Abstract
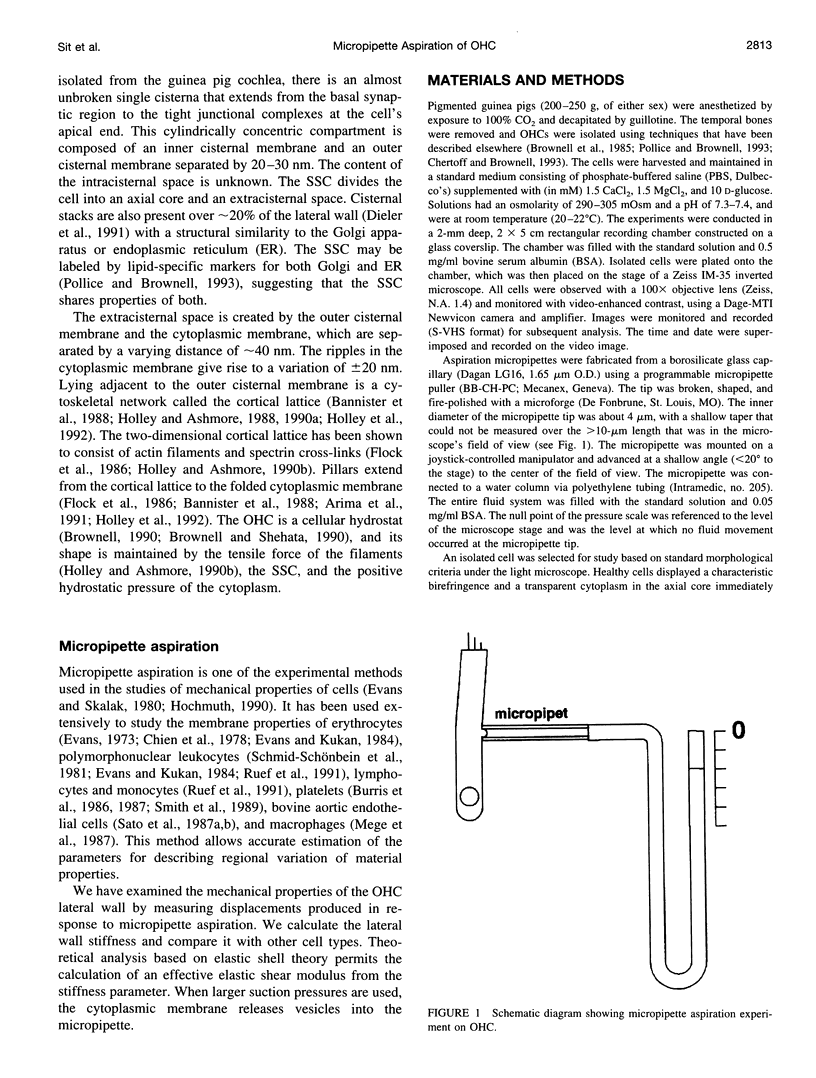
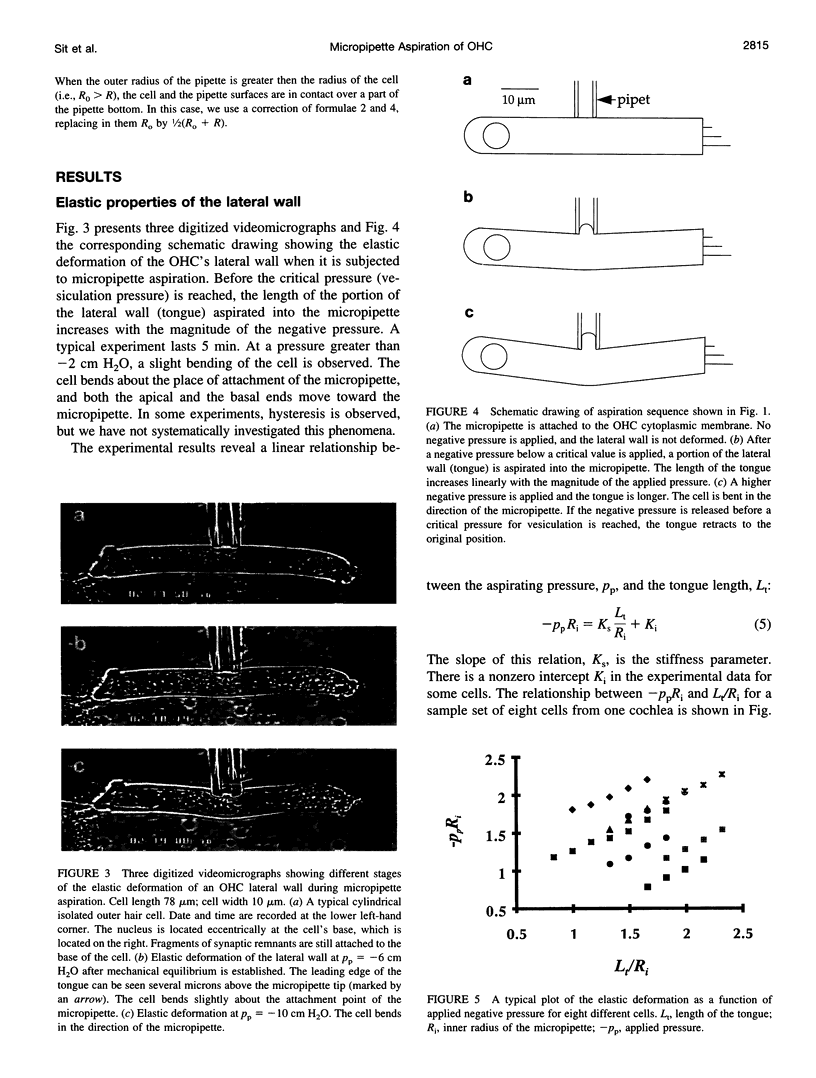
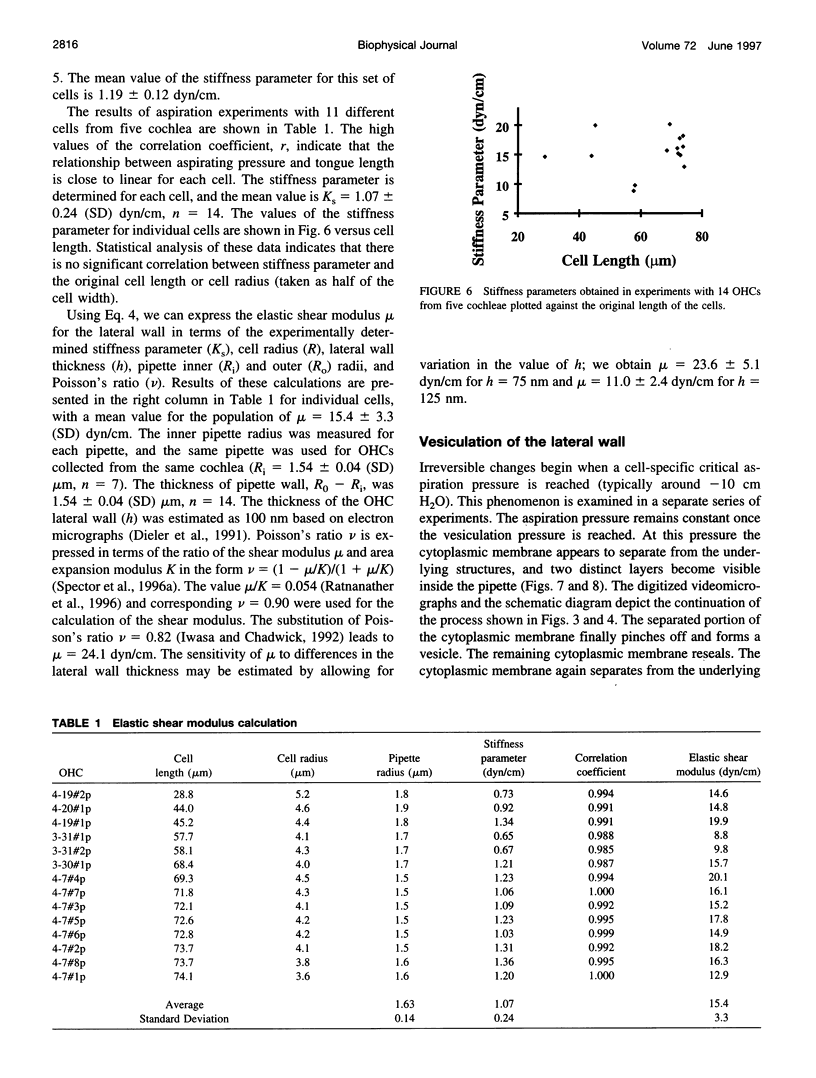
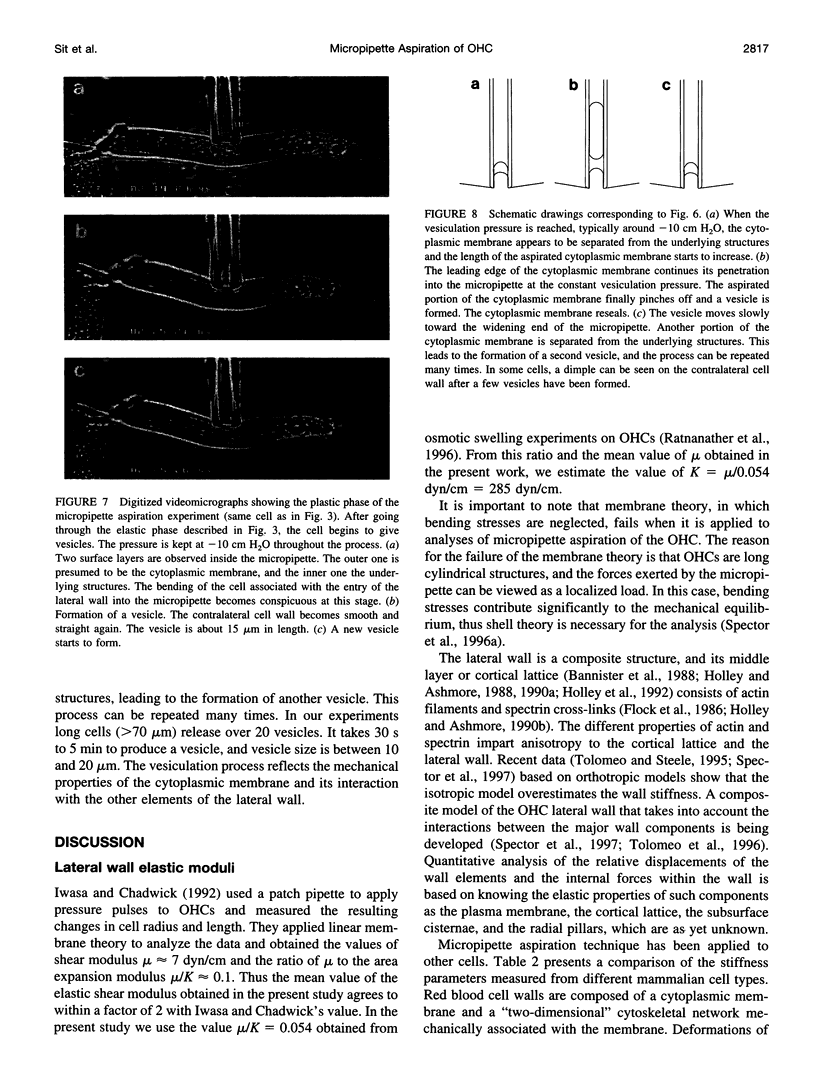
The mechanical properties of the lateral wall of the guinea pig cochlear outer hair cell were studied using the micropipette aspiration technique. A fire-polished micropipette with an inner diameter of approximately 4 microm was brought into contact with the lateral wall and negative pressure was applied. The resulting deformation of the lateral wall was recorded on videotape and subjected to morphometric analysis. The relation between the length of the aspirated portion of the cell and aspiration pressure is characterized by the stiffness parameter, K(s) = 1.07 +/- 0.24 (SD) dyn/cm (n = 14). Values of K(s) do not correlate with the original cell length, which ranges from 29 to 74 microm. Theoretical analysis based on elastic shell theory applied to the experimental data yields an estimate of the effective elastic shear modulus, mu = 15.4 +/- 3.3 dyn/cm. These data were obtained at subcritical aspiration pressures, typically less than 10 cm H2O. After reaching a critical (vesiculation) pressure, the cytoplasmic membrane appeared to separate from the underlying structures, a vesicle with a length of 10-20 microm was formed, and the cytoplasmic membrane resealed. This vesiculation process was repeated until a cell-specific limit was reached and no more vesicles were formed. Over 20 vesicles were formed from the longest cells in the experiment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima T., Kuraoka A., Toriya R., Shibata Y., Uemura T. Quick-freeze, deep-etch visualization of the 'cytoskeletal spring' of cochlear outer hair cells. Cell Tissue Res. 1991 Jan;263(1):91–97. doi: 10.1007/BF00318403. [DOI] [PubMed] [Google Scholar]
- Bannister L. H., Dodson H. C., Astbury A. R., Douek E. E. The cortical lattice: a highly ordered system of subsurface filaments in guinea pig cochlear outer hair cells. Prog Brain Res. 1988;74:213–219. doi: 10.1016/s0079-6123(08)63016-2. [DOI] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Brownell W. E. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990 Apr;11(2):82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris S. M., Smith C. M., 2nd, Rao G. H., White J. G. Aspirin treatment reduces platelet resistance to deformation. Arteriosclerosis. 1987 Jul-Aug;7(4):385–388. doi: 10.1161/01.atv.7.4.385. [DOI] [PubMed] [Google Scholar]
- Burris S. M., Smith C. M., 2nd, Tukey D., Clawson C. C., White J. G. Micropipette aspiration of human platelets: influence of rewarming on deformability of chilled cells. J Lab Clin Med. 1986 Mar;107(3):238–243. [PubMed] [Google Scholar]
- Cheng L. Y. Deformation analyses in cell and developmental biology. Part I--Formal methodology. J Biomech Eng. 1987 Feb;109(1):10–17. doi: 10.1115/1.3138634. [DOI] [PubMed] [Google Scholar]
- Chien S., Sung K. L., Skalak R., Usami S., Tözeren A. Theoretical and experimental studies on viscoelastic properties of erythrocyte membrane. Biophys J. 1978 Nov;24(2):463–487. doi: 10.1016/S0006-3495(78)85395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieler R., Shehata-Dieler W. E., Brownell W. E. Concomitant salicylate-induced alterations of outer hair cell subsurface cisternae and electromotility. J Neurocytol. 1991 Aug;20(8):637–653. doi: 10.1007/BF01187066. [DOI] [PubMed] [Google Scholar]
- Discher D. E., Mohandas N., Evans E. A. Molecular maps of red cell deformation: hidden elasticity and in situ connectivity. Science. 1994 Nov 11;266(5187):1032–1035. doi: 10.1126/science.7973655. [DOI] [PubMed] [Google Scholar]
- Engström K. G., Möller B., Meiselman H. J. Optical evaluation of red blood cell geometry using micropipette aspiration. Blood Cells. 1992;18(2):241–265. [PubMed] [Google Scholar]
- Evans B. N. Fatal contractions: ultrastructural and electromechanical changes in outer hair cells following transmembraneous electrical stimulation. Hear Res. 1990 May;45(3):265–282. doi: 10.1016/0378-5955(90)90126-a. [DOI] [PubMed] [Google Scholar]
- Evans E. A. New membrane concept applied to the analysis of fluid shear- and micropipette-deformed red blood cells. Biophys J. 1973 Sep;13(9):941–954. doi: 10.1016/S0006-3495(73)86036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Kukan B. Passive material behavior of granulocytes based on large deformation and recovery after deformation tests. Blood. 1984 Nov;64(5):1028–1035. [PubMed] [Google Scholar]
- Flock A., Flock B., Ulfendahl M. Mechanisms of movement in outer hair cells and a possible structural basis. Arch Otorhinolaryngol. 1986;243(2):83–90. doi: 10.1007/BF00453755. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M. Cell biomechanics: a brief overview. J Biomech Eng. 1990 Aug;112(3):233–234. doi: 10.1115/1.2891177. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. A cytoskeletal spring for the control of cell shape in outer hair cells isolated from the guinea pig cochlea. Eur Arch Otorhinolaryngol. 1990;247(1):4–7. doi: 10.1007/BF00240939. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. A cytoskeletal spring in cochlear outer hair cells. Nature. 1988 Oct 13;335(6191):635–637. doi: 10.1038/335635a0. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. Spectrin, actin and the structure of the cortical lattice in mammalian cochlear outer hair cells. J Cell Sci. 1990 Jun;96(Pt 2):283–291. doi: 10.1242/jcs.96.2.283. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Kalinec F., Kachar B. Structure of the cortical cytoskeleton in mammalian outer hair cells. J Cell Sci. 1992 Jul;102(Pt 3):569–580. doi: 10.1242/jcs.102.3.569. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H., Chadwick R. S. Elasticity and active force generation of cochlear outer hair cells. J Acoust Soc Am. 1992 Dec;92(6):3169–3173. doi: 10.1121/1.404194. [DOI] [PubMed] [Google Scholar]
- Mege J. L., Capo C., Benoliel A. M., Bongrand P. Use of cell contour analysis to evaluate the affinity between macrophages and glutaraldehyde-treated erythrocytes. Biophys J. 1987 Aug;52(2):177–186. doi: 10.1016/S0006-3495(87)83205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollice P. A., Brownell W. E. Characterization of the outer hair cell's lateral wall membranes. Hear Res. 1993 Nov;70(2):187–196. doi: 10.1016/0378-5955(93)90157-v. [DOI] [PubMed] [Google Scholar]
- Ratnanather J. T., Zhi M., Brownell W. E., Popel A. S. The ratio of elastic moduli of cochlear outer hair cells derived from osmotic experiments. J Acoust Soc Am. 1996 Feb;99(2):1025–1028. doi: 10.1121/1.414631. [DOI] [PubMed] [Google Scholar]
- Ruef P., Böhler T., Linderkamp O. Deformability and volume of neonatal and adult leukocytes. Pediatr Res. 1991 Feb;29(2):128–132. doi: 10.1203/00006450-199102000-00004. [DOI] [PubMed] [Google Scholar]
- Saito K. Fine structure of the sensory epithelium of guinea-pig organ of Corti: subsurface cisternae and lamellar bodies in the outer hair cells. Cell Tissue Res. 1983;229(3):467–481. doi: 10.1007/BF00207692. [DOI] [PubMed] [Google Scholar]
- Sato M., Levesque M. J., Nerem R. M. An application of the micropipette technique to the measurement of the mechanical properties of cultured bovine aortic endothelial cells. J Biomech Eng. 1987 Feb;109(1):27–34. doi: 10.1115/1.3138638. [DOI] [PubMed] [Google Scholar]
- Sato M., Levesque M. J., Nerem R. M. Micropipette aspiration of cultured bovine aortic endothelial cells exposed to shear stress. Arteriosclerosis. 1987 May-Jun;7(3):276–286. doi: 10.1161/01.atv.7.3.276. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Sung K. L., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys J. 1981 Oct;36(1):243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky N. B., Ligotti P. J. Characterization of inner ear sensory hair cells after rapid-freezing and freeze-substitution. J Neurocytol. 1992 May;21(5):374–381. doi: 10.1007/BF01191705. [DOI] [PubMed] [Google Scholar]
- Smith C. M., 2nd, Burris S. M., Weiss D. J., White J. G. Comparison of bovine and human platelet deformability, using micropipette elastimetry. Am J Vet Res. 1989 Jan;50(1):34–38. [PubMed] [Google Scholar]
- Spector A. A., Brownell W. E., Popel A. S. A model for cochlear outer hair cell deformations in micropipette aspiration experiments: an analytical solution. Ann Biomed Eng. 1996 Jul-Aug;24(4):241–249. doi: 10.1007/BF02648116. [DOI] [PubMed] [Google Scholar]
- Theret D. P., Levesque M. J., Sato M., Nerem R. M., Wheeler L. T. The application of a homogeneous half-space model in the analysis of endothelial cell micropipette measurements. J Biomech Eng. 1988 Aug;110(3):190–199. doi: 10.1115/1.3108430. [DOI] [PubMed] [Google Scholar]
- Tolomeo J. A., Steele C. R., Holley M. C. Mechanical properties of the lateral cortex of mammalian auditory outer hair cells. Biophys J. 1996 Jul;71(1):421–429. doi: 10.1016/S0006-3495(96)79244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolomeo J. A., Steele C. R. Orthotropic piezoelectric properties of the cochlear outer hair cell wall. J Acoust Soc Am. 1995 May;97(5 Pt 1):3006–3011. doi: 10.1121/1.411865. [DOI] [PubMed] [Google Scholar]
- Ulfendahl M., Slepecky N. Ultrastructural correlates of inner ear sensory cell shortening. J Submicrosc Cytol Pathol. 1988 Jan;20(1):47–51. [PubMed] [Google Scholar]




