Abstract
The well-characterized integral membrane protein lactose (lac) permease from Escherichia coli was reconstituted together with trace amounts (molar fraction X = 0.005 of the total phospholipid) of different pyrene-labeled phospholipid analogs into 1-palmitoyl-2-oleoyl-sn-glycero-3-sn-glycero-3-phospho-rac'-glycerol (POPG) liposomes. Effects of lac permease on bilayer lipid dynamics were investigated by measuring the excimer-to-monomer fluorescence intensity ratio IE/IM. Compared to control vesicles, the presence of lac permease (at a protein:phospholipid stoichiometry P/L of 1:4.000) increased the rate of excimer formation by 1-palmitoyl-2[6-(pyren-1-yl)]decanoyl-sn-glycero-3-phosphocholine (PPDPC) by approximately fivefold. Decreasing P/L from approximately 1:4.000 to 1:7.600 decreased the IE/IM for PPDPC from 0.16 to 0.05, respectively. An increase in bilayer fluidity due to permease is unlikely, thus implying that the augmented IE/IM should arise from partial lateral segregation of PPDPC in the vesicles. This notion is supported by the further 38% increase in IE/IM observed for the pyrene-labeled Cys-148 lac permease reconstituted into POPG vesicles at P/L 1:4000. The importance of the length of the lipid-protein boundary is implicated by the reduction in IE/IM resulting from the aggregation of the lac permease in vesicles by a monoclonal antibody. Interestingly, excimer formation by 1-palmitoyl-2[6-(pyren-1-yl)hexanoyl-sn-glycero-3-phosphocholine (PPHPC) was enhanced only fourfold in the presence of lac permease. Results obtained with the corresponding pyrenyl phosphatidylglycerols and -methanols were qualitatively similar to those above, thus indicating that lipid headgroup-protein interactions are not involved. Inclusion of 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamino-N-(5-fluoresce inthio- carbamoyl) (DPPF, X = 0.005) into reconstituted lactose permease vesicles containing PPDPC caused a nearly 90% decrease in excimer fluorescence, whereas in control vesicles lacking the reconstituted protein only 40% quenching was evident. The addition of 1,2-dipalmitoyl-sn-glycero-3-phospho-rac'-glycerol (DPPG) decreased IE/IM for PPDPC, revealing the driving force for the lateral segregation of this probe to become attenuated. More specifically for protein-free bilayers at XDPPG = 0.10 the rate of lateral diffusion of PPDPC in POPG is diminished, as evidenced by the 24% decrement in IE/IM, under these conditions the increase in IE/IM due to lac permease was strongly reduced, by approximately 84%. The present data are interpreted in terms of the hydrophobic mismatch theory, which predicts that integral membrane proteins will draw lipids of similar hydrophobic thickness into their vicinity. In brief, the approximate lengths of most of the predicted 12 hydrophobic, membrane-spanning alpha-helical segments of lactose permease range between 28.5 and 37.5 A and thus exceed the hydrophobic thickness of POPG of approximately 25.8 A. Therefore, to reduce the free energy of the assembly, longer lipids such as PPDPC and DPPF are accumulated in the immediate vicinity of lactose permease in fluid, liquid crystalline POPG bilayers.
Full text
PDF


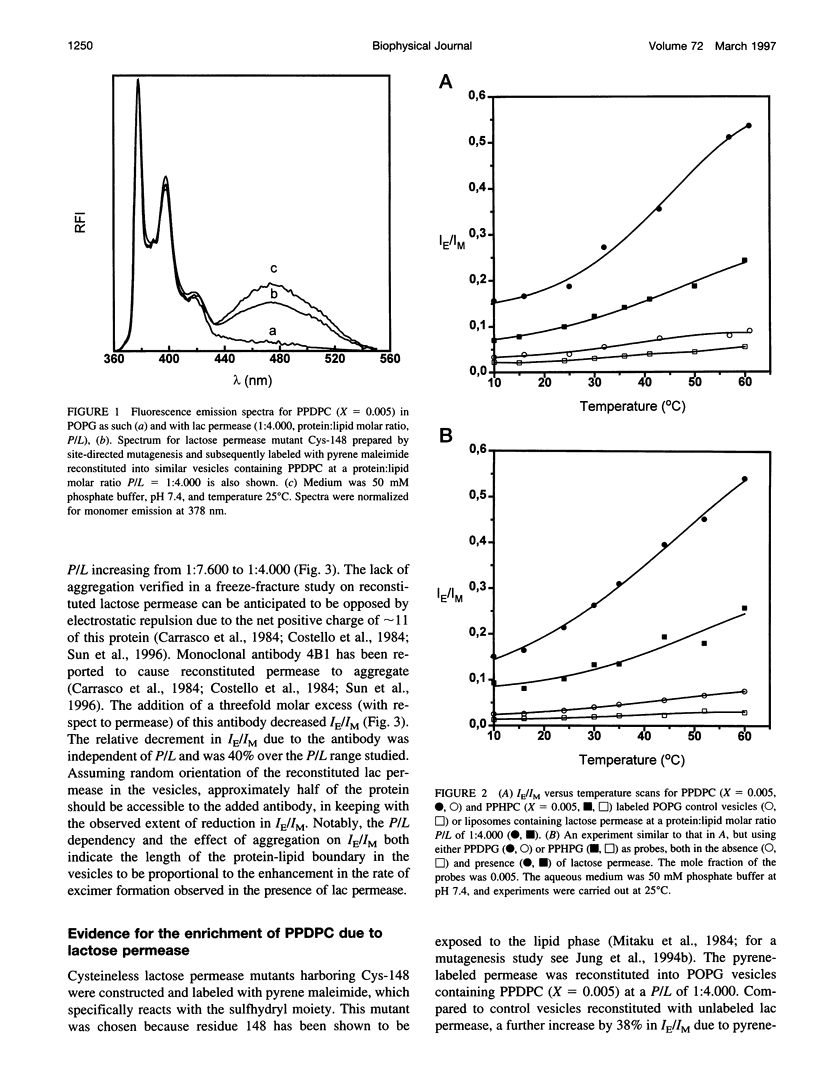
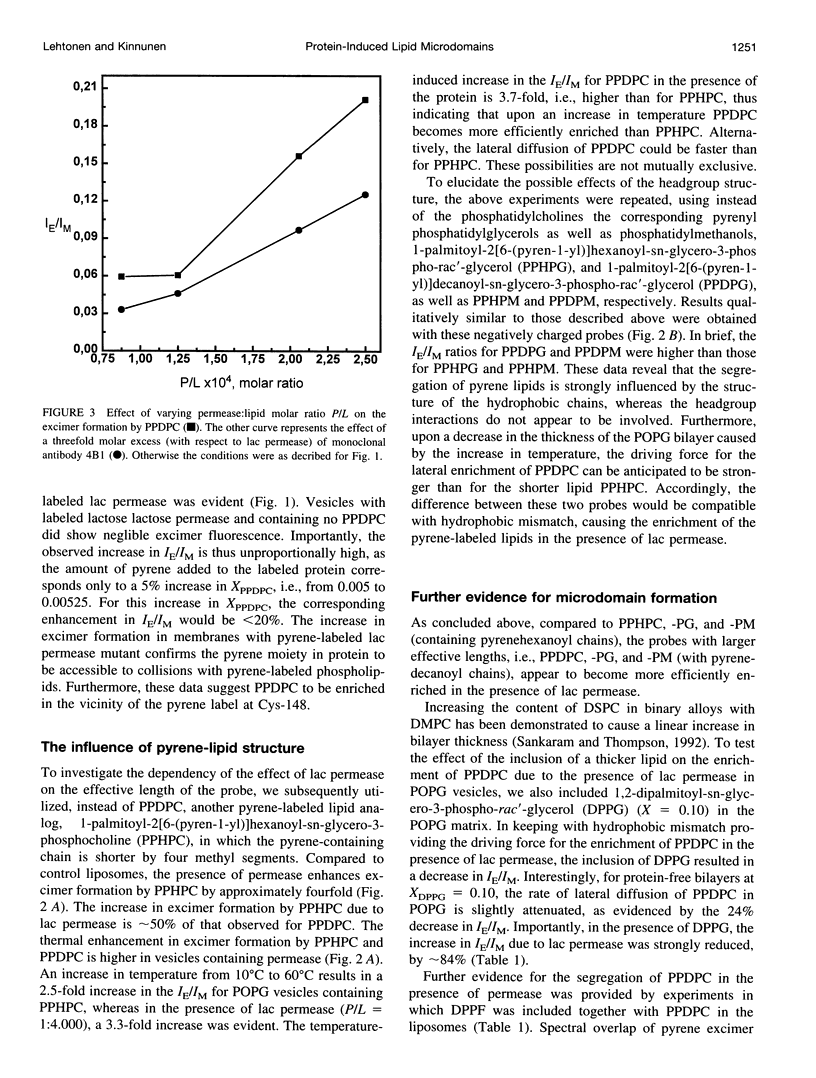
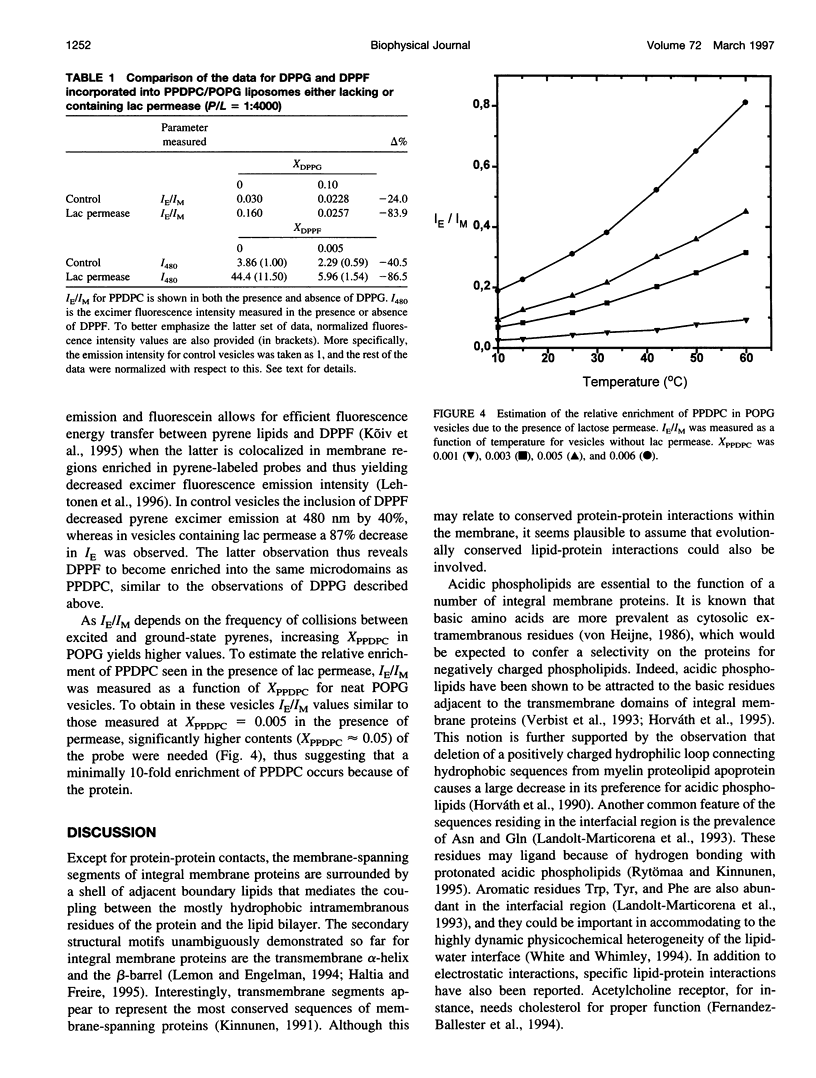





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Berclaz T., McConnell H. M. Phase Equilibria in binary mixtures of dimyristoylphosphatidylcholine and cardiolipin. Biochemistry. 1981 Nov 10;20(23):6635–6640. doi: 10.1021/bi00526a018. [DOI] [PubMed] [Google Scholar]
- Bogdanov M., Dowhan W. Phosphatidylethanolamine is required for in vivo function of the membrane-associated lactose permease of Escherichia coli. J Biol Chem. 1995 Jan 13;270(2):732–739. doi: 10.1074/jbc.270.2.732. [DOI] [PubMed] [Google Scholar]
- Carrasco N., Viitanen P., Herzlinger D., Kaback H. R. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 1. Functional studies. Biochemistry. 1984 Jul 31;23(16):3681–3687. doi: 10.1021/bi00311a017. [DOI] [PubMed] [Google Scholar]
- Chen C. C., Wilson T. H. The phospholipid requirement for activity of the lactose carrier of Escherichia coli. J Biol Chem. 1984 Aug 25;259(16):10150–10158. [PubMed] [Google Scholar]
- Chong P. L., Tang D., Sugar I. P. Exploration of physical principles underlying lipid regular distribution: effects of pressure, temperature, and radius of curvature on E/M dips in pyrene-labeled PC/DMPC binary mixtures. Biophys J. 1994 Jun;66(6):2029–2038. doi: 10.1016/S0006-3495(94)80996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consler T. G., Persson B. L., Jung H., Zen K. H., Jung K., Privé G. G., Verner G. E., Kaback H. R. Properties and purification of an active biotinylated lactose permease from Escherichia coli. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6934–6938. doi: 10.1073/pnas.90.15.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea R. L., Thomas D. D. Effects of membrane thickness on the molecular dynamics and enzymatic activity of reconstituted Ca-ATPase. Biochemistry. 1994 Mar 15;33(10):2912–2920. doi: 10.1021/bi00176a022. [DOI] [PubMed] [Google Scholar]
- Costello M. J., Viitanen P., Carrasco N., Foster D. L., Kaback H. R. Morphology of proteoliposomes reconstituted with purified lac carrier protein from Escherichia coli. J Biol Chem. 1984 Dec 25;259(24):15579–15586. [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Drake J. M., Klafter J., Levitz P. Chemical and biological microstructures as probed by dynamic processes. Science. 1991 Mar 29;251(5001):1574–1579. doi: 10.1126/science.2011737. [DOI] [PubMed] [Google Scholar]
- Eklund K. K., Vuorinen J., Mikkola J., Virtanen J. A., Kinnunen P. K. Ca2+-induced lateral phase separation in phosphatidic acid/phosphatidylcholine monolayers as revealed by fluorescence microscopy. Biochemistry. 1988 May 3;27(9):3433–3437. doi: 10.1021/bi00409a046. [DOI] [PubMed] [Google Scholar]
- Fattal D. R., Ben-Shaul A. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys J. 1993 Nov;65(5):1795–1809. doi: 10.1016/S0006-3495(93)81249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ballester G., Castresana J., Fernandez A. M., Arrondo J. L., Ferragut J. A., Gonzalez-Ros J. M. A role for cholesterol as a structural effector of the nicotinic acetylcholine receptor. Biochemistry. 1994 Apr 5;33(13):4065–4071. doi: 10.1021/bi00179a035. [DOI] [PubMed] [Google Scholar]
- Frillingos S., Sahin-Tóth M., Persson B., Kaback H. R. Cysteine-scanning mutagenesis of putative helix VII in the lactose permease of Escherichia coli. Biochemistry. 1994 Jul 5;33(26):8074–8081. doi: 10.1021/bi00192a012. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W. Excimer-forming lipids in membrane research. Chem Phys Lipids. 1980 Oct;27(3):199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Gibson N. J., Brown M. F. Lipid headgroup and acyl chain composition modulate the MI-MII equilibrium of rhodopsin in recombinant membranes. Biochemistry. 1993 Mar 9;32(9):2438–2454. doi: 10.1021/bi00060a040. [DOI] [PubMed] [Google Scholar]
- Haltia T., Freire E. Forces and factors that contribute to the structural stability of membrane proteins. Biochim Biophys Acta. 1995 Feb 14;1228(1):1–27. doi: 10.1016/0005-2728(94)00161-w. [DOI] [PubMed] [Google Scholar]
- Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986 Nov;5(11):3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderliter A. K., Huang J., Feigenson G. W. Detection of phase separation in fluid phosphatidylserine/phosphatidylcholine mixtures. Biophys J. 1994 Nov;67(5):1906–1911. doi: 10.1016/S0006-3495(94)80673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Horváth L. I., Brophy P. J., Marsh D. Influence of polar residue deletions on lipid-protein interactions with the myelin proteolipid protein. Spin-label ESR studies with DM-20/lipid recombinants. Biochemistry. 1990 Mar 20;29(11):2635–2638. doi: 10.1021/bi00463a002. [DOI] [PubMed] [Google Scholar]
- Horváth L. I., Heimburg T., Kovachev P., Findlay J. B., Hideg K., Marsh D. Integration of a K+ channel-associated peptide in a lipid bilayer: conformation, lipid-protein interactions, and rotational diffusion. Biochemistry. 1995 Mar 28;34(12):3893–3898. doi: 10.1021/bi00012a004. [DOI] [PubMed] [Google Scholar]
- Hresko R. C., Sugár I. P., Barenholz Y., Thompson T. E. Lateral distribution of a pyrene-labeled phosphatidylcholine in phosphatidylcholine bilayers: fluorescence phase and modulation study. Biochemistry. 1986 Jul 1;25(13):3813–3823. doi: 10.1021/bi00361a012. [DOI] [PubMed] [Google Scholar]
- Huang H. W. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys J. 1986 Dec;50(6):1061–1070. doi: 10.1016/S0006-3495(86)83550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In 't Veld G., Driessen A. J., Op den Kamp J. A., Konings W. N. Hydrophobic membrane thickness and lipid-protein interactions of the leucine transport system of Lactococcus lactis. Biochim Biophys Acta. 1991 Jun 18;1065(2):203–212. doi: 10.1016/0005-2736(91)90231-v. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Chaponnier C. Medical aspects of the actin cytoskeleton. Curr Opin Cell Biol. 1995 Feb;7(1):111–117. doi: 10.1016/0955-0674(95)80052-2. [DOI] [PubMed] [Google Scholar]
- Johannsson A., Keightley C. A., Smith G. A., Richards C. D., Hesketh T. R., Metcalfe J. C. The effect of bilayer thickness and n-alkanes on the activity of the (Ca2+ + Mg2+)-dependent ATPase of sarcoplasmic reticulum. J Biol Chem. 1981 Feb 25;256(4):1643–1650. [PubMed] [Google Scholar]
- Johannsson A., Smith G. A., Metcalfe J. C. The effect of bilayer thickness on the activity of (Na+ + K+)-ATPase. Biochim Biophys Acta. 1981 Mar 6;641(2):416–421. doi: 10.1016/0005-2736(81)90498-3. [DOI] [PubMed] [Google Scholar]
- Jung H., Jung K., Kaback H. R. Cysteine 148 in the lactose permease of Escherichia coli is a component of a substrate binding site. 1. Site-directed mutagenesis studies. Biochemistry. 1994 Oct 11;33(40):12160–12165. doi: 10.1021/bi00206a019. [DOI] [PubMed] [Google Scholar]
- Jung K., Jung H., Colacurcio P., Kaback H. R. Role of glycine residues in the structure and function of lactose permease, an Escherichia coli membrane transport protein. Biochemistry. 1995 Jan 24;34(3):1030–1039. doi: 10.1021/bi00003a038. [DOI] [PubMed] [Google Scholar]
- Jung K., Jung H., Wu J., Privé G. G., Kaback H. R. Use of site-directed fluorescence labeling to study proximity relationships in the lactose permease of Escherichia coli. Biochemistry. 1993 Nov 23;32(46):12273–12278. doi: 10.1021/bi00097a001. [DOI] [PubMed] [Google Scholar]
- Jung K., Jung H., Wu J., Privé G. G., Kaback H. R. Use of site-directed fluorescence labeling to study proximity relationships in the lactose permease of Escherichia coli. Biochemistry. 1993 Nov 23;32(46):12273–12278. doi: 10.1021/bi00097a001. [DOI] [PubMed] [Google Scholar]
- Junker M., Creutz C. E. Endonexin (annexin IV)-mediated lateral segregation of phosphatidylglycerol in phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry. 1993 Sep 28;32(38):9968–9974. doi: 10.1021/bi00089a012. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Active transport in Escherichia coli: passage to permease. Annu Rev Biophys Biophys Chem. 1986;15:279–319. doi: 10.1146/annurev.bb.15.060186.001431. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Frillingos S., Jung H., Jung K., Privé G. G., Ujwal M. L., Weitzman C., Wu J., Zen K. The lactose permease meets Frankenstein. J Exp Biol. 1994 Nov;196:183–195. doi: 10.1242/jeb.196.1.183. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. In and out and up and down with lac permease. Int Rev Cytol. 1992;137:97–125. doi: 10.1016/s0074-7696(08)62674-1. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Use of site-directed mutagenesis to study the mechanism of a membrane transport protein. Biochemistry. 1987 Apr 21;26(8):2071–2076. doi: 10.1021/bi00382a001. [DOI] [PubMed] [Google Scholar]
- Killian J. A., Salemink I., de Planque M. R., Lindblom G., Koeppe R. E., 2nd, Greathouse D. V. Induction of nonbilayer structures in diacylphosphatidylcholine model membranes by transmembrane alpha-helical peptides: importance of hydrophobic mismatch and proposed role of tryptophans. Biochemistry. 1996 Jan 23;35(3):1037–1045. doi: 10.1021/bi9519258. [DOI] [PubMed] [Google Scholar]
- Kinnunen P. K. On the principles of functional ordering in biological membranes. Chem Phys Lipids. 1991 Mar;57(2-3):375–399. doi: 10.1016/0009-3084(91)90087-r. [DOI] [PubMed] [Google Scholar]
- Kurrle A., Rieber P., Sackmann E. Reconstitution of transferrin receptor in mixed lipid vesicles. An example of the role of elastic and electrostatic forces for protein/lipid assembly. Biochemistry. 1990 Sep 11;29(36):8274–8282. doi: 10.1021/bi00488a011. [DOI] [PubMed] [Google Scholar]
- Kõiv A., Palvimo J., Kinnunen P. K. Evidence for ternary complex formation by histone H1, DNA, and liposomes. Biochemistry. 1995 Jun 27;34(25):8018–8027. doi: 10.1021/bi00025a007. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landolt-Marticorena C., Williams K. A., Deber C. M., Reithmeier R. A. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J Mol Biol. 1993 Feb 5;229(3):602–608. doi: 10.1006/jmbi.1993.1066. [DOI] [PubMed] [Google Scholar]
- Lehtonen J. Y., Holopainen J. M., Kinnunen P. K. Evidence for the formation of microdomains in liquid crystalline large unilamellar vesicles caused by hydrophobic mismatch of the constituent phospholipids. Biophys J. 1996 Apr;70(4):1753–1760. doi: 10.1016/S0006-3495(96)79738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen J. Y., Kinnunen P. K. Poly(ethylene glycol)-induced and temperature-dependent phase separation in fluid binary phospholipid membranes. Biophys J. 1995 Feb;68(2):525–535. doi: 10.1016/S0006-3495(95)80214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M. A., Engelman D. M. Specificity and promiscuity in membrane helix interactions. Q Rev Biophys. 1994 May;27(2):157–218. doi: 10.1017/s0033583500004522. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Bacteriorhodopsin remains dispersed in fluid phospholipid bilayers over a wide range of bilayer thicknesses. J Mol Biol. 1983 May 15;166(2):203–210. doi: 10.1016/s0022-2836(83)80006-0. [DOI] [PubMed] [Google Scholar]
- Litman B. J., Lewis E. N., Levin I. W. Packing characteristics of highly unsaturated bilayer lipids: Raman spectroscopic studies of multilamellar phosphatidylcholine dispersions. Biochemistry. 1991 Jan 15;30(2):313–319. doi: 10.1021/bi00216a001. [DOI] [PubMed] [Google Scholar]
- Ludtke S., He K., Huang H. Membrane thinning caused by magainin 2. Biochemistry. 1995 Dec 26;34(51):16764–16769. doi: 10.1021/bi00051a026. [DOI] [PubMed] [Google Scholar]
- Maneri L. R., Low P. S. Structural stability of the erythrocyte anion transporter, band 3, in different lipid environments. A differential scanning calorimetric study. J Biol Chem. 1988 Nov 5;263(31):16170–16178. [PubMed] [Google Scholar]
- Mayer L. D., Nelsestuen G. L. Calcium and prothrombin-induced lateral phase separation in membranes. Biochemistry. 1981 Apr 28;20(9):2457–2463. doi: 10.1021/bi00512a015. [DOI] [PubMed] [Google Scholar]
- Melchior D. L. Lipid domains in fluid membranes: a quick-freeze differential scanning calorimetry study. Science. 1986 Dec 19;234(4783):1577–1580. doi: 10.1126/science.3787264. [DOI] [PubMed] [Google Scholar]
- Michelangeli F., Orlowski S., Champeil P., Grimes E. A., East J. M., Lee A. G. Effects of phospholipids on binding of calcium to (Ca2(+)-Mg2(+)-ATPase. Biochemistry. 1990 Sep 11;29(36):8307–8312. doi: 10.1021/bi00488a015. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Wright J. K., Best L., Jähnig F. Localization of the galactoside binding site in the lactose carrier of Escherichia coli. Biochim Biophys Acta. 1984 Oct 3;776(2):247–258. doi: 10.1016/0005-2736(84)90214-1. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Smith G. A., Dabbeni-sala F., Johannsson A., Galante Y. M., Bisson R. Bilayer thickness and enzymatic activity in the mitochondrial cytochrome c oxidase and ATPase complex. FEBS Lett. 1982 Jul 19;144(1):145–148. doi: 10.1016/0014-5793(82)80588-7. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984 Aug;46(2):141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen O. G., Jørgensen K. Dynamical order and disorder in lipid bilayers. Chem Phys Lipids. 1994 Sep 6;73(1-2):3–25. doi: 10.1016/0009-3084(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Kinnunen P. K. On the reversal by deoxyribonucleic acid of the binding of adriamycin to cardiolipin-containing liposomes. J Biol Chem. 1993 Jan 15;268(2):1074–1080. [PubMed] [Google Scholar]
- Mustonen P., Lehtonen J., Kõiv A., Kinnunen P. K. Effects of sphingosine on peripheral membrane interactions: comparison of adriamycin, cytochrome c, and phospholipase A2. Biochemistry. 1993 May 25;32(20):5373–5380. doi: 10.1021/bi00071a012. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Virtanen J. A., Somerharju P. J., Kinnunen P. K. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987 Jun 2;26(11):2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- Nezil F. A., Bloom M. Combined influence of cholesterol and synthetic amphiphillic peptides upon bilayer thickness in model membranes. Biophys J. 1992 May;61(5):1176–1183. doi: 10.1016/S0006-3495(92)81926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Jørgensen K., Baekmark T. R., Mouritsen O. G. Indirect evidence for lipid-domain formation in the transition region of phospholipid bilayers by two-probe fluorescence energy transfer. Biophys J. 1996 Aug;71(2):554–560. doi: 10.1016/S0006-3495(96)79279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegler J., Möhwald H. Elastic interactions of photosynthetic reaction center proteins affecting phase transitions and protein distributions. Biophys J. 1986 Jun;49(6):1111–1118. doi: 10.1016/S0006-3495(86)83740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe E. S. Induction of lateral phase separations in binary lipid mixtures by alcohol. Biochemistry. 1987 Jan 13;26(1):46–51. doi: 10.1021/bi00375a007. [DOI] [PubMed] [Google Scholar]
- Rytömaa M., Kinnunen P. K. Dissociation of cytochrome c from liposomes by histone H1. Comparison with basic peptides. Biochemistry. 1996 Apr 9;35(14):4529–4539. doi: 10.1021/bi952413w. [DOI] [PubMed] [Google Scholar]
- Rytömaa M., Kinnunen P. K. Reversibility of the binding of cytochrome c to liposomes. Implications for lipid-protein interactions. J Biol Chem. 1995 Feb 17;270(7):3197–3202. doi: 10.1074/jbc.270.7.3197. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Deuterium magnetic resonance study of phase equilibria and membrane thickness in binary phospholipid mixed bilayers. Biochemistry. 1992 Sep 8;31(35):8258–8268. doi: 10.1021/bi00150a020. [DOI] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Sperotto M. M., Mouritsen O. G. Monte Carlo simulation studies of lipid order parameter profiles near integral membrane proteins. Biophys J. 1991 Feb;59(2):261–270. doi: 10.1016/S0006-3495(91)82219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Sun J., Wu J., Carrasco N., Kaback H. R. Identification of the epitope for monoclonal antibody 4B1 which uncouples lactose and proton translocation in the lactose permease of Escherichia coli. Biochemistry. 1996 Jan 23;35(3):990–998. doi: 10.1021/bi952166w. [DOI] [PubMed] [Google Scholar]
- Tang D., Chong P. L. E/M dips. Evidence for lipids regularly distributed into hexagonal super-lattices in pyrene-PC/DMPC binary mixtures at specific concentrations. Biophys J. 1992 Oct;63(4):903–910. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Wieb van der Meer B., Chen S. Y. Evidence for a regular distribution of cholesterol in phospholipid bilayers from diphenylhexatriene fluorescence. Biophys J. 1995 May;68(5):1944–1951. doi: 10.1016/S0006-3495(95)80371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Thilo L., Träuble H., Overath P. Mechanistic interpretation of the influence of lipid phase transitions on transport functions. Biochemistry. 1977 Apr 5;16(7):1283–1290. doi: 10.1021/bi00626a007. [DOI] [PubMed] [Google Scholar]
- Thurmond R. L., Niemi A. R., Lindblom G., Wieslander A., Rilfors L. Membrane thickness and molecular ordering in Acholeplasma laidlawii strain A studied by 2H NMR spectroscopy. Biochemistry. 1994 Nov 15;33(45):13178–13188. doi: 10.1021/bi00249a004. [DOI] [PubMed] [Google Scholar]
- Verbist J., Gadella T. W., Jr, Raeymaekers L., Wuytack F., Wirtz K. W., Casteels R. Phosphoinositide-protein interactions of the plasma-membrane Ca2(+)-transport ATPase as revealed by fluorescence energy transfer. Biochim Biophys Acta. 1991 Mar 18;1063(1):1–6. doi: 10.1016/0005-2736(91)90345-9. [DOI] [PubMed] [Google Scholar]
- Viitanen P., Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Abele U., Weckesser J., Welte W., Schiltz E., Schulz G. E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991 Dec 13;254(5038):1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- Welti R., Glaser M. Lipid domains in model and biological membranes. Chem Phys Lipids. 1994 Sep 6;73(1-2):121–137. doi: 10.1016/0009-3084(94)90178-3. [DOI] [PubMed] [Google Scholar]
- Wiedmann T. S., Pates R. D., Beach J. M., Salmon A., Brown M. F. Lipid-protein interactions mediate the photochemical function of rhodopsin. Biochemistry. 1988 Aug 23;27(17):6469–6474. doi: 10.1021/bi00417a041. [DOI] [PubMed] [Google Scholar]
- Wiener M. C., Suter R. M., Nagle J. F. Structure of the fully hydrated gel phase of dipalmitoylphosphatidylcholine. Biophys J. 1989 Feb;55(2):315–325. doi: 10.1016/S0006-3495(89)82807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Frillingos S., Kaback H. R. Dynamics of lactose permease of Escherichia coli determined by site-directed chemical labeling and fluorescence spectroscopy. Biochemistry. 1995 Jul 4;34(26):8257–8263. doi: 10.1021/bi00026a007. [DOI] [PubMed] [Google Scholar]
- Yang L., Glaser M. Membrane domains containing phosphatidylserine and substrate can be important for the activation of protein kinase C. Biochemistry. 1995 Feb 7;34(5):1500–1506. doi: 10.1021/bi00005a005. [DOI] [PubMed] [Google Scholar]
- Zhang Y. P., Lewis R. N., Hodges R. S., McElhaney R. N. Interaction of a peptide model of a hydrophobic transmembrane alpha-helical segment of a membrane protein with phosphatidylcholine bilayers: differential scanning calorimetric and FTIR spectroscopic studies. Biochemistry. 1992 Nov 24;31(46):11579–11588. doi: 10.1021/bi00161a042. [DOI] [PubMed] [Google Scholar]
- van Iwaarden P. R., Pastore J. C., Konings W. N., Kaback H. R. Construction of a functional lactose permease devoid of cysteine residues. Biochemistry. 1991 Oct 8;30(40):9595–9600. doi: 10.1021/bi00104a005. [DOI] [PubMed] [Google Scholar]


