Abstract
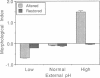
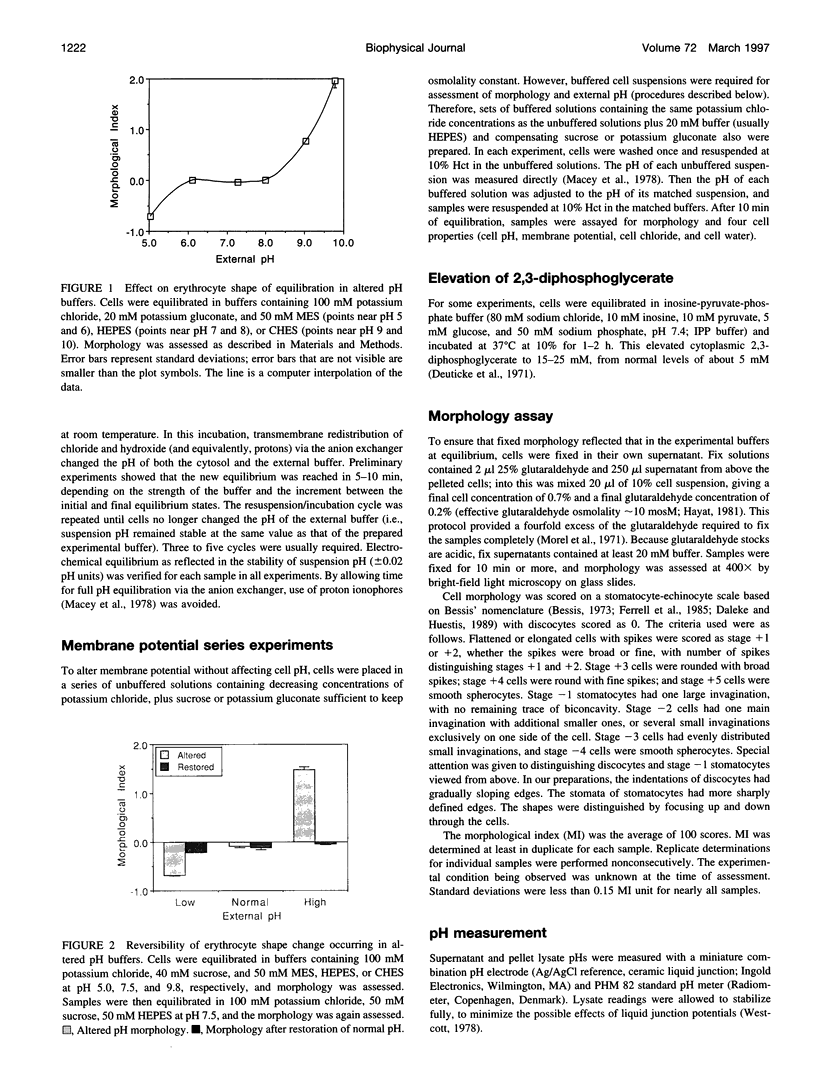
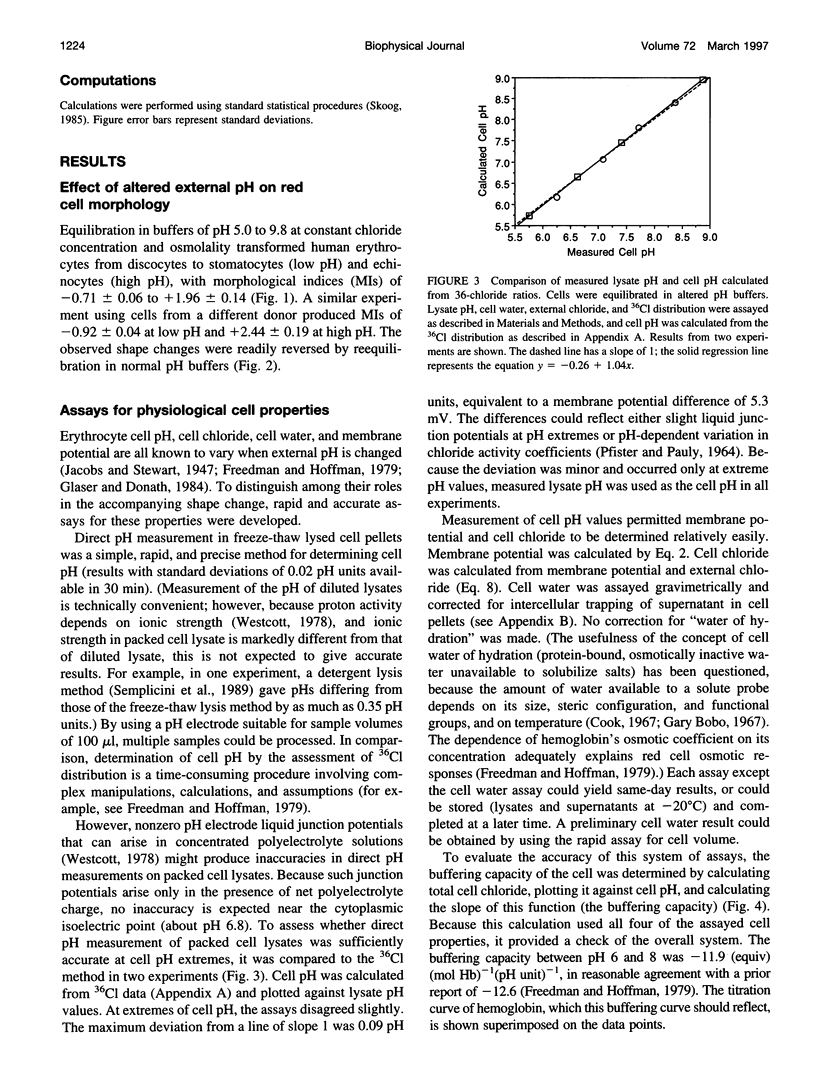
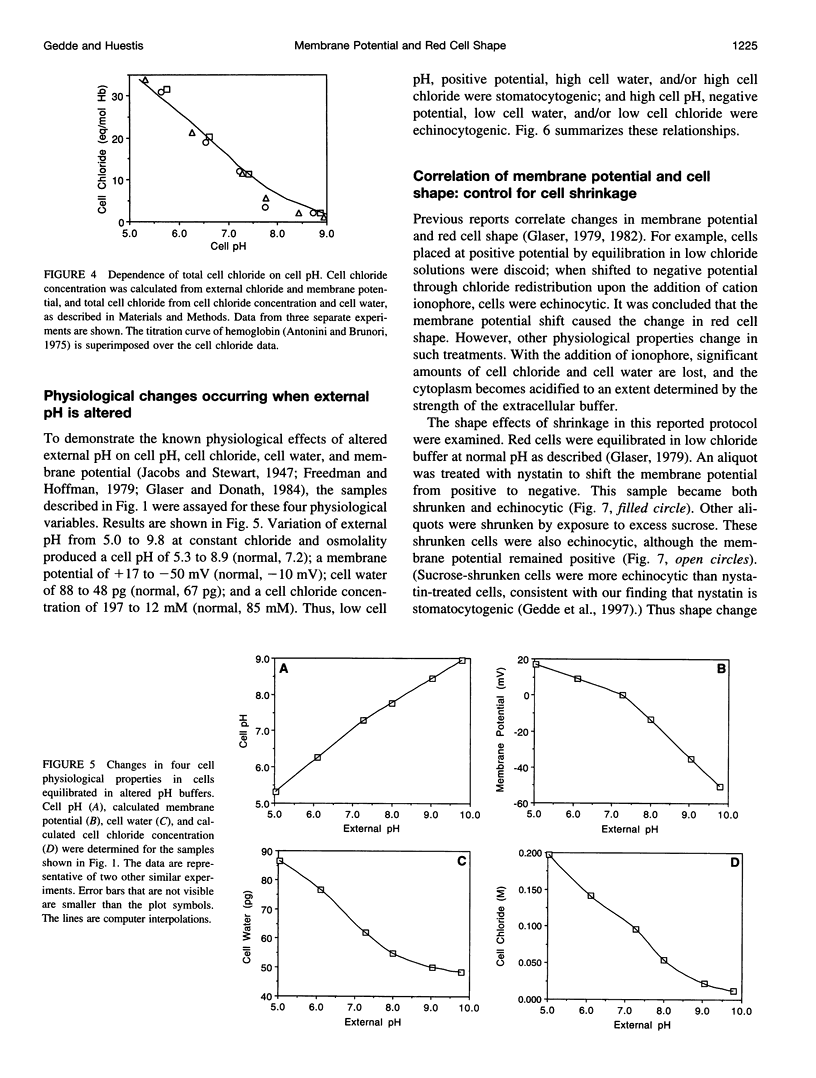
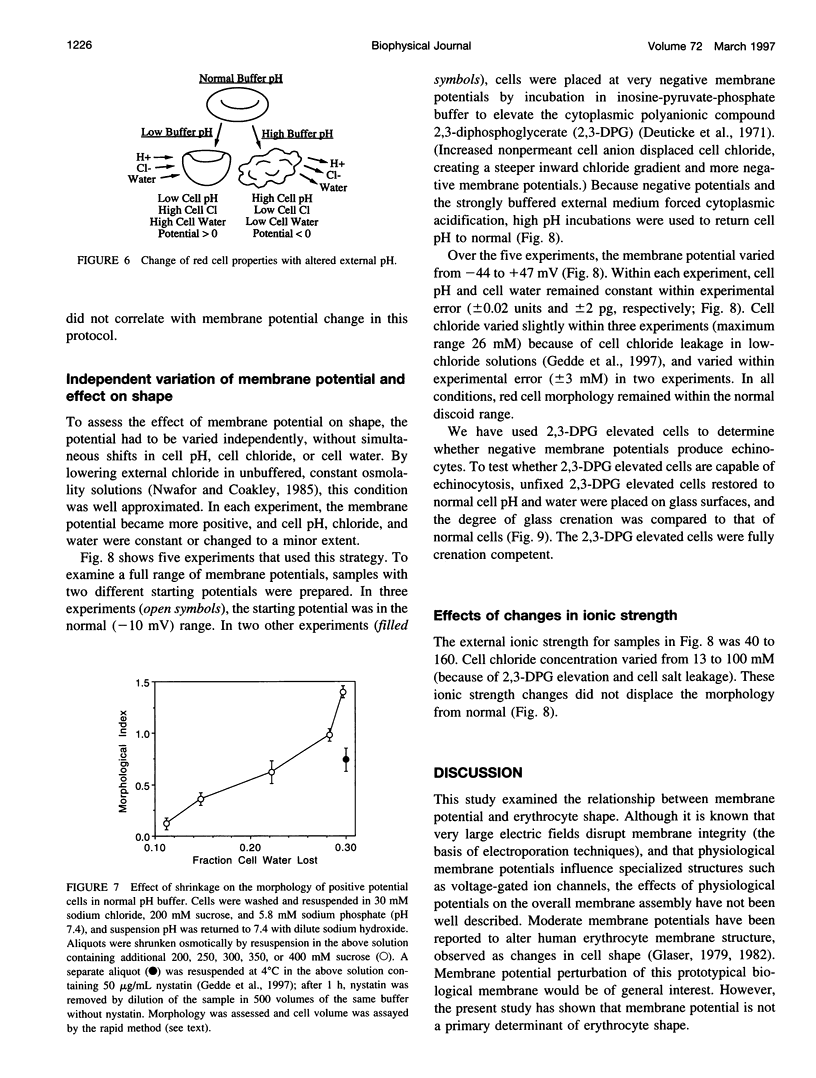
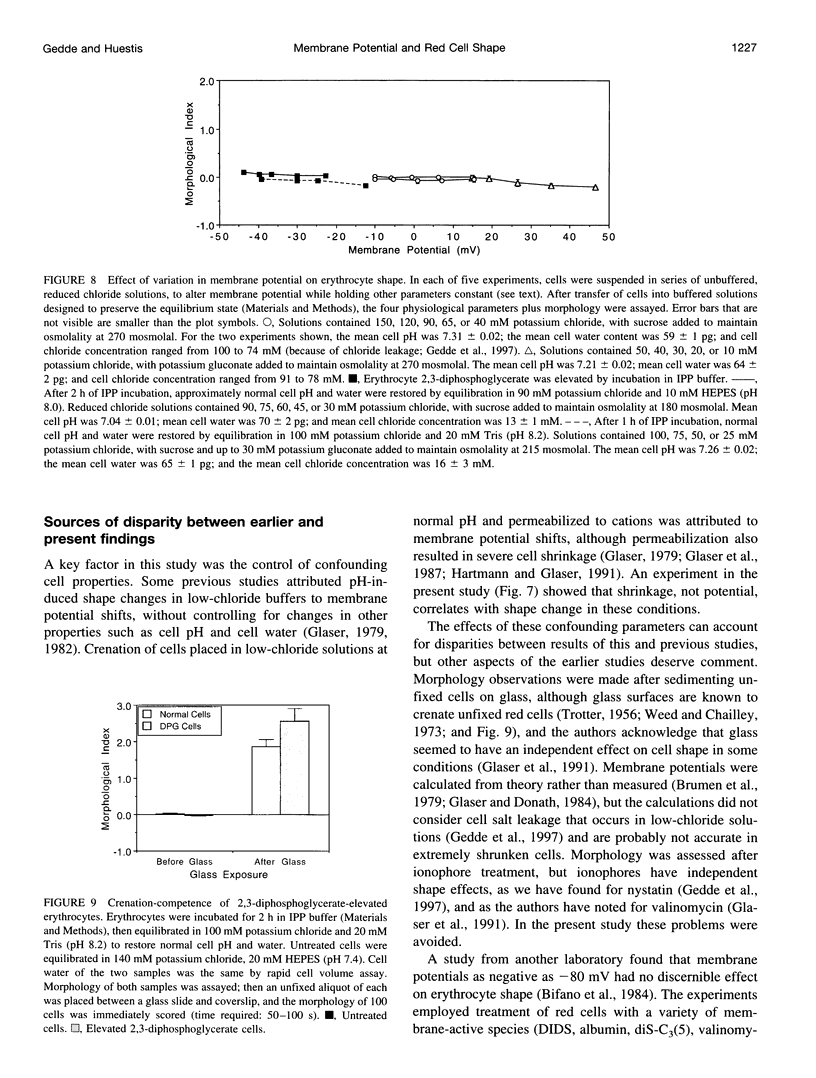
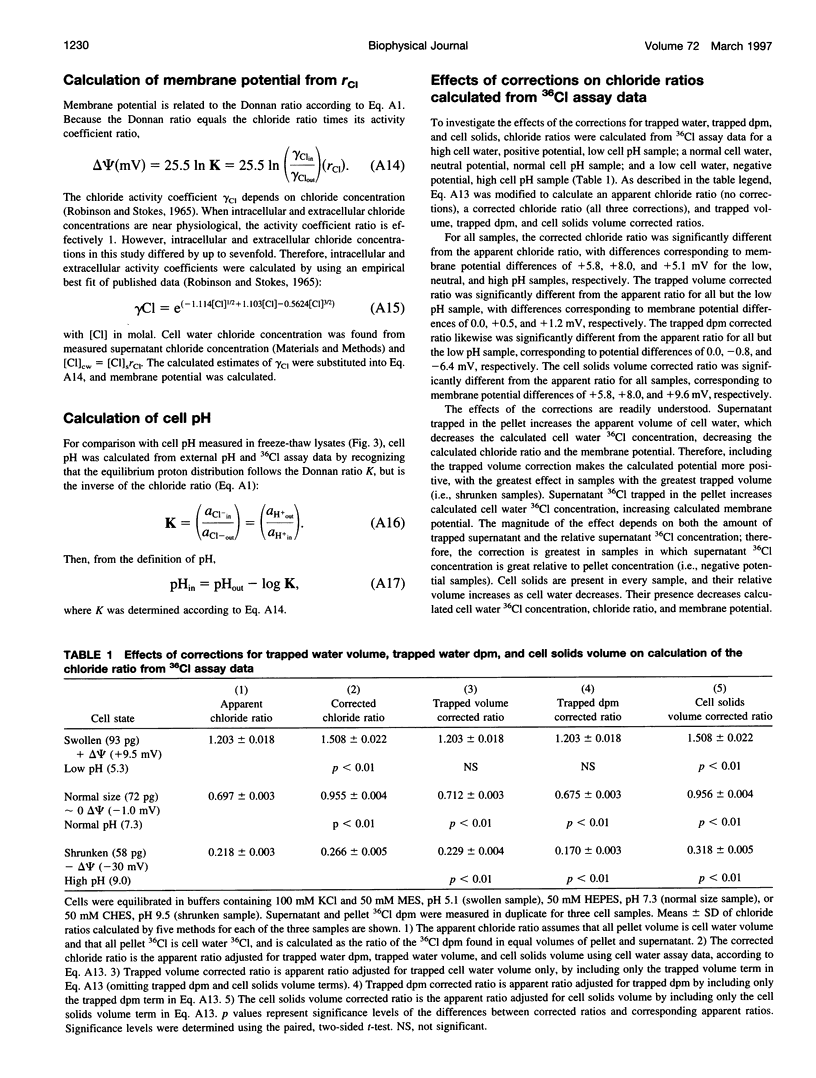
Altered external pH transforms human erythrocytes from discocytes to stomatocytes (low pH) or echinocytes (high pH). The process is fast and reversible at room temperature, so it seems to involve shifts in weak inter- or intramolecular bonds. This shape change has been reported to depend on changes in membrane potential, but control experiments excluding roles for other simultaneously varying cell properties (cell pH, cell water, and cell chloride concentration) were not reported. The present study examined the effect of independent variation of membrane potential on red cell shape. Red cells were equilibrated in a set of solutions with graduated chloride concentrations, producing in them a wide range of membrane potentials at normal cell pH and cell water. By using assays that were rapid and accurate, cell pH, cell water, cell chloride, and membrane potential were measured in each sample. Cells remained discoid over the entire range of membrane potentials examined (-45 to +45 mV). It was concluded that membrane potential has no independent effect on red cell shape and does not mediate the membrane curvature changes known to occur in red cells equilibrated at altered pH.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessis M., Prenant M. Topographie de l'apparition des spicules dans les érythrocytes crénelés (échinocytes. Nouv Rev Fr Hematol. 1972 May-Jun;12(3):351–364. [PubMed] [Google Scholar]
- Bifano E. M., Novak T. S., Freedman J. C. Relationship between the shape and the membrane potential of human red blood cells. J Membr Biol. 1984;82(1):1–13. doi: 10.1007/BF01870727. [DOI] [PubMed] [Google Scholar]
- Bobo C. M. Nonsolvent water in human erythrocytes and hemoglobin solutions. J Gen Physiol. 1967 Dec;50(11):2547–2564. doi: 10.1085/jgp.50.11.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986 Aug;103(2):343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. S. Nonsolvent water in human erythrocytes. J Gen Physiol. 1967 May;50(5):1311–1325. doi: 10.1085/jgp.50.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daleke D. L., Huestis W. H. Erythrocyte morphology reflects the transbilayer distribution of incorporated phospholipids. J Cell Biol. 1989 Apr;108(4):1375–1385. doi: 10.1083/jcb.108.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M. Chloride and water distribution in human red cells. J Physiol. 1975 Aug;250(1):65–84. doi: 10.1113/jphysiol.1975.sp011043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B., Duhm J., Dierkesmann R. Maximal elevation of 2,3-diphosphoglycerate concentrations in human erythrocytes: influence on glycolytic metabolism and intracellular pH. Pflugers Arch. 1971;326(1):15–34. doi: 10.1007/BF00586792. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A., Stokke B. T., Mikkelsen A., Branton D. The molecular basis of erythrocyte shape. Science. 1986 Dec 5;234(4781):1217–1223. doi: 10.1126/science.3775380. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Huestis W. H. Phosphoinositide metabolism and the morphology of human erythrocytes. J Cell Biol. 1984 Jun;98(6):1992–1998. doi: 10.1083/jcb.98.6.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Lee K. J., Huestis W. H. Membrane bilayer balance and erythrocyte shape: a quantitative assessment. Biochemistry. 1985 Jun 4;24(12):2849–2857. doi: 10.1021/bi00333a006. [DOI] [PubMed] [Google Scholar]
- Freedman J. C., Hoffman J. F. Ionic and osmotic equilibria of human red blood cells treated with nystatin. J Gen Physiol. 1979 Aug;74(2):157–185. doi: 10.1085/jgp.74.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Sato T., Tamura A., Wakatsuki M., Kanaho Y. Shape changes of human erythrocytes induced by various amphipathic drugs acting on the membrane of the intact cells. Biochem Pharmacol. 1979 Mar 1;28(5):613–620. doi: 10.1016/0006-2952(79)90144-8. [DOI] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Potassium, sodium, and water in normal human red blood cells. Scand J Clin Lab Invest. 1966;18(2):167–180. doi: 10.3109/00365516609051812. [DOI] [PubMed] [Google Scholar]
- Glaser R. Echinocyte formation induced by potential changes of human red blood cells. J Membr Biol. 1982;66(2):79–85. doi: 10.1007/BF01868484. [DOI] [PubMed] [Google Scholar]
- Glaser R., Fujii T., Müller P., Tamura E., Herrmann A. Erythrocyte shape dynamics: influence of electrolyte conditions and membrane potential. Biomed Biochim Acta. 1987;46(2-3):S327–S333. [PubMed] [Google Scholar]
- Glaser R., Gengnagel C., Donath J. The influence of valinomycin induced membrane potential on erythrocyte shape. Biomed Biochim Acta. 1991;50(7):869–877. [PubMed] [Google Scholar]
- Glaser R. The shape of red blood cells as a function of membrane potential and temperature. J Membr Biol. 1979 Dec 31;51(3-4):217–228. doi: 10.1007/BF01869085. [DOI] [PubMed] [Google Scholar]
- Hartmann J., Glaser R. The influence of chlorpromazine on the potential-induced shape change of human erythrocyte. Biosci Rep. 1991 Aug;11(4):213–221. doi: 10.1007/BF01136855. [DOI] [PubMed] [Google Scholar]
- Jinbu Y., Sato S., Nakao T., Nakao M., Tsukita S., Tsukita S., Ishikawa H. The role of ankyrin in shape and deformability change of human erythrocyte ghosts. Biochim Biophys Acta. 1984 Jun 27;773(2):237–245. doi: 10.1016/0005-2736(84)90087-7. [DOI] [PubMed] [Google Scholar]
- Lin S., Yang E., Huestis W. H. Relationship of phospholipid distribution to shape change in Ca(2+)-crenated and recovered human erythrocytes. Biochemistry. 1994 Jun 14;33(23):7337–7344. doi: 10.1021/bi00189a039. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Derick L. H. Molecular anatomy of the red blood cell membrane skeleton: structure-function relationships. Semin Hematol. 1992 Oct;29(4):231–243. [PubMed] [Google Scholar]
- Lovrien R. E., Anderson R. A. Stoichiometry of wheat germ agglutinin as a morphology controlling agent and as a morphology controlling agent and as a morphology protective agent for the human erythrocyte. J Cell Biol. 1980 Jun;85(3):534–548. doi: 10.1083/jcb.85.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIZELS M., REMINGTON M. Percentage of intercellular medium in human erythrocytes centrifuged from albumin and other media. J Physiol. 1959 Mar 12;145(3):658–666. doi: 10.1113/jphysiol.1959.sp006169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCONAGHEY P. D., MAIZELS M. The osmotic coefficients of haemoglobin in red cells under varying conditions. J Physiol. 1961 Jan;155:28–45. doi: 10.1113/jphysiol.1961.sp006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey R. I., Adorante J. S., Orme F. W. Erythrocyte membrane potentials determined by hydrogen ion distribution. Biochim Biophys Acta. 1978 Sep 22;512(2):284–295. doi: 10.1016/0005-2736(78)90253-5. [DOI] [PubMed] [Google Scholar]
- Morel F. M., Baker R. F., Wayland H. Quantitation of human red blood cell fixation by glutaraldehyde. J Cell Biol. 1971 Jan;48(1):91–100. doi: 10.1083/jcb.48.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwafor A., Coakley W. T. Drug-induced shape change in erythrocytes correlates with membrane potential change and is independent of glycocalyx charge. Biochem Pharmacol. 1985 Sep 15;34(18):3329–3336. doi: 10.1016/0006-2952(85)90354-5. [DOI] [PubMed] [Google Scholar]
- Raftos J. E., Bulliman B. T., Kuchel P. W. Evaluation of an electrochemical model of erythrocyte pH buffering using 31P nuclear magnetic resonance data. J Gen Physiol. 1990 Jun;95(6):1183–1204. doi: 10.1085/jgp.95.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval P. J., Carter D. P., Fairbanks G. Relationship of hemolysis buffer structure, pH and ionic strength to spontaneous contour smoothing of isolated erythrocyte membranes. Biochim Biophys Acta. 1989 Aug 7;983(2):230–240. doi: 10.1016/0005-2736(89)90238-1. [DOI] [PubMed] [Google Scholar]
- SCHOENFELD R. G., LEWELLEN C. J. A COLORIMETRIC METHOD FOR DETERMINATION OF SERUM CHLORIDE. Clin Chem. 1964 Jun;10:533–539. [PubMed] [Google Scholar]
- Schindler M., Koppel D. E., Sheetz M. P. Modulation of membrane protein lateral mobility by polyphosphates and polyamines. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1457–1461. doi: 10.1073/pnas.77.3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semplicini A., Spalvins A., Canessa M. Kinetics and stoichiometry of the human red cell Na+/H+ exchanger. J Membr Biol. 1989 Mar;107(3):219–228. doi: 10.1007/BF01871937. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Casaly J. 2,3-Diphosphoglycerate and ATP dissociate erythrocyte membrane skeletons. J Biol Chem. 1980 Oct 25;255(20):9955–9960. [PubMed] [Google Scholar]
- Sheetz M. P., Casaly J. Phosphate metabolite regulation of spectrin interactions. Scand J Clin Lab Invest Suppl. 1981;156:117–122. doi: 10.3109/00365518109097443. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Nakajima T., Shiga T., Maeda N. Influence of 2,3-diphosphoglycerate on the deformability of human erythrocytes. Biochim Biophys Acta. 1990 Nov 2;1029(1):85–90. doi: 10.1016/0005-2736(90)90439-u. [DOI] [PubMed] [Google Scholar]
- TROTTER W. D. The slide-coverslip disc-sphere transformation in mammalian erythrocytes. Br J Haematol. 1956 Jan;2(1):65–74. doi: 10.1111/j.1365-2141.1956.tb06685.x. [DOI] [PubMed] [Google Scholar]
- Waugh R. E. Effects of 2,3-diphosphoglycerate on the mechanical properties of erythrocyte membrane. Blood. 1986 Jul;68(1):231–238. [PubMed] [Google Scholar]