Abstract
Takayasu arteritis is a rare vasculitis commonly seen in young women. Amaurosis fugax and headache are visual presentations of Takayasu arteritis. Migraine with visual aura is a common condition affecting young women, and differentiating migraine from Takayasu presenting with visual symptoms and headache can be challenging. We present a case of a young woman initially misdiagnosed with migraine with aura, who was later found to have amaurosis fugax as an early manifestation of Takayasu arteritis.
Keywords: Case report, large-vessel vasculitis, migraine, Takayasu arteritis
KEY POINTS
Takayasu arteritis is a rare form of large vessel vasculitis that is notoriously difficult to diagnose given the nonspecific presentation and lack of reliable laboratory markers. Thus, diagnosis is often delayed, which can lead to complications depending on the involved vessel territories.
Diagnosis of Takayasu arteritis relies on multimodal vessel imaging tests to demonstrate involvement of multiple vessel territories and absence of atherosclerotic changes in involved vessels.
CME
CME Information: https://ce.bswhealth.com/BUMC_Proceedings_CME_info.
Credit Claim Process: To claim CME for this activity, read the entire article and go to ce.bswhealth.com/2025BUMC_Proceedings_Mar_Takayasu. You will register for the course, pay any relevant fee, take the quiz, complete the evaluation, and claim your CME credit.
Dates for credit: March 1, 2025, to March 1, 2026.
For questions about CME credit, visit our website, ce.bswhealth.com/contact-us.
CASE SUMMARY
A 34-year-old African American woman presented to the emergency department with complaints of chest pain following an episode of alcohol consumption and physical exertion. The patient reported experiencing sharp, right-sided chest pain, which was not associated with shortness of breath or palpitations. This was her first episode, and the pain resolved within 30 minutes. She had a significant history of three episodes of sudden-onset vision loss in the right eye approximately 2 years earlier, accompanied by a headache. She described those episodes as a gray shade covering the right eye, followed by gradual restoration of vision to normal within approximately 15 minutes. These episodes were associated with bilateral headaches over the vertex. Additionally, she had a significant medical history of migraine headaches since the age of 16. The patient was not on any medications. Her family history included a 38-year-old sister who had lupus and Sjogren’s disease and her father who died in his 40s with a myocardial infarction.
The patient was normotensive and afebrile. Physical examination revealed an abdominal bruit and bilaterally diminished femoral and popliteal pulses; no cervical bruits were present and pulses were symmetric in upper extremities. Visual acuity was measured to be 20/20. Pertinent laboratory data included a negative troponin and creatine phosphokinase-MB test, normal white blood cell count (6500 cells/mm3) with elevated lymphocyte differential (50%), normal erythrocyte sedimentation rate (10 mm/hr), and slightly elevated C-reactive protein (1.6 mg/dL). An electrocardiogram and chest x-ray did not reveal any abnormalities.
An ultrasound of the carotid system showed peak systolic velocities of the right common, internal, and external carotid arteries of 261.4 cm/s, 236.1 cm/s, and 176.2 cm/s, respectively. Left common carotid and left internal and external carotid artery peak systolic velocities were 132.8 cm/s, showing 70% stenosis of the right common carotid system with no left carotid involvement.
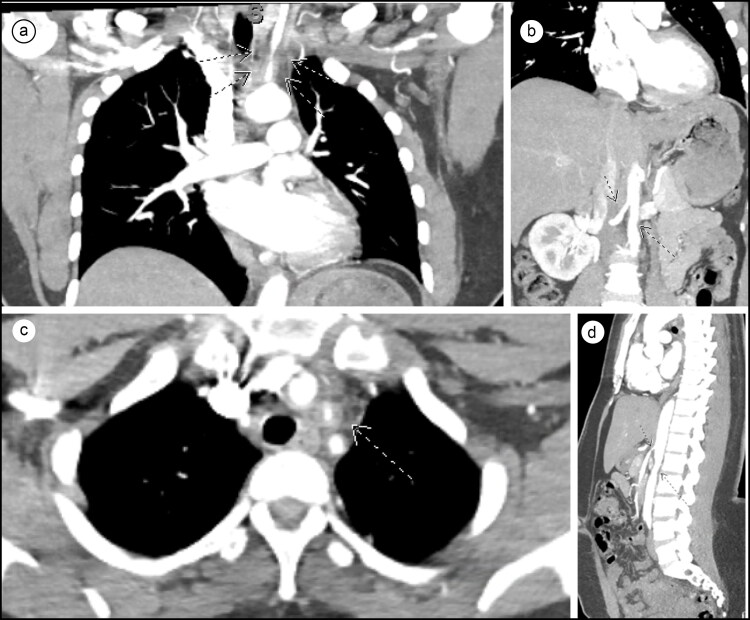
Computed tomography angiography (CTA) of the chest and abdomen showed 90% stenosis with significant smooth tapering of the proximal left common carotid artery, 30% stenosis and wall thickening of the proximal right common carotid artery, normal subclavian arteries bilaterally, and 50% tapered narrowing of the infrarenal aorta and right renal artery (Figure 1). No significant calcifications were seen in these territories. The inferior mesenteric artery was also occluded, and a bovine arch configuration of the aorta was seen. No pulmonary embolism was identified.
Figure 1.
(a) CTA of the thorax showing significant smooth tapering of the left common carotid artery. (b) CTA of the abdomen showing tapering stenosis of the infrarenal aorta and right renal artery. (c) CTA of the thorax showing left common carotid stenosis, with no calcification. (d) Sagittal section of CTA of the abdomen further demonstrating tapering stenosis of the infrarenal aorta and right renal artery.
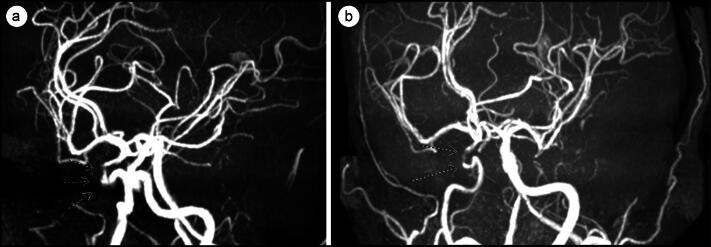
This was followed by magnetic resonance angiography (MRA) of the head and neck, which showed narrowing of the distal left common carotid artery by 30% and 75% stenosis of the cavernous portion of the suprasellar right internal carotid arteries (Figure 2). Compared with the MRA from 2 years earlier, there was an increase in stenosis of the cavernous portion of the right internal carotid artery. Middle cerebral, vertebral, and basilar arteries had normal flow. Ultrasound and CTA/MRA findings of the carotid arteries were divergent, and since vascular imaging with CTA/MRA was already performed, repeat ultrasound was not done at the time. There were no ischemic findings on the MRI.
Figure 2.
(a) Initial MRI from April 2022 showing stenosis of the right internal carotid artery siphon. (b) MRI from October 2023 showing increased stenosis of the right internal carotid artery siphon.
These imaging studies were consistent with findings seen in Takayasu arteritis (TA). Pertinently, when the patient presented to the emergency department 2 years earlier with a headache and amaurosis fugax, a CT scan of the head was unremarkable and an MRA showed around 50% stenosis of the cavernous portion of the suprasellar right internal carotid artery, but the study was deemed technically limited. The patient was diagnosed with recurrent migraine episodes and discharged at that time.
During this admission, further diagnostic studies were conducted to exclude other vasculitic processes, including hepatitis C, syphilis, hepatitis B, rheumatoid factor, C-ANCA and P-ANCA, ANA, anti dsDNA, anti-SS, and complement levels (C3 and C4), all of which returned negative results. A lipid profile revealed an elevated direct low-density lipoprotein of 101 mg/dL and hemoglobin A1c was 6.1%. The patient was diagnosed with TA based on a score of 9 on the American College of Rheumatology 2022 revised criteria (positive score for female sex, abdominal aorta bruit, involvement of three or more territories, abdominal aorta with renal and mesenteric involvement), the absolute requirements of age < 60, and evidence of vasculitis on imaging. Cardiology advised against catheterization due to low suspicion of acute coronary syndrome and limited benefit as a diagnostic modality in TA. The chest pain was deemed likely to be due to gastritis secondary to alcohol consumption.
The patient was initiated on a treatment regimen of prednisone 40 mg twice daily, methotrexate 10 mg daily, and atorvastatin 40 mg daily. The patient experienced no chest pain or vision abnormalities during her hospitalization. She was discharged on the third day of hospitalization with a follow-up appointment with rheumatology.
CLINICAL QUESTIONS
-
A 56-year-old Hispanic man presents to the outpatient clinic with reddish-blue lesions on his lower extremities, fever, muscle pain, and weight loss. He reported a history of acute viral hepatitis B 3 months earlier. Physical examination reveals multiple red-purple nodules on the skin of both legs. Laboratory results for P- and C-ANCA are negative. Urinalysis reveals hematuria and proteinuria. Abdominal ultrasound reveals a few 1 to 2 cm fluid-filled cavities in both kidneys. Which of the following is the most likely diagnosis?
Polyarteritis nodosa
Microscopic polyangiitis
Churg-Strauss syndrome
Henoch-Schonlein purpura
-
Which of the following is an absolute requirement to classify a patient as having TA according to the American College of Rheumatology 2022 revised criteria?
Blood pressure difference between arms of ≥20 mm Hg
Age ≤60 years
Reduced pulse or tenderness of carotid artery
Presence of limb claudication or arterial bruit
Answers are provided at the end of the article.
DISCUSSION
TA is a large vessel vasculitis with a predilection toward young females; the female-to-male ratio is 4:1 in epidemiologic studies.1,2 The disease is more common in Eastern Asian countries like Japan. In the United States, Caucasian women are more likely to be affected. The incidence rate in the US is estimated to range from 0.9 to 8.4 cases per million.2 The pathogenesis of the disease is unclear, but autoimmune, infectious, and genetic factors have been implicated. Correlation with tuberculosis infection,3 syphilis,4 and heat shock proteins3,5 has been described in the literature, and further investigation is ongoing. Emerging evidence pointing to an altered vascular microbiome is also being actively considered.6
The clinical presentation of TA is often nonspecific. The common complaints elucidated in a recent literature review include fever (20.93%), chest pain (13.95%), claudication (13.95%), and headache (13.95%).2 Another common finding at initial diagnosis is decreased pulses, with the radial artery most frequently affected. Common complications include secondary hypertension, ischemic strokes, pulmonary hypertension, coronary syndromes, and heart failure. The degree and extent of involvement of various vascular beds dictate the symptomatology and complication profile, resulting in significant variability between patients.2
Laboratory abnormalities in TA are nonspecific, with erythrocyte sedimentation rate being the most commonly elevated inflammatory marker. However, studies have described many cases with normal erythrocyte sedimentation rates during active disease. Novel biomarkers like PTX3, MMP, and TIMP are under evaluation and are promising candidates for laboratory-based diagnosis.7 Currently, the mainstay of diagnosis is clinical data along with vascular imaging, with CTA and MRA commonly used to delineate vascular territory involvement.7 18 F-fluorodeoxyglucose positron emission tomography (PET) is increasingly being used, with increased vessel wall uptake signifying increased disease activity in the regional territory, and is an area of active research.8 In patients with suspicion of coronary involvement, coronary CTA is also increasingly being used, and in appropriate cases, catheter-mediated revascularization has been shown to be successful.9
Ocular manifestations of TA are usually seen late in the disease course, but case reports have outlined early onset of ocular findings.10 The most common eye disorder accompanying the disease is retinal ischemia (present in 57.4% of patients), followed by optic neuropathy (18%), cataract (14.8%), and retinal artery occlusion (12.3%). Amaurosis fugax was a common complaint (25.4%), with patients describing it as transient blurring, fogging, dimming, seeing shades/curtains, or “white out.”10 The likely cause is hypoperfusion secondary to the involvement of central retinal artery.
The primary treatment for active TA disease is systemic glucocorticoid therapy, and patients frequently require long-term steroid administration. Agents that are added routinely include methotrexate and cyclophosphamide.11 Newer biologics like anti–tumor necrosis factor drugs and tocilizumab are being studied and are an area of active interest.11 Other drugs like statins and aspirin have also demonstrated varying degrees of efficacy. In patients with amaurosis fugax and high-grade stenosis, close monitoring with imaging (MRA and PET scans) and clinical findings are important and are best undertaken with a collaborative effort from a primary care physician and the rheumatologist and/or neurologist.7 If deemed clinically appropriate, revascularization procedures using bypass grafting techniques or stent placements are useful and can be life or limb-saving.
Fortunately, once TA is diagnosed and treated appropriately, patients have a good expectation of survival. The overall survival in a cohort of cases followed up over 6.1 years was over 95% with event-free survival being 48.2% and 36.1%.12 Another factor to consider in these patients is complications associated with long-term steroid use and its adverse effects, including reduced bone mass density and increased overall mortality.12 The recent research regarding the utilization of biologic therapies for TA offers a glimpse of future possible steroid-free management strategies.11
ANSWERS TO CLINICAL QUESTIONS
Question 1, a. Necrotizing vasculitis involves multiple organs; the lungs are spared. It classically presents in young adults as hypertension (renal artery involvement), abdominal pain with melena (mesenteric artery involvement), neurologic disturbances, and skin lesions. It is associated with serum HBsAg, and lesions of multiple stages are present at the same time. The early lesion consists of transmural inflammation with fibrinoid necrosis and eventually heals with fibrosis, producing a “string-of-pearls” appearance on imaging. Treatment is corticosteroids and cyclophosphamide. The condition is fatal if not treated.
Question 2, b. American College of Rheumatology 2022 revised criteria highlight age ≤60 years and imaging evidence of large‐vessel vasculitis as absolute requirements to classify a patient as having TA. Other points-based criteria include the following: female sex (+1), presence of angina (+2), limb claudication (+2), arterial bruit (+2), reduced upper extremity pulse (+2), reduced pulse or tenderness of a carotid artery (+2), blood pressure difference between arms of ≥20 mm Hg (+1), number of affected arterial territories (+1 to +3), paired artery involvement (+1), and abdominal aorta plus renal or mesenteric involvement (+3). A patient could be classified as having TA with a cumulative score of ≥5 points.
Disclosure statement/Funding
The planners and faculty for this activity have no relevant financial relationships to disclose. The authors report no funding. The patient consented to publication of this case report.
References
- 1.Sanchez-Alvarez C, Crowson CS, Koster MJ, et al.. Prevalence of Takayasu arteritis: a population-based study. J Rheumatol. 2021;48(6):952–952. doi: 10.3899/jrheum.201463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez-Alvarez C, Mertz LE, Thomas CS, et al.. Demographic, clinical, and radiologic characteristics of a cohort of patients with Takayasu arteritis. Am J Med. 2019;132(5):647–651. doi: 10.1016/j.amjmed.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal A, Chag M, Sinha N, Naik S.. Takayasu’s arteritis: role of Mycobacterium tuberculosis and its 65 kDa heat shock protein. Int J Cardiol. 1996;55(1):49–55. doi: 10.1016/0167-5273(96)02660-5. [DOI] [PubMed] [Google Scholar]
- 4.Maffei S, Di Renzo M, Bova G, et al.. Takayasu’s arteritis: a review of the literature. Intern Emerg Med. 2006;1(2):105–112. doi: 10.1007/BF02936534. [DOI] [PubMed] [Google Scholar]
- 5.Seko Y, Minota S, Kawasaki A, et al. Perforin-secreting killer cell infiltration and expression of a 65-kD heat-shock protein in aortic tissue of patients with Takayasu’s arteritis. J Clin Invest. 1994;93(2):750–758. doi: 10.1172/JCI117029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinoza JL, Ai S, Matsumura I.. New insights on the pathogenesis of Takayasu arteritis: revisiting the microbial theory. Pathogens. 2018;7(3):73. doi: 10.3390/pathogens7030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson L, Brogan P, Peart I, et al.. Diagnosis and assessment of disease activity in Takayasu arteritis: a childhood case illustrating the challenge. Case Rep Rheumatol. 2014;2014:603171. doi: 10.1155/2014/603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sağer S, Yılmaz S, Ozhan M, et al. F-18 Fdg PET/CT findings of a patient with Takayasu arteritis before and after therapy. Mol Imaging Radionucl Ther. 2012;21(1):32–34. doi: 10.4274/Mirt.021896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soto ME, Meléndez-Ramírez G, Kimura-Hayama E, et al. Coronary CT angiography in Takayasu arteritis. JACC Cardiovasc Imaging. 2011;4(9):958–966. doi: 10.1016/j.jcmg.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Szydełko-Paśko U, Przeździecka-Dołyk J, Nowak Ł, et al.. Ocular manifestations of Takayasu’s arteritis—a case-based systematic review and meta-analysis. J Clin Med. 2023;12(11):3745. doi: 10.3390/jcm12113745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regola F, Uzzo M, Toniati P, et al.. Novel therapies in Takayasu arteritis. Front Med. 2021;8:814075. doi: 10.3389/fmed.2021.814075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirouse A, Biard L, Comarmond C, et al. Overall survival and mortality risk factors in Takayasu’s arteritis: a multicenter study of 318 patients. J Autoimmun. 2019;96:35–39. doi: 10.1016/j.jaut.2018.08.001. [DOI] [PubMed] [Google Scholar]