Abstract
Epifluorescence microscopy was used to investigate the effect of cholesterol on monolayers of dipalmitoylphosphatidylcholine (DPPC) and 1 -palmitoyl-2-oleoyl phosphatidylcholine (POPC) at 21 +/- 2 degrees C using 1 mol% 1-palmitoyl-2-[12-[(7-nitro-2-1, 3-benzoxadizole-4-yl)amino]dodecanoyl]phosphatidylcholine (NBD-PC) as a fluorophore. Up to 30 mol% cholesterol in DPPC monolayers decreased the amounts of probe-excluded liquid-condensed (LC) phase at all surface pressures (pi), but did not effect the monolayers of POPC, which remained in the liquid-expanded (LE) phase at all pi. At low pi (2-5 mN/m), 10 mol% or more cholesterol in DPPC induced a lateral phase separation into dark probe-excluded and light probe-rich regions. In POPC monolayers, phase separation was observed at low pi when > or =40 mol% or more cholesterol was present. The lateral phase separation observed with increased cholesterol concentrations in these lipid monolayers may be a result of the segregation of cholesterol-rich domains in ordered fluid phases that preferentially exclude the fluorescent probe. With increasing pi, monolayers could be transformed from a heterogeneous dark and light appearance into a homogeneous fluorescent phase, in a manner that was dependent on pi and cholesterol content. The packing density of the acyl chains may be a determinant in the interaction of cholesterol with phosphatidylcholine (PC), because the transformations in monolayer surface texture were observed in phospholipid (PL)/sterol mixtures having similar molecular areas. At high pi (41 mN/m), elongated crystal-like structures were observed in monolayers containing 80-100 mol% cholesterol, and these structures grew in size when the monolayers were compressed after collapse. This observation could be associated with the segregation and crystallization of cholesterol after monolayer collapse.
Full text
PDF
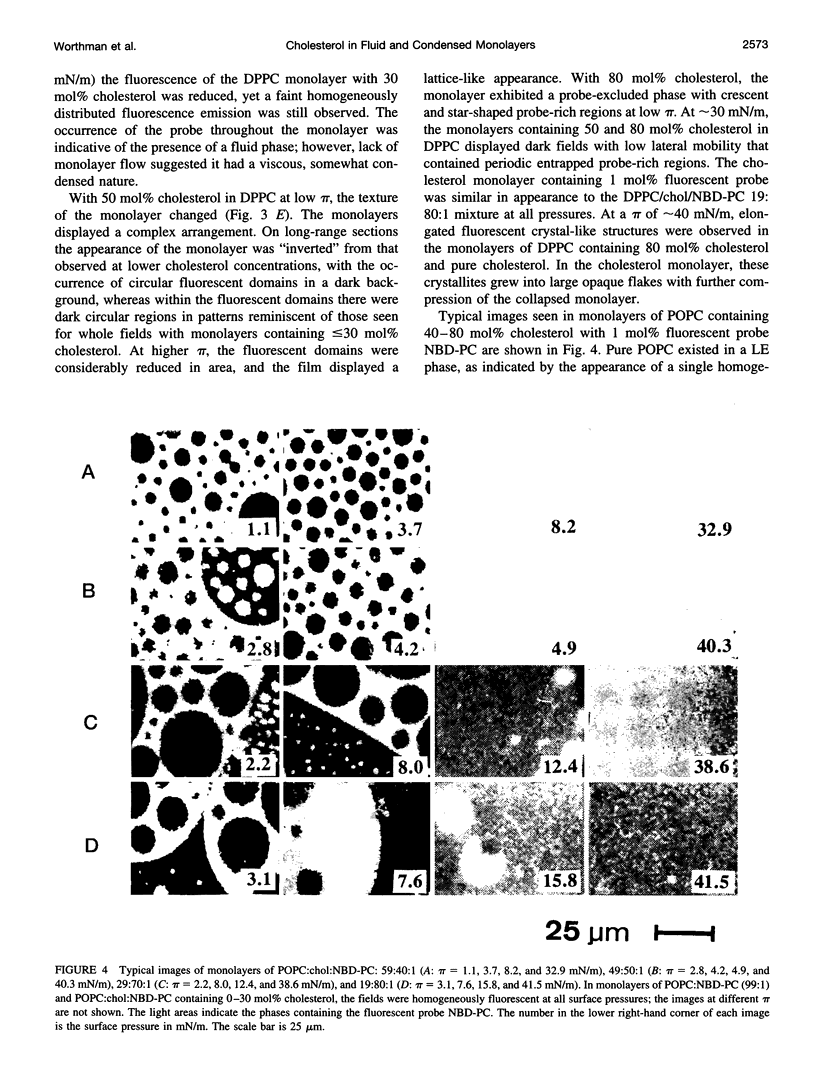
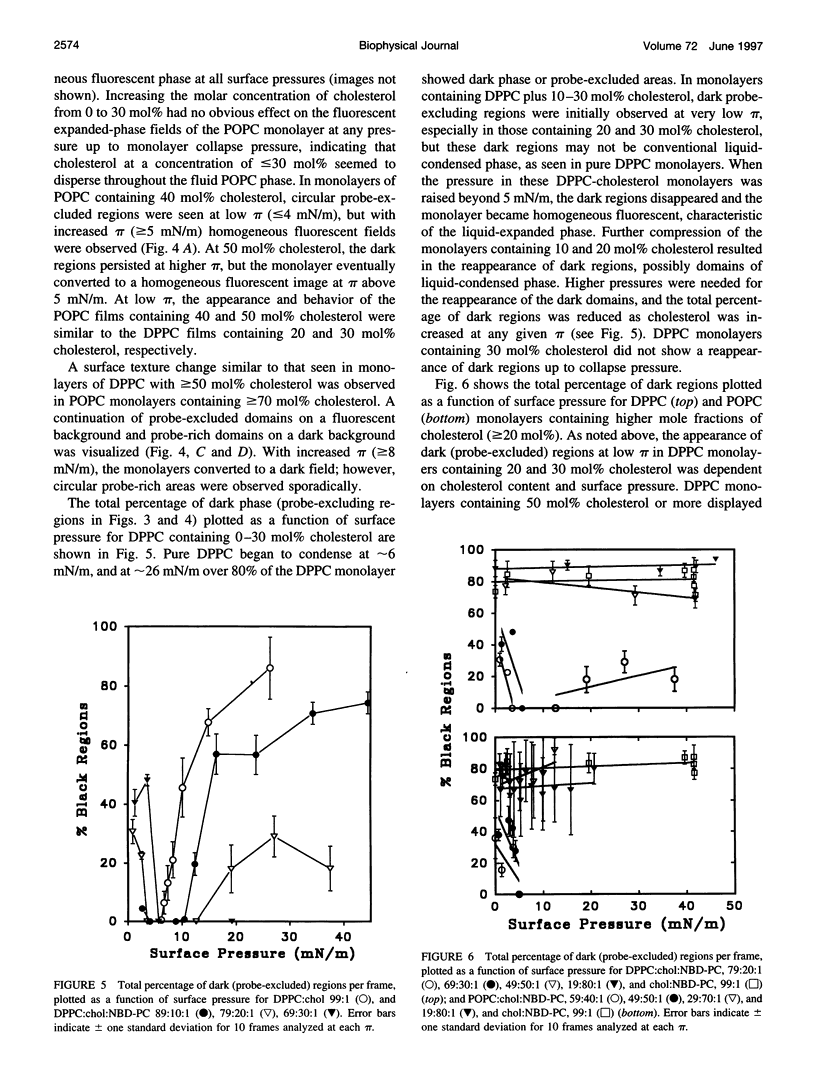
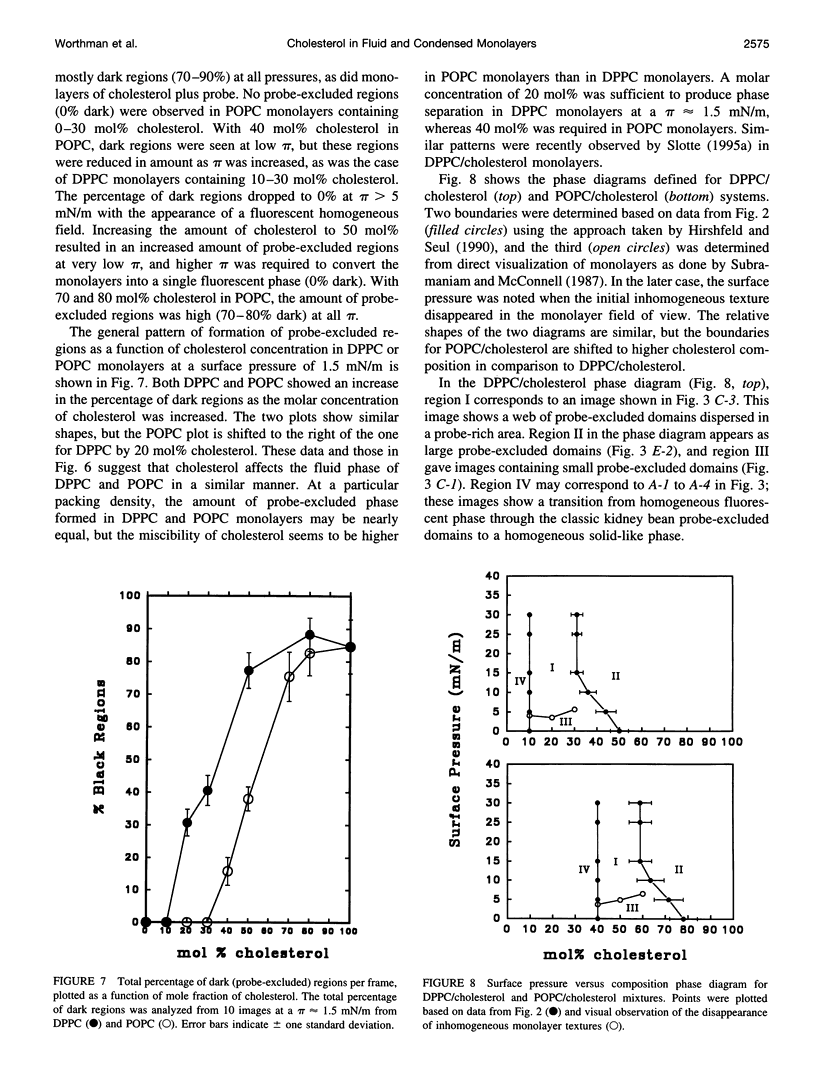





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Chao F. F., Amende L. M., Blanchette-Mackie E. J., Skarlatos S. I., Gamble W., Resau J. H., Mergner W. T., Kruth H. S. Unesterified cholesterol-rich lipid particles in atherosclerotic lesions of human and rabbit aortas. Am J Pathol. 1988 Apr;131(1):73–83. [PMC free article] [PubMed] [Google Scholar]
- Chapman D., Owens N. F., Phillips M. C., Walker D. A. Mixed monolayers of phospholipids and cholesterol. Biochim Biophys Acta. 1969;183(3):458–465. doi: 10.1016/0005-2736(69)90160-6. [DOI] [PubMed] [Google Scholar]
- Collins J. J., Phillips M. C. The stability and structure of cholesterol-rich codispersions of cholesterol and phosphatidylcholine. J Lipid Res. 1982 Feb;23(2):291–298. [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Tinoco J. Monolayer interactions of individual lecithins with natural sterols. Biochim Biophys Acta. 1972 Apr 14;266(1):41–49. doi: 10.1016/0005-2736(72)90117-4. [DOI] [PubMed] [Google Scholar]
- Heckl W. M., Lösche M., Cadenhead D. A., Möhwald H. Electrostatically induced growth of spiral lipid domains in the presence of cholesterol. Eur Biophys J. 1986;14(1):11–17. doi: 10.1007/BF00260398. [DOI] [PubMed] [Google Scholar]
- Huang T. H., Lee C. W., Das Gupta S. K., Blume A., Griffin R. G. A 13C and 2H nuclear magnetic resonance study of phosphatidylcholine/cholesterol interactions: characterization of liquid-gel phases. Biochemistry. 1993 Dec 7;32(48):13277–13287. doi: 10.1021/bi00211a041. [DOI] [PubMed] [Google Scholar]
- Keough K. M., Kariel N. Differential scanning calorimetric studies of aqueous dispersions of phosphatidylcholines containing two polyenoic chains. Biochim Biophys Acta. 1987 Aug 7;902(1):11–18. doi: 10.1016/0005-2736(87)90130-1. [DOI] [PubMed] [Google Scholar]
- Knobler C. M. Seeing phenomena in flatland: studies of monolayers by fluorescence microscopy. Science. 1990 Aug 24;249(4971):870–874. doi: 10.1126/science.249.4971.870. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Klingler J. F., McConnell H. M. Electric field-induced concentration gradients in lipid monolayers. Science. 1994 Feb 4;263(5147):655–658. doi: 10.1126/science.8303272. [DOI] [PubMed] [Google Scholar]
- Lund-Katz S., Laboda H. M., McLean L. R., Phillips M. C. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry. 1988 May 3;27(9):3416–3423. doi: 10.1021/bi00409a044. [DOI] [PubMed] [Google Scholar]
- Mattjus P., Slotte J. P. Availability for enzyme-catalyzed oxidation of cholesterol in mixed monolayers containing both phosphatidylcholine and sphingomyelin. Chem Phys Lipids. 1994 May 6;71(1):73–81. doi: 10.1016/0009-3084(94)02306-9. [DOI] [PubMed] [Google Scholar]
- McMullen T. P., McElhaney R. N. New aspects of the interaction of cholesterol with dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim Biophys Acta. 1995 Mar 8;1234(1):90–98. doi: 10.1016/0005-2736(94)00266-r. [DOI] [PubMed] [Google Scholar]
- Möhwald H. Phospholipid and phospholipid-protein monolayers at the air/water interface. Annu Rev Phys Chem. 1990;41:441–476. doi: 10.1146/annurev.pc.41.100190.002301. [DOI] [PubMed] [Google Scholar]
- Nag K., Boland C., Rich N., Keough K. M. Epifluorescence microscopic observation of monolayers of dipalmitoylphosphatidylcholine: dependence of domain size on compression rates. Biochim Biophys Acta. 1991 Sep 30;1068(2):157–160. doi: 10.1016/0005-2736(91)90204-l. [DOI] [PubMed] [Google Scholar]
- Nag K., Keough K. M. Epifluorescence microscopic studies of monolayers containing mixtures of dioleoyl- and dipalmitoylphosphatidylcholines. Biophys J. 1993 Sep;65(3):1019–1026. doi: 10.1016/S0006-3495(93)81155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C., Chapman D. Monolayer characteristics of saturated 1,2,-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Rice P. A., McConnell H. M. Critical shape transitions of monolayer lipid domains. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6445–6448. doi: 10.1073/pnas.86.17.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder F., Woodford J. K., Kavecansky J., Wood W. G., Joiner C. Cholesterol domains in biological membranes. Mol Membr Biol. 1995 Jan-Mar;12(1):113–119. doi: 10.3109/09687689509038505. [DOI] [PubMed] [Google Scholar]
- Seul M, Sammon MJ. Competing interactions and domain-shape instabilities in a monomolecular film at an air-water interface. Phys Rev Lett. 1990 Apr 16;64(16):1903–1906. doi: 10.1103/PhysRevLett.64.1903. [DOI] [PubMed] [Google Scholar]
- Slotte J. P. Lateral domain formation in mixed monolayers containing cholesterol and dipalmitoylphosphatidylcholine or N-palmitoylsphingomyelin. Biochim Biophys Acta. 1995 May 4;1235(2):419–427. doi: 10.1016/0005-2736(95)80031-a. [DOI] [PubMed] [Google Scholar]
- Slotte J. P., Mattjus P. Visualization of lateral phases in cholesterol and phosphatidylcholine monolayers at the air/water interface--a comparative study with two different reporter molecules. Biochim Biophys Acta. 1995 Jan 3;1254(1):22–29. doi: 10.1016/0005-2760(94)00159-v. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L., Brown R. E. Cholesterol's interfacial interactions with sphingomyelins and phosphatidylcholines: hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 1994 Aug 9;33(31):9135–9142. doi: 10.1021/bi00197a016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutzer G., Yeagle P. L. A fluorescence anisotropy study on the phase behavior of dimyristoylphosphatidylcholine/cholesterol mixtures. Biochim Biophys Acta. 1985 Apr 11;814(2):274–280. doi: 10.1016/0005-2736(85)90445-6. [DOI] [PubMed] [Google Scholar]
- Stine K. J. Investigations of monolayers by fluorescence microscopy. Microsc Res Tech. 1994 Apr 1;27(5):439–450. doi: 10.1002/jemt.1070270510. [DOI] [PubMed] [Google Scholar]
- Thewalt J. L., Bloom M. Phosphatidylcholine: cholesterol phase diagrams. Biophys J. 1992 Oct;63(4):1176–1181. doi: 10.1016/S0006-3495(92)81681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vist M. R., Davis J. H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990 Jan 16;29(2):451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- Weis R. M. Fluorescence microscopy of phospholipid monolayer phase transitions. Chem Phys Lipids. 1991 Mar;57(2-3):227–239. doi: 10.1016/0009-3084(91)90078-p. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- Yu H., Hui S. W. Methylation effects on the microdomain structures of phosphatidylethanolamine monolayers. Chem Phys Lipids. 1992 Jul;62(1):69–78. doi: 10.1016/0009-3084(92)90055-t. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., McConnell H. M. An alternative view of phospholipid phase behavior at the air-water interface. Microscope and film balance studies. Biophys J. 1981 Nov;36(2):409–419. doi: 10.1016/S0006-3495(81)84740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]