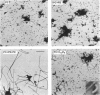
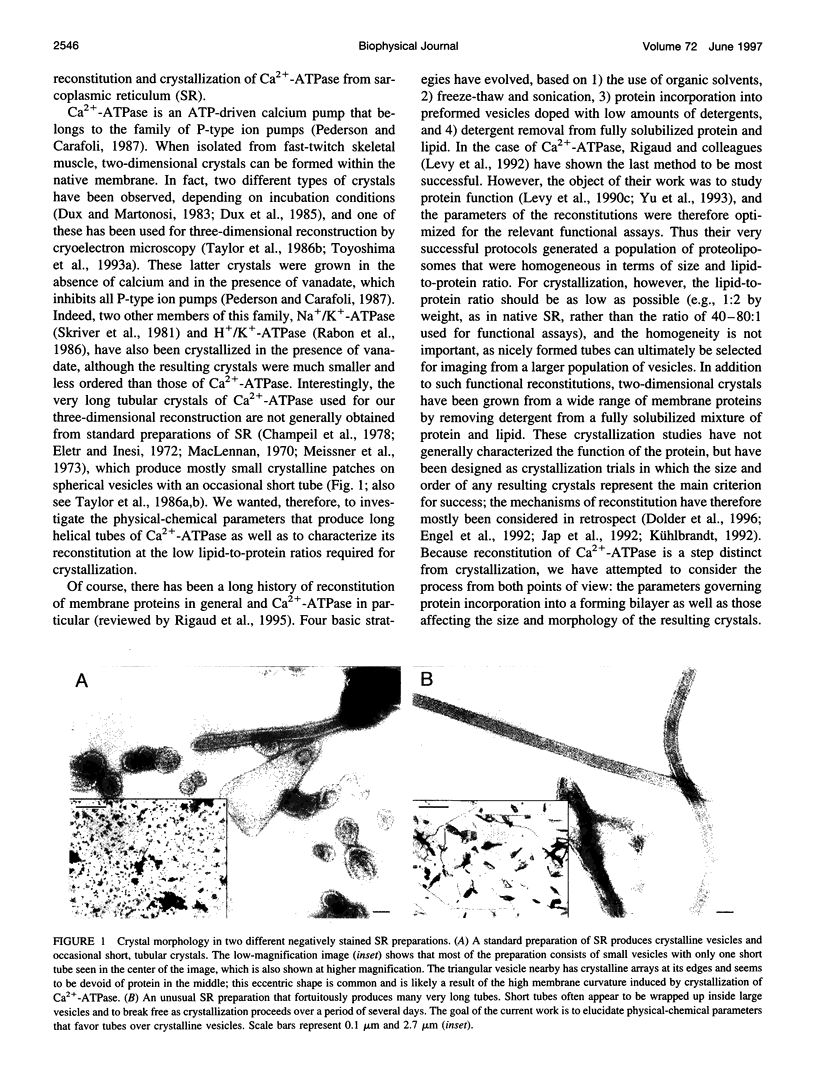
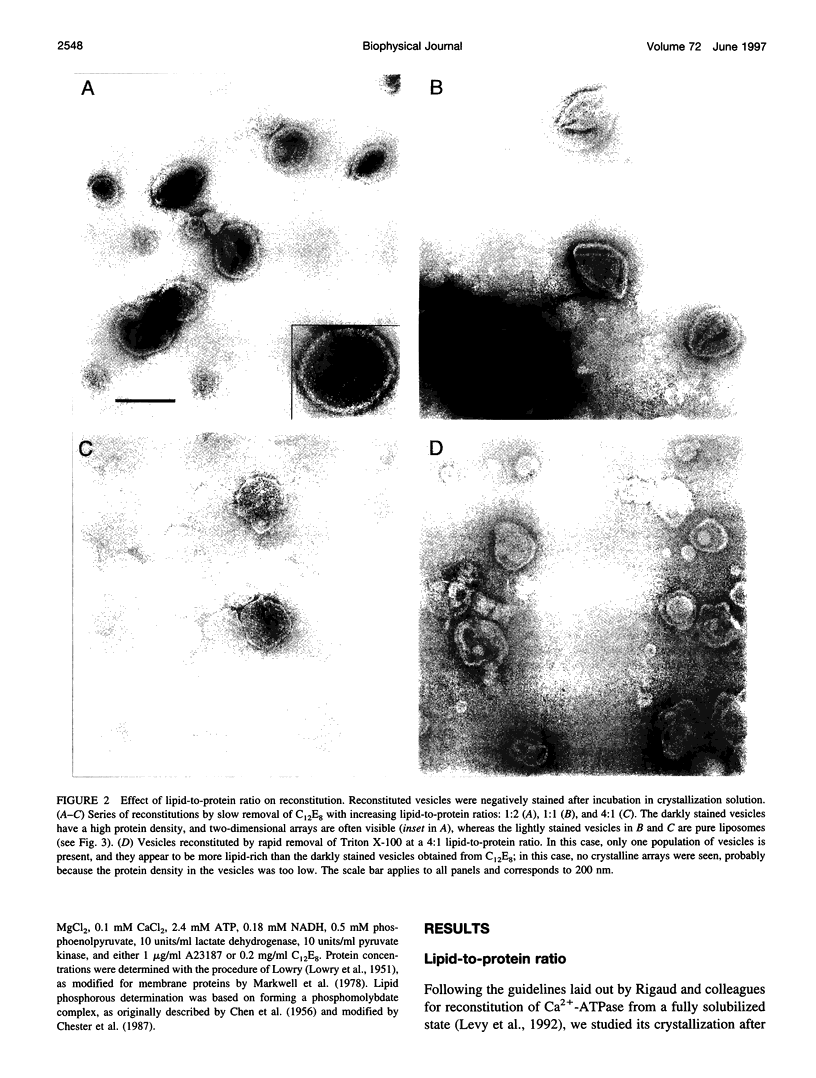
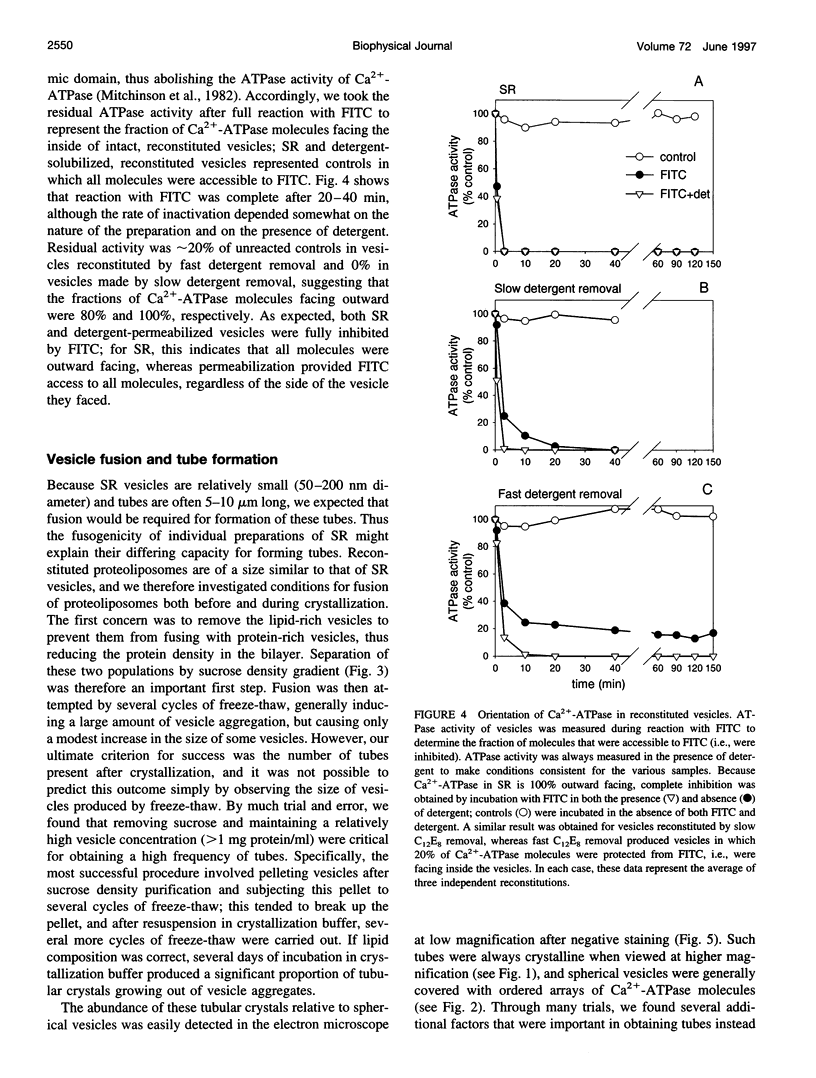
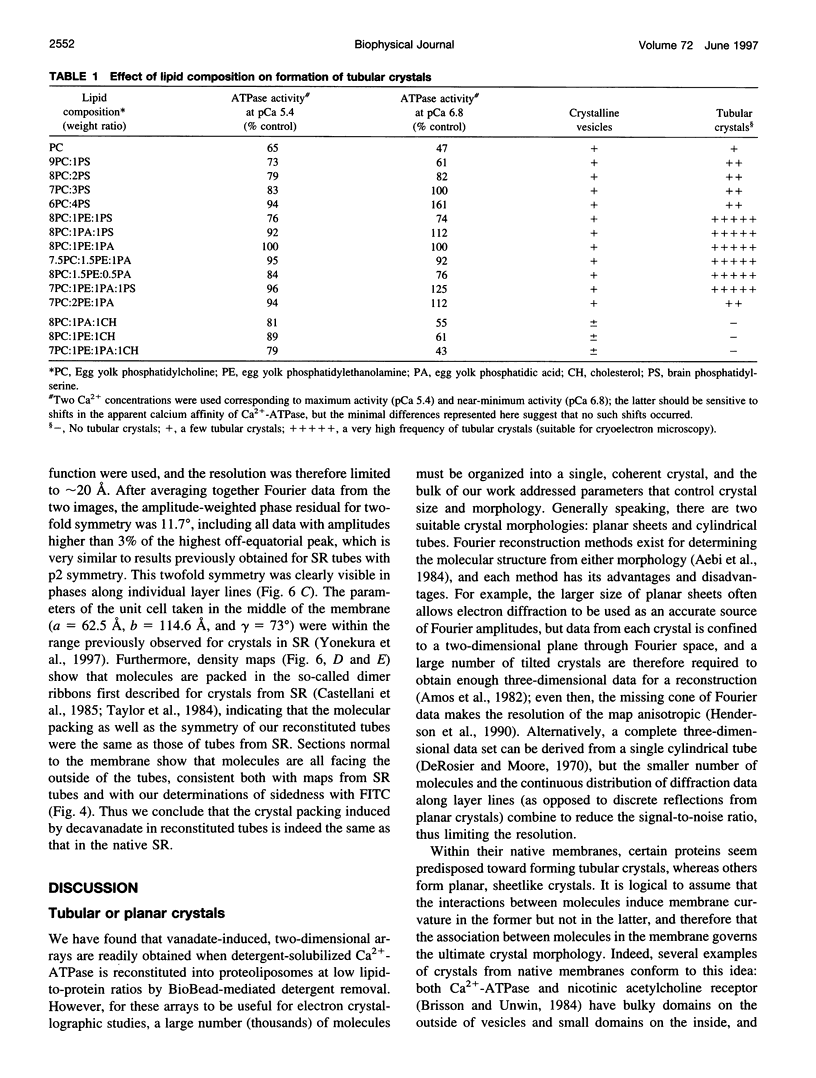
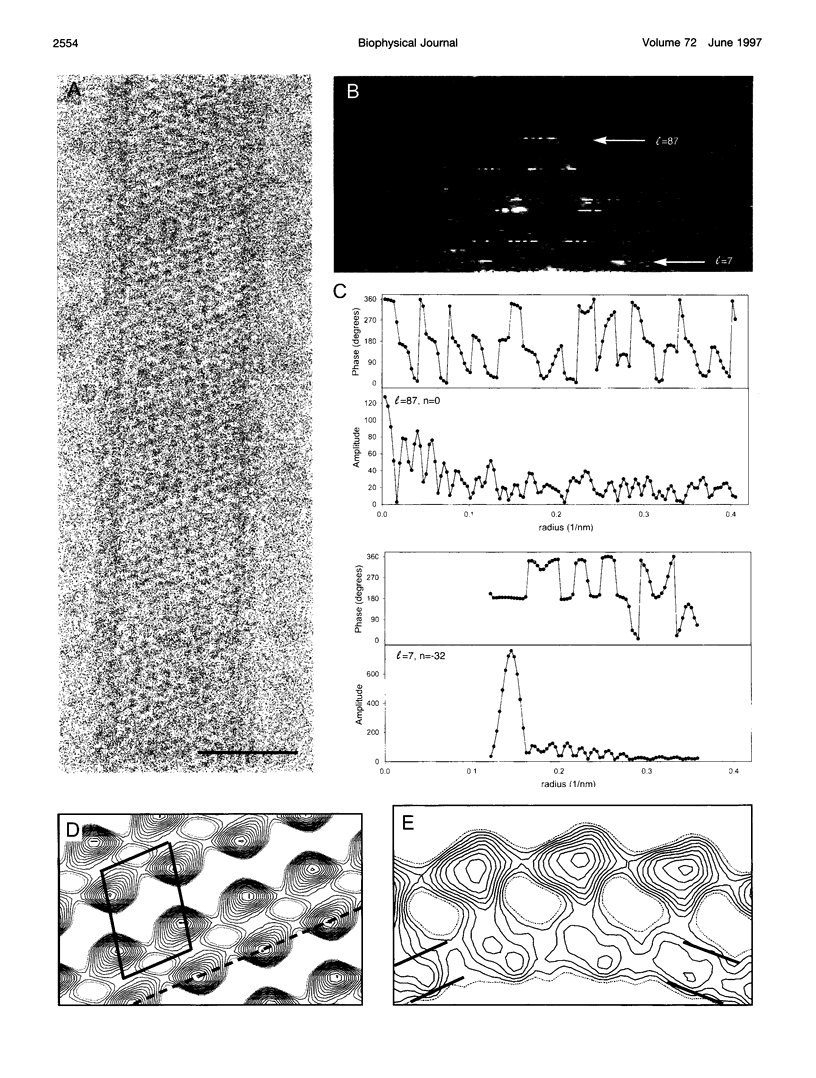
In an attempt to better define the parameters governing reconstitution and two-dimensional crystallization of membrane proteins, we have studied Ca2(+)-ATPase from rabbit sarcoplasmic reticulum. This ion pump forms vanadate-induced crystals in its native membrane and has previously been reconstituted at high lipid-to-protein ratios for functional studies. We have characterized the reconstitution of purified Ca2(+)-ATPase at low lipid-to-protein ratios and discovered procedures that produce long, tubular crystals suitable for helical reconstruction. C12E8 (n-dodecyl-octaethylene-glycol monoether) was used to fully solubilize various mixtures of lipid and purified Ca2(+)-ATPase, and BioBeads were then used to remove the C12E8. Slow removal resulted in two populations of vesicles, and the proteoliposome population was separated from the liposome population on a sucrose density gradient. These proteoliposomes had a lipid-to-protein ratio of 1:2, and virtually 100% of molecules faced the outside of vesicles, as determined by fluorescein isothiocyanate labeling. Cycles of freeze-thaw caused considerable aggregation of these proteoliposomes, and, if phosphatidyl ethanolamine and phosphatidic acid were included, or if the bilayers were doped with small amounts of C12E8, vanadate-induced tubular crystals grew from the aggregates. Thus our procedure comprised two steps-reconstitution followed by crystallization-allowing us to consider mechanisms of bilayer formation separately from those of crystallization and tube formation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Fowler W. E., Buhle E. L., Jr, Smith P. R. Electron microscopy and image processing applied to the study of protein structure and protein-protein interactions. J Ultrastruct Res. 1984 Aug;88(2):143–176. doi: 10.1016/s0022-5320(84)80006-4. [DOI] [PubMed] [Google Scholar]
- Akiba T., Toyoshima C., Matsunaga T., Kawamoto M., Kubota T., Fukuyama K., Namba K., Matsubara H. Three-dimensional structure of bovine cytochrome bc1 complex by electron cryomicroscopy and helical image reconstruction. Nat Struct Biol. 1996 Jun;3(6):553–561. doi: 10.1038/nsb0696-553. [DOI] [PubMed] [Google Scholar]
- Amos L. A., Henderson R., Unwin P. N. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog Biophys Mol Biol. 1982;39(3):183–231. doi: 10.1016/0079-6107(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Brisson A., Unwin P. N. Tubular crystals of acetylcholine receptor. J Cell Biol. 1984 Oct;99(4 Pt 1):1202–1211. doi: 10.1083/jcb.99.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani L., Hardwicke P. M., Vibert P. Dimer ribbons in the three-dimensional structure of sarcoplasmic reticulum. J Mol Biol. 1985 Oct 5;185(3):579–594. doi: 10.1016/0022-2836(85)90073-7. [DOI] [PubMed] [Google Scholar]
- Champeil P., Büschlen-Boucly S., Bastide F., Gary-Bobo C. Sarcoplasmic reticulum ATPase. Spin labeling detection of ligand-induced changes in the relative reactivities of certain sulfhydryl groups. J Biol Chem. 1978 Feb 25;253(4):1179–1186. [PubMed] [Google Scholar]
- Chester D. W., Herbette L. G., Mason R. P., Joslyn A. F., Triggle D. J., Koppel D. E. Diffusion of dihydropyridine calcium channel antagonists in cardiac sarcolemmal lipid multibilayers. Biophys J. 1987 Dec;52(6):1021–1030. doi: 10.1016/S0006-3495(87)83295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius F. Functional reconstitution of the sodium pump. Kinetics of exchange reactions performed by reconstituted Na/K-ATPase. Biochim Biophys Acta. 1991 Mar 7;1071(1):19–66. doi: 10.1016/0304-4157(91)90011-k. [DOI] [PubMed] [Google Scholar]
- DeRosier D. J., Moore P. B. Reconstruction of three-dimensional images from electron micrographs of structures with helical symmetry. J Mol Biol. 1970 Sep 14;52(2):355–369. doi: 10.1016/0022-2836(70)90036-7. [DOI] [PubMed] [Google Scholar]
- Dolder M., Engel A., Zulauf M. The micelle to vesicle transition of lipids and detergents in the presence of a membrane protein: towards a rationale for 2D crystallization. FEBS Lett. 1996 Mar 11;382(1-2):203–208. doi: 10.1016/0014-5793(96)00180-9. [DOI] [PubMed] [Google Scholar]
- Dux L., Martonosi A. Two-dimensional arrays of proteins in sarcoplasmic reticulum and purified Ca2+-ATPase vesicles treated with vanadate. J Biol Chem. 1983 Feb 25;258(4):2599–2603. [PubMed] [Google Scholar]
- Dux L., Taylor K. A., Ting-Beall H. P., Martonosi A. Crystallization of the Ca2+-ATPase of sarcoplasmic reticulum by calcium and lanthanide ions. J Biol Chem. 1985 Sep 25;260(21):11730–11743. [PubMed] [Google Scholar]
- Eletr S., Inesi G. Phospholipid orientation in sarcoplasmic membranes: spin-label ESR and proton MNR studies. Biochim Biophys Acta. 1972 Sep 1;282(1):174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- Engel A., Hoenger A., Hefti A., Henn C., Ford R. C., Kistler J., Zulauf M. Assembly of 2-D membrane protein crystals: dynamics, crystal order, and fidelity of structure analysis by electron microscopy. J Struct Biol. 1992 Nov-Dec;109(3):219–234. doi: 10.1016/1047-8477(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Picot D., Loll P. J. Strategies for crystallizing membrane proteins. J Bioenerg Biomembr. 1996 Feb;28(1):13–27. [PubMed] [Google Scholar]
- Gruner S. M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Herbert H., Skriver E., Maunsbach A. B. Three-dimensional structure of renal Na,K-ATPase determined by electron microscopy of membrane crystals. FEBS Lett. 1985 Jul 22;187(1):182–186. doi: 10.1016/0014-5793(85)81238-2. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Iwata S., Ostermeier C., Ludwig B., Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995 Aug 24;376(6542):660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Zulauf M., Scheybani T., Hefti A., Baumeister W., Aebi U., Engel A. 2D crystallization: from art to science. Ultramicroscopy. 1992 Oct;46(1-4):45–84. doi: 10.1016/0304-3991(92)90007-7. [DOI] [PubMed] [Google Scholar]
- Karon B. S., Mahaney J. E., Thomas D. D. Halothane and cyclopiazonic acid modulate Ca-ATPase oligomeric state and function in sarcoplasmic reticulum. Biochemistry. 1994 Nov 22;33(46):13928–13937. doi: 10.1021/bi00250a048. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N. Three-dimensional structure of plant light-harvesting complex determined by electron crystallography. Nature. 1991 Mar 14;350(6314):130–134. doi: 10.1038/350130a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy D., Gulik A., Seigneuret M., Rigaud J. L. Phospholipid vesicle solubilization and reconstitution by detergents. Symmetrical analysis of the two processes using octaethylene glycol mono-n-dodecyl ether. Biochemistry. 1990 Oct 9;29(40):9480–9488. doi: 10.1021/bi00492a022. [DOI] [PubMed] [Google Scholar]
- Levy D., Seigneuret M., Bluzat A., Rigaud J. L. Evidence for proton countertransport by the sarcoplasmic reticulum Ca2(+)-ATPase during calcium transport in reconstituted proteoliposomes with low ionic permeability. J Biol Chem. 1990 Nov 15;265(32):19524–19534. [PubMed] [Google Scholar]
- Li J., Hollingshead C. Formation of crystalline arrays of chlorophyll a/b - light-harvesting protein by membrane reconstitution. Biophys J. 1982 Jan;37(1):363–370. doi: 10.1016/S0006-3495(82)84684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy D., Bluzat A., Seigneuret M., Rigaud J. L. A systematic study of liposome and proteoliposome reconstitution involving Bio-Bead-mediated Triton X-100 removal. Biochim Biophys Acta. 1990 Jun 27;1025(2):179–190. doi: 10.1016/0005-2736(90)90096-7. [DOI] [PubMed] [Google Scholar]
- Lévy D., Gulik A., Bluzat A., Rigaud J. L. Reconstitution of the sarcoplasmic reticulum Ca(2+)-ATPase: mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. Biochim Biophys Acta. 1992 Jun 30;1107(2):283–298. doi: 10.1016/0005-2736(92)90415-i. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- Mannella C. A. Phospholipase-induced crystallization of channels in mitochondrial outer membranes. Science. 1984 Apr 13;224(4645):165–166. doi: 10.1126/science.6322311. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Mitchinson C., Wilderspin A. F., Trinnaman B. J., Green N. M. Identification of a labelled peptide after stoicheiometric reaction of fluorescein isothiocyanate with the Ca2+ -dependent adenosine triphosphatase of sarcoplasmic reticulum. FEBS Lett. 1982 Sep 6;146(1):87–92. doi: 10.1016/0014-5793(82)80710-2. [DOI] [PubMed] [Google Scholar]
- Mohraz M., Simpson M. V., Smith P. R. The three-dimensional structure of the Na,K-ATPase from electron microscopy. J Cell Biol. 1987 Jul;105(1):1–8. doi: 10.1083/jcb.105.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot D., Loll P. J., Garavito R. M. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994 Jan 20;367(6460):243–249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- Pikuła S., Wrzosek A., Famulski K. S. Long-term stabilization and crystallization of (Ca2+ + Mg2+)-ATPase of detergent-solubilized erythrocyte plasma membrane. Biochim Biophys Acta. 1991 Jan 30;1061(2):206–214. doi: 10.1016/0005-2736(91)90286-h. [DOI] [PubMed] [Google Scholar]
- Rabon E., Wilke M., Sachs G., Zampighi G. Crystallization of the gastric H,K-ATPase. J Biol Chem. 1986 Jan 25;261(3):1434–1439. [PubMed] [Google Scholar]
- Reddy L. G., Jones L. R., Cala S. E., O'Brian J. J., Tatulian S. A., Stokes D. L. Functional reconstitution of recombinant phospholamban with rabbit skeletal Ca(2+)-ATPase. J Biol Chem. 1995 Apr 21;270(16):9390–9397. doi: 10.1074/jbc.270.16.9390. [DOI] [PubMed] [Google Scholar]
- Reddy L. G., Jones L. R., Pace R. C., Stokes D. L. Purified, reconstituted cardiac Ca2+-ATPase is regulated by phospholamban but not by direct phosphorylation with Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1996 Jun 21;271(25):14964–14970. doi: 10.1074/jbc.271.25.14964. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud J. L., Mosser G., Lacapere J. J., Olofsson A., Levy D., Ranck J. L. Bio-Beads: an efficient strategy for two-dimensional crystallization of membrane proteins. J Struct Biol. 1997 Apr;118(3):226–235. doi: 10.1006/jsbi.1997.3848. [DOI] [PubMed] [Google Scholar]
- Rigaud J. L., Pitard B., Levy D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim Biophys Acta. 1995 Oct 10;1231(3):223–246. doi: 10.1016/0005-2728(95)00091-v. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Wade J. B., Inesi G. A conformational mechanism for formation of a dead-end complex by the sarcoplasmic reticulum ATPase with thapsigargin. J Biol Chem. 1992 Jan 15;267(2):1286–1292. [PubMed] [Google Scholar]
- Skriver E., Maunsbach A. B., Jørgensen P. L. Formation of two-dimensional crystals in pure membrane-bound Na+,K+-ATPase. FEBS Lett. 1981 Aug 31;131(2):219–222. doi: 10.1016/0014-5793(81)80371-7. [DOI] [PubMed] [Google Scholar]
- Stokes D. L., Green N. M. Three-dimensional crystals of CaATPase from sarcoplasmic reticulum. Symmetry and molecular packing. Biophys J. 1990 Jan;57(1):1–14. doi: 10.1016/S0006-3495(90)82501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Kadoma M. Regulation of the Ca2+ pump ATPase by cAMP-dependent phosphorylation of phospholamban. Bioessays. 1989 May;10(5):157–163. doi: 10.1002/bies.950100505. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Dux L., Martonosi A. Three-dimensional reconstruction of negatively stained crystals of the Ca2+-ATPase from muscle sarcoplasmic reticulum. J Mol Biol. 1986 Feb 5;187(3):417–427. doi: 10.1016/0022-2836(86)90442-0. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Ho M. H., Martonosi A. Image analysis of the Ca2+-ATPase from sarcoplasmic reticulum. Ann N Y Acad Sci. 1986;483:31–43. doi: 10.1111/j.1749-6632.1986.tb34493.x. [DOI] [PubMed] [Google Scholar]
- Taylor K., Dux L., Martonosi A. Structure of the vanadate-induced crystals of sarcoplasmic reticulum Ca2+-ATPase. J Mol Biol. 1984 Mar 25;174(1):193–204. doi: 10.1016/0022-2836(84)90372-3. [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Sasabe H., Stokes D. L. Three-dimensional cryo-electron microscopy of the calcium ion pump in the sarcoplasmic reticulum membrane. Nature. 1993 Apr 1;362(6419):467–471. doi: 10.1038/362469a0. [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Unwin N. Contrast transfer for frozen-hydrated specimens: determination from pairs of defocused images. Ultramicroscopy. 1988;25(4):279–291. doi: 10.1016/0304-3991(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Unwin N. Three-dimensional structure of the acetylcholine receptor by cryoelectron microscopy and helical image reconstruction. J Cell Biol. 1990 Dec;111(6 Pt 1):2623–2635. doi: 10.1083/jcb.111.6.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. N., Kühlbrandt W., Sarabia V. E., Reithmeier R. A. Two-dimensional structure of the membrane domain of human band 3, the anion transport protein of the erythrocyte membrane. EMBO J. 1993 Jun;12(6):2233–2239. doi: 10.1002/j.1460-2075.1993.tb05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne A., Wang D. N., Saraste M. Purification and two-dimensional crystallization of bacterial cytochrome oxidases. Eur J Biochem. 1995 Dec 1;234(2):443–451. doi: 10.1111/j.1432-1033.1995.443_b.x. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reconstitution of a calcium pump using defined membrane components. Proc Natl Acad Sci U S A. 1974 Mar;71(3):622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. S., Wacker T., Weckesser J., Welte W., Schulz G. E. The three-dimensional structure of porin from Rhodobacter capsulatus at 3 A resolution. FEBS Lett. 1990 Jul 16;267(2):268–272. doi: 10.1016/0014-5793(90)80942-c. [DOI] [PubMed] [Google Scholar]
- Wingfield P., Arad T., Leonard K., Weiss H. Membrane crystals of ubiquinone:cytochrome c reductase from Neurospora mitochondria. Nature. 1979 Aug 23;280(5724):696–697. doi: 10.1038/280696a0. [DOI] [PubMed] [Google Scholar]
- Yonekura K., Stokes D. L., Sasabe H., Toyoshima C. The ATP-binding site of Ca(2+)-ATPase revealed by electron image analysis. Biophys J. 1997 Mar;72(3):997–1005. doi: 10.1016/S0006-3495(97)78752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Carroll S., Rigaud J. L., Inesi G. H+ countertransport and electrogenicity of the sarcoplasmic reticulum Ca2+ pump in reconstituted proteoliposomes. Biophys J. 1993 Apr;64(4):1232–1242. doi: 10.1016/S0006-3495(93)81489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]