Abstract
The energetic determinants of the distribution of anionic phospholipids across a phosphatidylcholine (PtdCho) bilayer with different packing constraints in the two leaflets were studied, using (13)CH2-ethyl-labeled phosphatidylethanol (PtdEth) as a (13)C NMR membrane probe. PtdEth is unique in exhibiting a split (13)CH2-ethyl resonance in sonicated vesicles, the two components originating from the inner and outer leaflets, thus permitting the determination of the PtdEth concentration in each leaflet. Small and large unilamellar PtdEth-PtdCho vesicles were prepared in solutions of different ionic strengths. A quantitative expression for the transbilayer distribution of PtdEth, based on the balance between steric and electrostatic factors, was derived. The transbilayer difference in packing constraints was obtained from the magnitude of the PtdEth signal splitting. The electrostatic contribution could be satisfactorily described by the transmembrane difference in Gouy-Chapman surface potentials. At low (0.1-0.25%) PtdEth levels and high (up to 500 mM) salt concentrations, PtdEth had a marked fivefold preference for the inner leaflet, presumably because of its small headgroup, which favors tighter packing. At higher PtdEth content (4.8-9.1%) and low salt concentrations, where electrostatic repulsion becomes a dominant factor, the asymmetry was markedly reduced and an almost even distribution across the bilayer was obtained. In less curved, large vesicles, where packing constraints in the two leaflets are approximately the same, the PtdEth distribution was almost symmetrical. This study is the first quantitative analysis of the balance between steric and electrostatic factors that determines the equilibrium transbilayer distribution of charged membrane constituents.
Full text
PDF



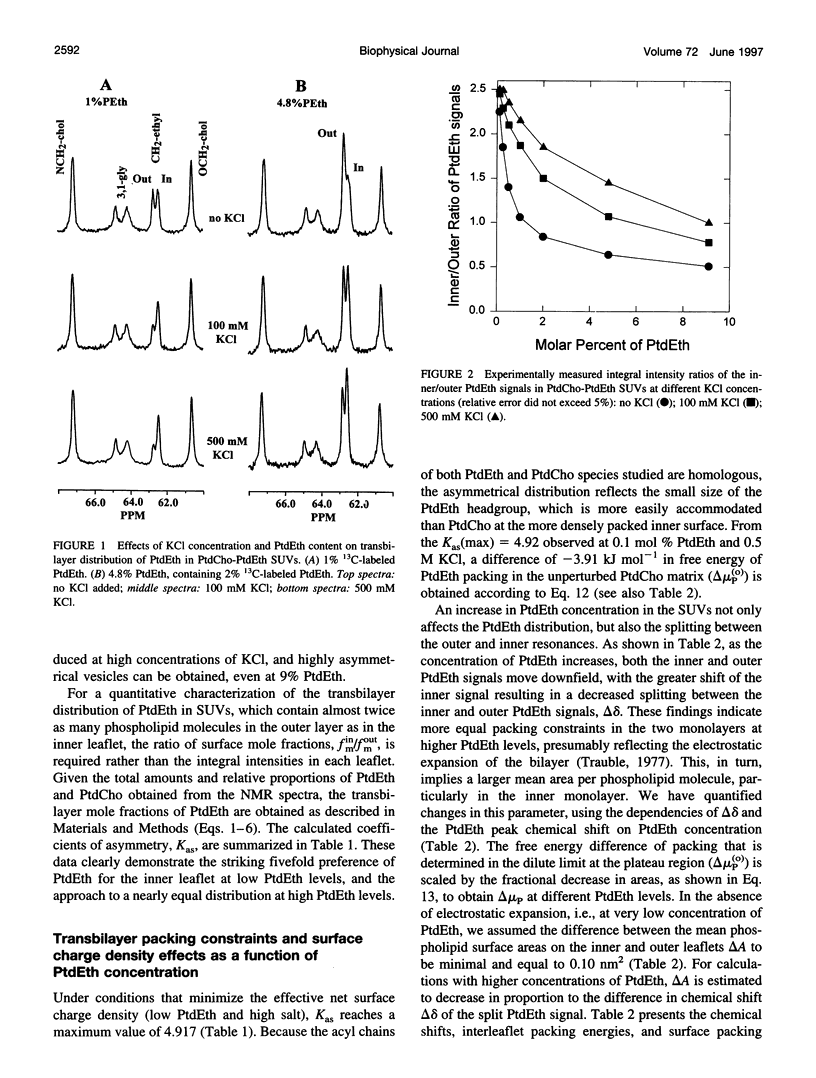
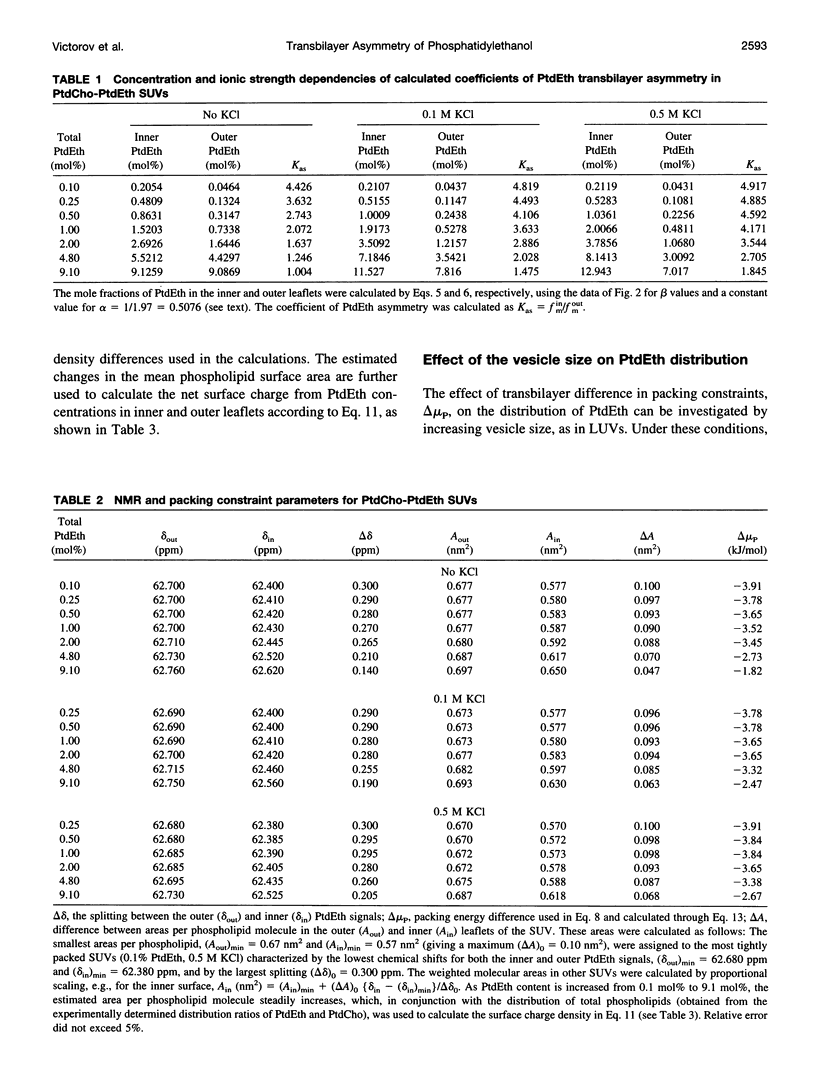
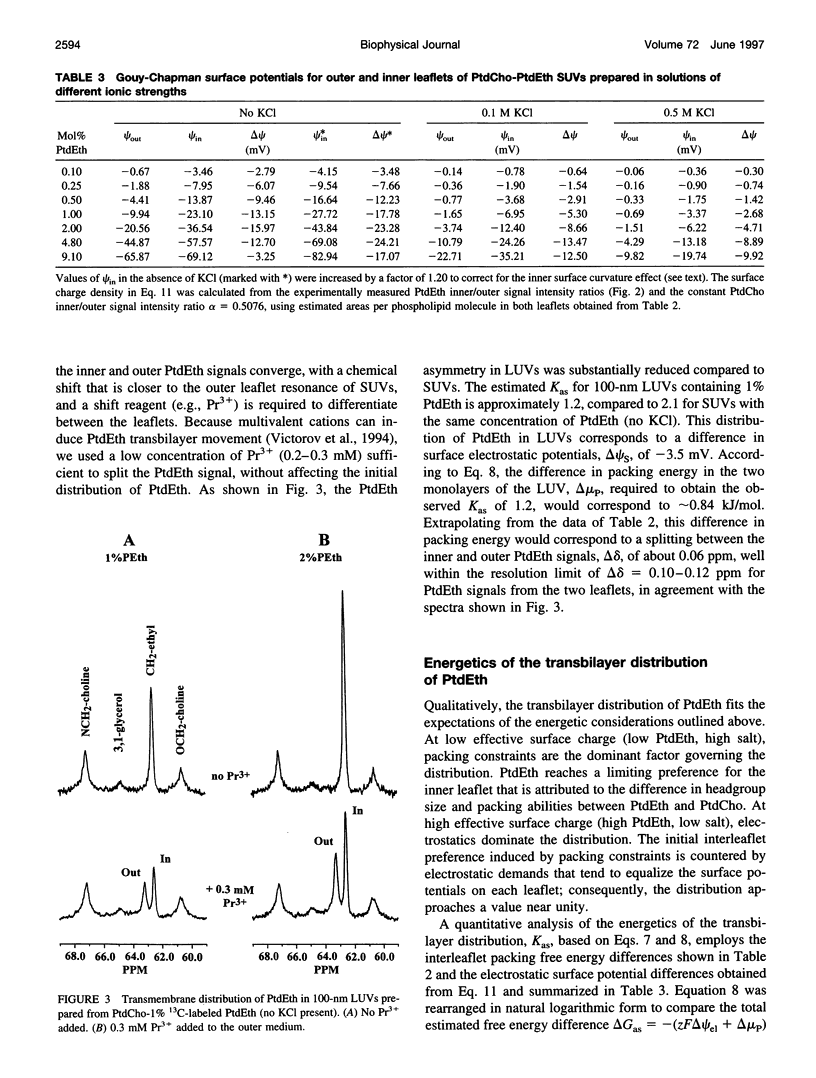
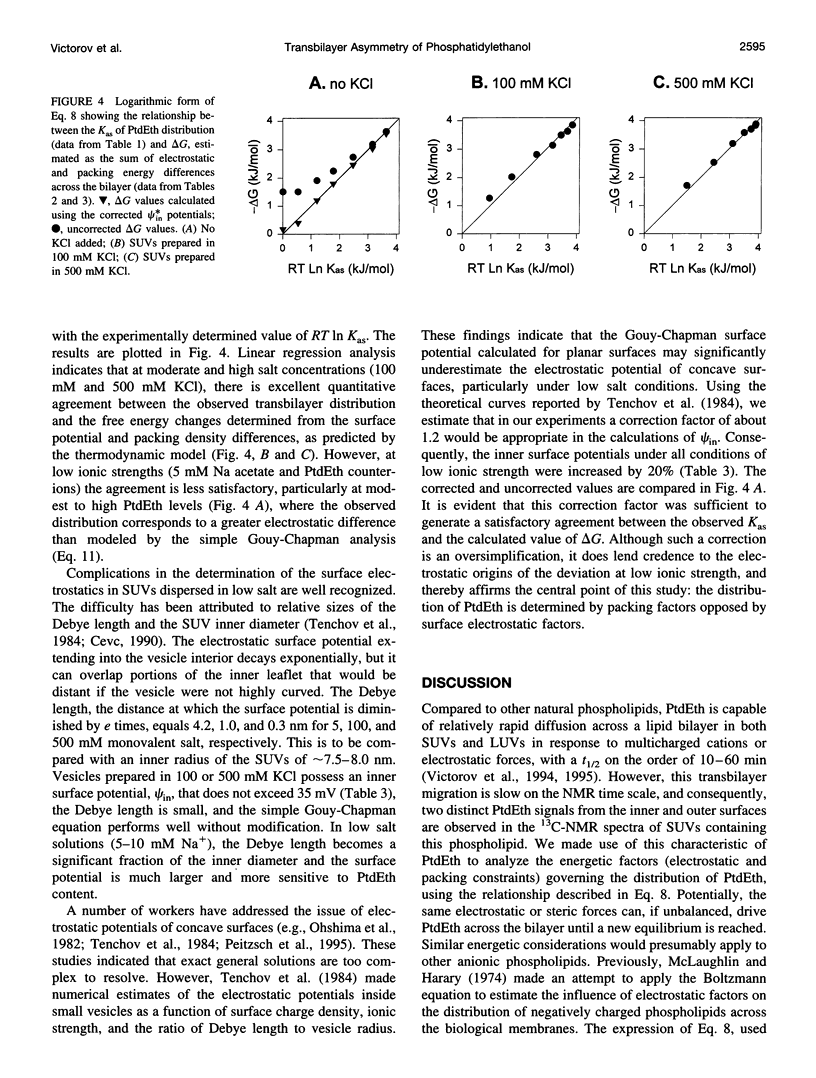



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alling C., Gustavsson L., Månsson J. E., Benthin G., Anggård E. Phosphatidylethanol formation in rat organs after ethanol treatment. Biochim Biophys Acta. 1984 Mar 27;793(1):119–122. doi: 10.1016/0005-2760(84)90060-2. [DOI] [PubMed] [Google Scholar]
- Asaoka Y., Kikkawa U., Sekiguchi K., Shearman M. S., Kosaka Y., Nakano Y., Satoh T., Nishizuka Y. Activation of a brain-specific protein kinase C subspecies in the presence of phosphatidylethanol. FEBS Lett. 1988 Apr 11;231(1):221–224. doi: 10.1016/0014-5793(88)80735-x. [DOI] [PubMed] [Google Scholar]
- Barsukov L. I., Victorov A. V., Vasilenko I. A., Evstigneeva R. P., Bergelson L. D. Investigation of the inside-outside distribution, intermembrane exchange and transbilayer movement of phospholipids in sonicated vesicles by shift reagent NMR. Biochim Biophys Acta. 1980 May 8;598(1):153–168. doi: 10.1016/0005-2736(80)90273-4. [DOI] [PubMed] [Google Scholar]
- Berden J. A., Barker R. W., Radda G. K. NMR studies on phospholipid bilayers. Some factors affecting lipid distribution. Biochim Biophys Acta. 1975 Jan 28;375(2):186–208. doi: 10.1016/0005-2736(75)90188-1. [DOI] [PubMed] [Google Scholar]
- Browning J. L. Motions and interactions of phospholipid head groups at the membrane surface. 2. Simple alkyl head groups. Biochemistry. 1981 Dec 8;20(25):7123–7133. doi: 10.1021/bi00528a012. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Phosphoinositides in vesicular traffic. Trends Biochem Sci. 1994 Feb;19(2):55–57. doi: 10.1016/0968-0004(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990 Oct 8;1031(3):311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Thomas G. M., Fensome A., Geny B., Cunningham E., Gout I., Hiles I., Totty N. F., Truong O., Hsuan J. J. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994 Jan 28;263(5146):523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- Devaux P. F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991 Feb 5;30(5):1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Eastman S. J., Hope M. J., Cullis P. R. Transbilayer transport of phosphatidic acid in response to transmembrane pH gradients. Biochemistry. 1991 Feb 19;30(7):1740–1745. doi: 10.1021/bi00221a002. [DOI] [PubMed] [Google Scholar]
- Eigenberg K. E., Chan S. I. The effect of surface curvature on the head-group structure and phase transition properties of phospholipid bilayer vesicles. Biochim Biophys Acta. 1980 Jun 20;599(1):330–335. doi: 10.1016/0005-2736(80)90079-6. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994 Apr 14;1212(1):26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson L. ESBRA 1994 Award Lecture. Phosphatidylethanol formation: specific effects of ethanol mediated via phospholipase D. Alcohol Alcohol. 1995 Jul;30(4):391–406. [PubMed] [Google Scholar]
- Homan R., Pownall H. J. Transbilayer diffusion of phospholipids: dependence on headgroup structure and acyl chain length. Biochim Biophys Acta. 1988 Feb 18;938(2):155–166. doi: 10.1016/0005-2736(88)90155-1. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Redelmeier T. E., Wong K. F., Rodrigueza W., Cullis P. R. Phospholipid asymmetry in large unilamellar vesicles induced by transmembrane pH gradients. Biochemistry. 1989 May 16;28(10):4181–4187. doi: 10.1021/bi00436a009. [DOI] [PubMed] [Google Scholar]
- Huang C., Mason J. T. Geometric packing constraints in egg phosphatidylcholine vesicles. Proc Natl Acad Sci U S A. 1978 Jan;75(1):308–310. doi: 10.1073/pnas.75.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton W. C., Yeagle P. L., Martin R. B. The interaction of lanthanide and calcium salts with phospholipid bilayer vesicles: the validity of the nuclear magnetic resonance method for determination of vesicle bilayer phospholipid surface ratios. Chem Phys Lipids. 1977 Jul;19(3):255–265. doi: 10.1016/0009-3084(77)90047-0. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N. Theoretical considerations on the asymmetric distribution of charged phospholipid molecules on the inner and outer layers of curved bilayer membranes. Biochim Biophys Acta. 1973 Nov 16;323(4):659–663. doi: 10.1016/0005-2736(73)90179-x. [DOI] [PubMed] [Google Scholar]
- Kumar A., Gupta C. M. Transbilayer distributions of red cell membrane phospholipids in unilamellar vesicles. Biochim Biophys Acta. 1984 Jan 25;769(2):419–428. doi: 10.1016/0005-2736(84)90326-2. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Taraschi T. F., Janes N. Support for the shape concept of lipid structure based on a headgroup volume approach. Biophys J. 1993 Oct;65(4):1429–1432. doi: 10.1016/S0006-3495(93)81206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. C., Zheng Y. O., Taraschi T. F., Janes N. Hydrophobic alkyl headgroups strongly promote membrane curvature and violate the headgroup volume correlation due to "headgroup" insertion. Biochemistry. 1996 Mar 26;35(12):3677–3684. doi: 10.1021/bi9517502. [DOI] [PubMed] [Google Scholar]
- Liscovitch M., Chalifa V., Pertile P., Chen C. S., Cantley L. C. Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem. 1994 Aug 26;269(34):21403–21406. [PubMed] [Google Scholar]
- Low M. G., Zilversmit D. B. Phosphatidylinositol distribution and translocation in sonicated vesicles. A study with exchange protein and phospholipase C. Biochim Biophys Acta. 1980 Feb 28;596(2):223–234. doi: 10.1016/0005-2736(80)90357-0. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Range of the solvation pressure between lipid membranes: dependence on the packing density of solvent molecules. Biochemistry. 1989 Sep 19;28(19):7904–7912. doi: 10.1021/bi00445a053. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Harary H. Phospholipid flip-flop and the distribution of surface charges in excitable membranes. Biophys J. 1974 Mar;14(3):200–208. doi: 10.1016/S0006-3495(74)85907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuarrie D. A., Mulás P. Asymmetric charge distributions in planar bilayer systems. Biophys J. 1977 Feb;17(2):103–109. doi: 10.1016/S0006-3495(77)85629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K. Flippases. Trends Cell Biol. 1995 Sep;5(9):355–360. doi: 10.1016/s0962-8924(00)89069-8. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M., Horwitz A. F., Klein M. P. Transbilayer asymmetry and surface homogeneity of mixed phospholipids in cosonicated vesicles. Biochemistry. 1973 Jul 3;12(14):2637–2645. doi: 10.1021/bi00738a014. [DOI] [PubMed] [Google Scholar]
- Moehren G., Gustavsson L., Hoek J. B. Activation and desensitization of phospholipase D in intact rat hepatocytes. J Biol Chem. 1994 Jan 14;269(2):838–848. [PubMed] [Google Scholar]
- Nagle J. F. Area/lipid of bilayers from NMR. Biophys J. 1993 May;64(5):1476–1481. doi: 10.1016/S0006-3495(93)81514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nordlund J. R., Schmidt C. F., Dicken S. N., Thompson T. E. Transbilayer distribution of phosphatidylethanolamine in large and small unilamellar vesicles. Biochemistry. 1981 May 26;20(11):3237–3241. doi: 10.1021/bi00514a039. [DOI] [PubMed] [Google Scholar]
- Omodeo-Salé F., Lindi C., Palestini P., Masserini M. Role of phosphatidylethanol in membranes. Effects on membrane fluidity, tolerance to ethanol, and activity of membrane-bound enzymes. Biochemistry. 1991 Mar 5;30(9):2477–2482. doi: 10.1021/bi00223a026. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Martin O. C., Schroit A. J., Struck D. K. Formation of asymmetric phospholipid membranes via spontaneous transfer of fluorescent lipid analogues between vesicle populations. Biochemistry. 1981 Aug 18;20(17):4920–4927. doi: 10.1021/bi00520a018. [DOI] [PubMed] [Google Scholar]
- Peitzsch R. M., Eisenberg M., Sharp K. A., McLaughlin S. Calculations of the electrostatic potential adjacent to model phospholipid bilayers. Biophys J. 1995 Mar;68(3):729–738. doi: 10.1016/S0006-3495(95)80253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelmeier T. E., Hope M. J., Cullis P. R. On the mechanism of transbilayer transport of phosphatidylglycerol in response to transmembrane pH gradients. Biochemistry. 1990 Mar 27;29(12):3046–3053. doi: 10.1021/bi00464a022. [DOI] [PubMed] [Google Scholar]
- Sears B., Hutton W. C., Thompson T. E. Effects of paramagnetic shift reagents on the 13C nuclear magnetic resonance spectra of egg phosphatidylcholine enriched with 13C in the N-methyl carbons. Biochemistry. 1976 Apr 20;15(8):1635–1639. doi: 10.1021/bi00653a007. [DOI] [PubMed] [Google Scholar]
- Sundberg S. A., Hubbell W. L. Investigation of surface potential asymmetry in phospholipid vesicles by a spin label relaxation method. Biophys J. 1986 Feb;49(2):553–562. doi: 10.1016/S0006-3495(86)83665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenchov B. G., Koynova R. D. The effect of nonideal lateral mixing on the transmembrane lipid asymmetry. Biochim Biophys Acta. 1985 May 28;815(3):380–391. doi: 10.1016/0005-2736(85)90364-5. [DOI] [PubMed] [Google Scholar]
- Victorov A. V., Taraschi T. F., Hoek J. B. Phosphatidylethanol as a 13C-NMR probe for reporting packing constraints in phospholipid membranes. Biochim Biophys Acta. 1996 Sep 4;1283(2):151–162. doi: 10.1016/0005-2736(96)00096-x. [DOI] [PubMed] [Google Scholar]


