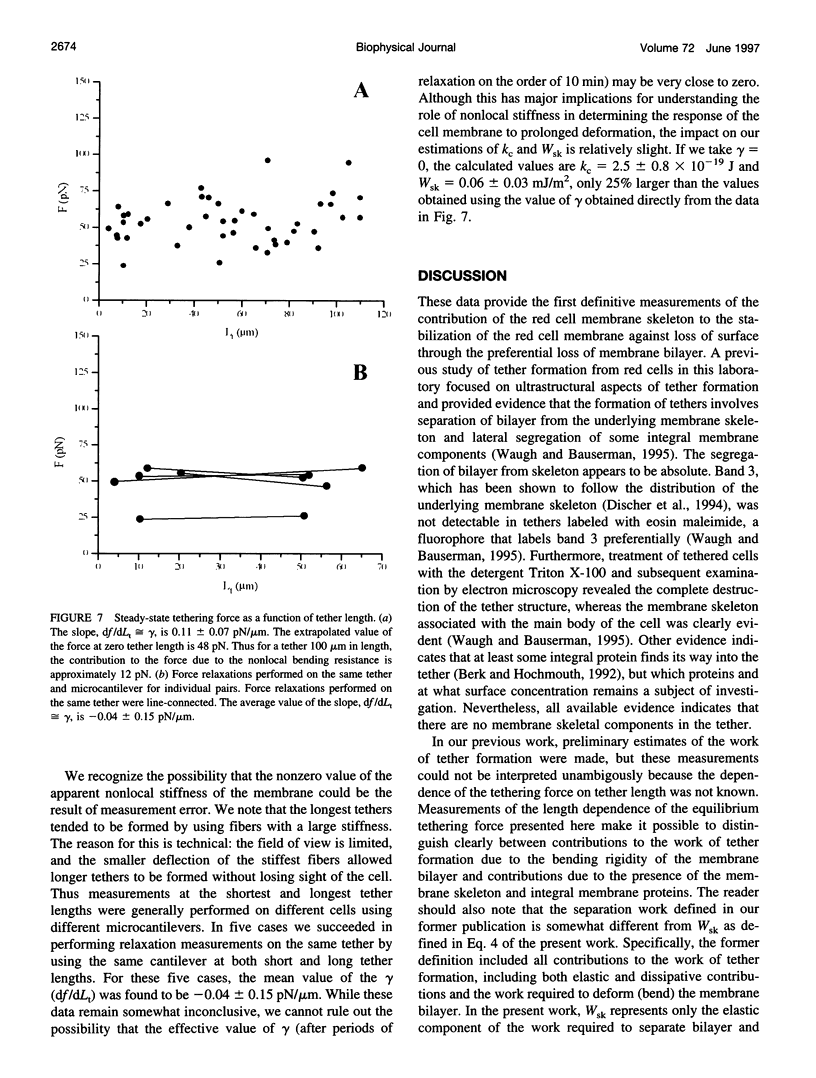
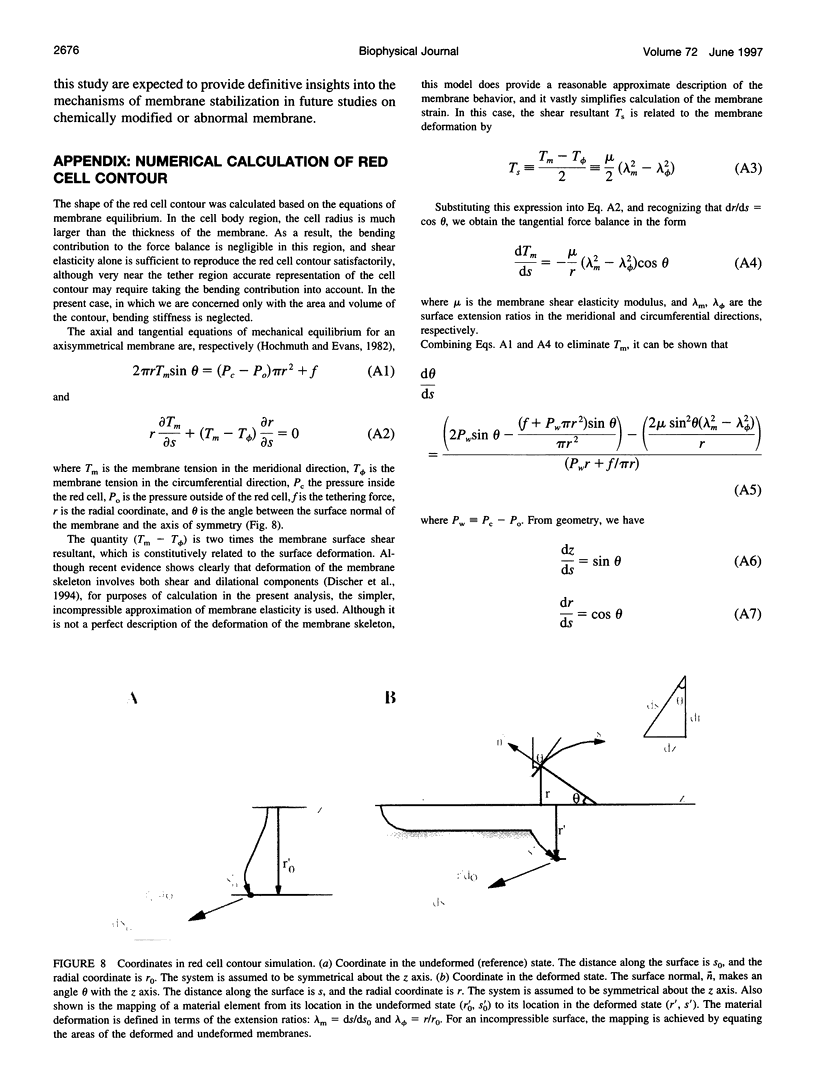
Abstract
The association between the lipid bilayer and the membrane skeleton is important to cell function. In red blood cells, defects in this association can lead to various forms of hemolytic anemia. Although proteins involved in this association have been well characterized biochemically, the physical strength of this association is only beginning to be studied. Formation of a small cylindrical strand of membrane material (tether) from the membrane involves separation of the lipid bilayer from the membrane skeleton. By measuring the force required to form a tether, and knowing the contribution to the force due to the deformation of a lipid bilayer, it is possible to calculate the additional contribution to the work of tether formation due to the separation of membrane skeleton from the lipid bilayer. In the present study, we measured the tethering force during tether formation using a microcantilever (a thin, flexible glass fiber) as a force transducer. Numerical calculations of the red cell contour were performed to examine how the shape of the contour affects the calculation of tether radius, and subsequently separation work per unit area W(sk) and bending stiffness k(c). At high aspiration pressure and small external force, the red cell contour can be accurately modeled as a sphere, but at low aspiration pressure and large external force, the contour deviates from a sphere and may affect the calculation. Based on an energy balance and numerical calculations of the cell contour, values of the membrane bending stiffness k(c) = 2.0 x 10(-19) Nm and the separation work per unit area W(sk) = 0.06 mJ/m2 were obtained.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Berk D. A., Hochmuth R. M. Lateral mobility of integral proteins in red blood cell tethers. Biophys J. 1992 Jan;61(1):9–18. doi: 10.1016/S0006-3495(92)81811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic B., Svetina S., Zeks B., Waugh R. E. Role of lamellar membrane structure in tether formation from bilayer vesicles. Biophys J. 1992 Apr;61(4):963–973. doi: 10.1016/S0006-3495(92)81903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Sheetz M. P. Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys J. 1995 Mar;68(3):988–996. doi: 10.1016/S0006-3495(95)80274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P. F. Protein involvement in transmembrane lipid asymmetry. Annu Rev Biophys Biomol Struct. 1992;21:417–439. doi: 10.1146/annurev.bb.21.060192.002221. [DOI] [PubMed] [Google Scholar]
- Discher D. E., Mohandas N., Evans E. A. Molecular maps of red cell deformation: hidden elasticity and in situ connectivity. Science. 1994 Nov 11;266(5187):1032–1035. doi: 10.1126/science.7973655. [DOI] [PubMed] [Google Scholar]
- Evans E., Berk D., Leung A. Detachment of agglutinin-bonded red blood cells. I. Forces to rupture molecular-point attachments. Biophys J. 1991 Apr;59(4):838–848. doi: 10.1016/S0006-3495(91)82296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Fung Y. C. Improved measurements of the erythrocyte geometry. Microvasc Res. 1972 Oct;4(4):335–347. doi: 10.1016/0026-2862(72)90069-6. [DOI] [PubMed] [Google Scholar]
- Evans E., Metcalfe M. Free energy potential for aggregation of mixed phosphatidylcholine/phosphatidylserine lipid vesicles in glucose polymer (dextran) solutions. Biophys J. 1984 Apr;45(4):715–720. doi: 10.1016/S0006-3495(84)84213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. C., Tsang W. C., Patitucci P. High-resolution data on the geometry of red blood cells. Biorheology. 1981;18(3-6):369–385. doi: 10.3233/bir-1981-183-606. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Evans E. A. Extensional flow of erythrocyte membrane from cell body to elastic tether. I. Analysis. Biophys J. 1982 Jul;39(1):71–81. doi: 10.1016/S0006-3495(82)84492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Y., Dolde J., Steck T. L. The rate of transmembrane movement of cholesterol in the human erythrocyte. J Biol Chem. 1981 Jun 10;256(11):5321–5323. [PubMed] [Google Scholar]
- Leckband D., Müller W., Schmitt F. J., Ringsdorf H. Molecular mechanisms determining the strength of receptor-mediated intermembrane adhesion. Biophys J. 1995 Sep;69(3):1162–1169. doi: 10.1016/S0006-3495(95)79990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palek J., Lambert S. Genetics of the red cell membrane skeleton. Semin Hematol. 1990 Oct;27(4):290–332. [PubMed] [Google Scholar]
- Pinder J. C., Chung A., Reid M. E., Gratzer W. B. Membrane attachment sites for the membrane cytoskeletal protein 4.1 of the red blood cell. Blood. 1993 Dec 1;82(11):3482–3488. [PubMed] [Google Scholar]
- Raphael R. M., Waugh R. E. Accelerated interleaflet transport of phosphatidylcholine molecules in membranes under deformation. Biophys J. 1996 Sep;71(3):1374–1388. doi: 10.1016/S0006-3495(96)79340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J. Y., Hochmuth R. M. Micropipette suction for measuring piconewton forces of adhesion and tether formation from neutrophil membranes. Biophys J. 1996 Nov;71(5):2892–2901. doi: 10.1016/S0006-3495(96)79486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E., Agre P. Reductions of erythrocyte membrane viscoelastic coefficients reflect spectrin deficiencies in hereditary spherocytosis. J Clin Invest. 1988 Jan;81(1):133–141. doi: 10.1172/JCI113284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E., Bauserman R. G. Physical measurements of bilayer-skeletal separation forces. Ann Biomed Eng. 1995 May-Jun;23(3):308–321. doi: 10.1007/BF02584431. [DOI] [PubMed] [Google Scholar]
- Waugh R. E., Hochmuth R. M. Mechanical equilibrium of thick, hollow, liquid membrane cylinders. Biophys J. 1987 Sep;52(3):391–400. doi: 10.1016/S0006-3495(87)83227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E., Narla M., Jackson C. W., Mueller T. J., Suzuki T., Dale G. L. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood. 1992 Mar 1;79(5):1351–1358. [PubMed] [Google Scholar]
- Waugh R. E., Song J., Svetina S., Zeks B. Local and nonlocal curvature elasticity in bilayer membranes by tether formation from lecithin vesicles. Biophys J. 1992 Apr;61(4):974–982. doi: 10.1016/S0006-3495(92)81904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]