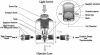
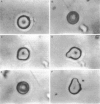
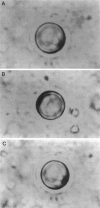
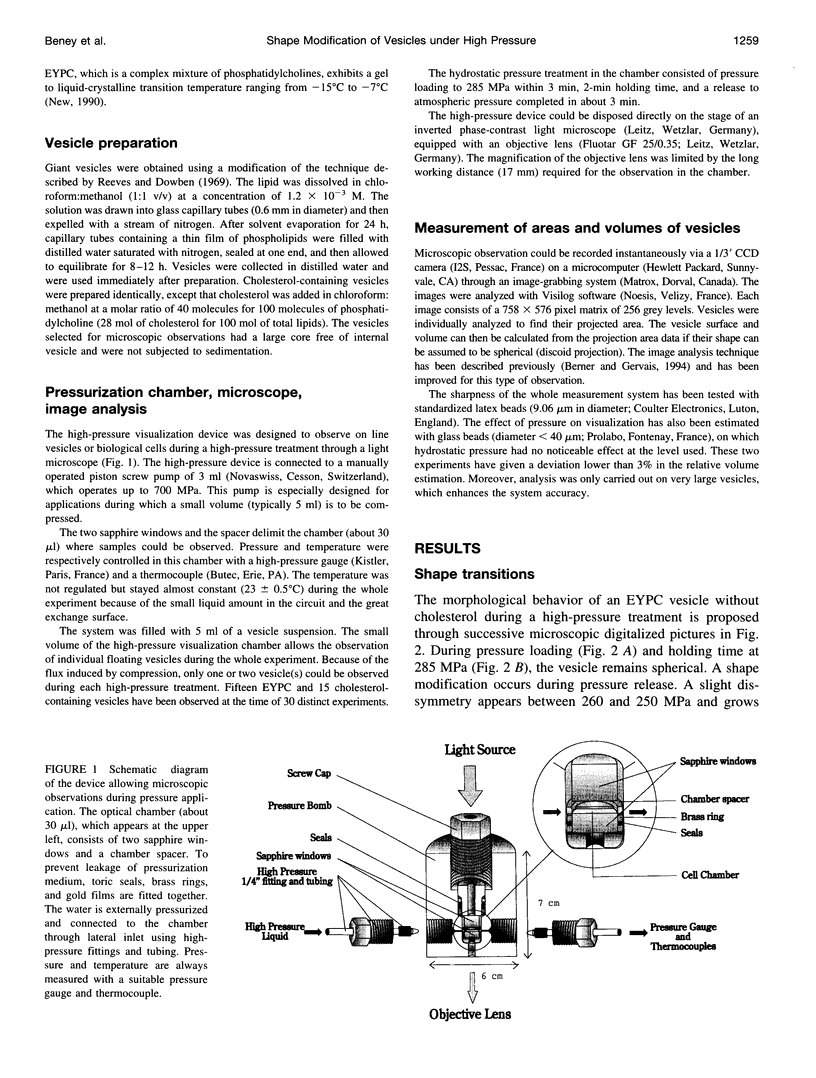
Abstract
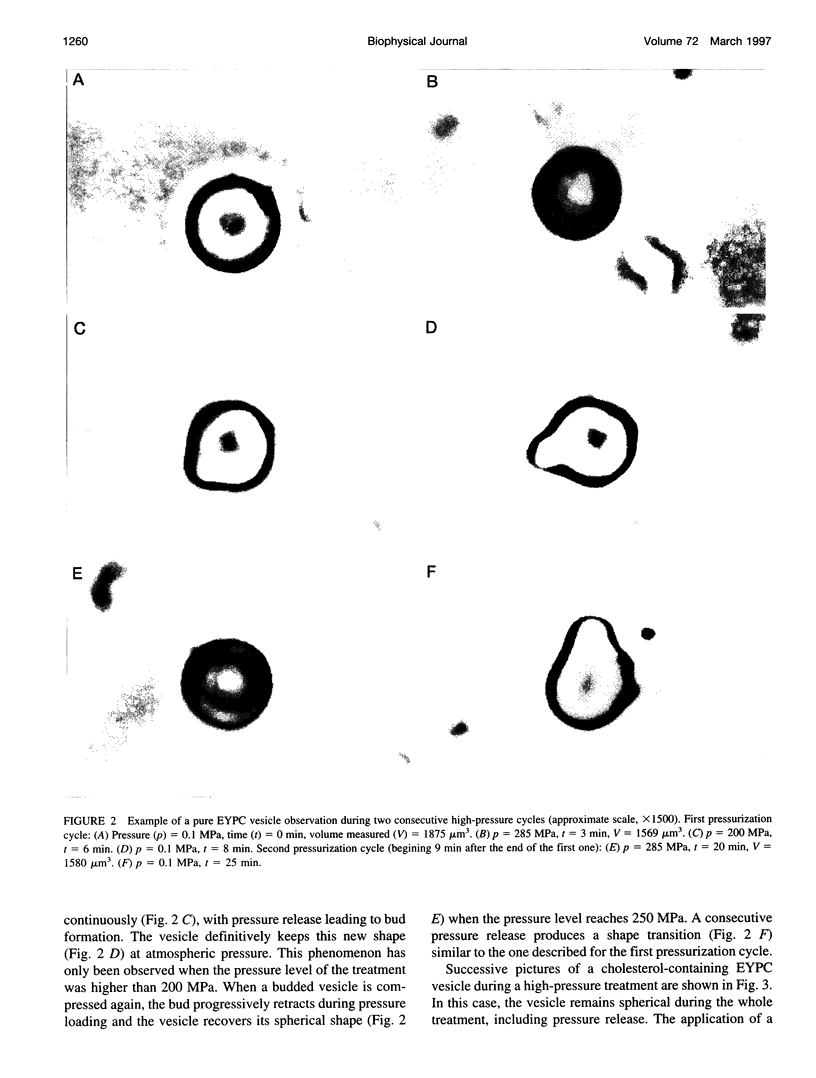
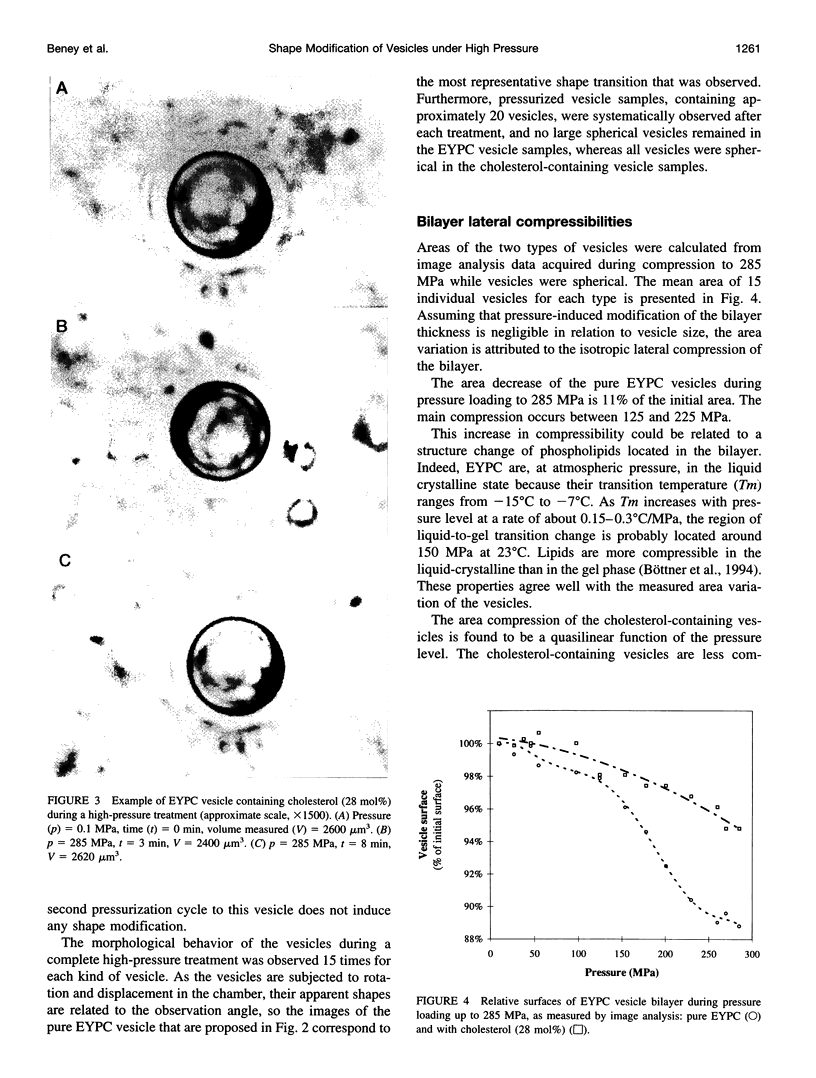
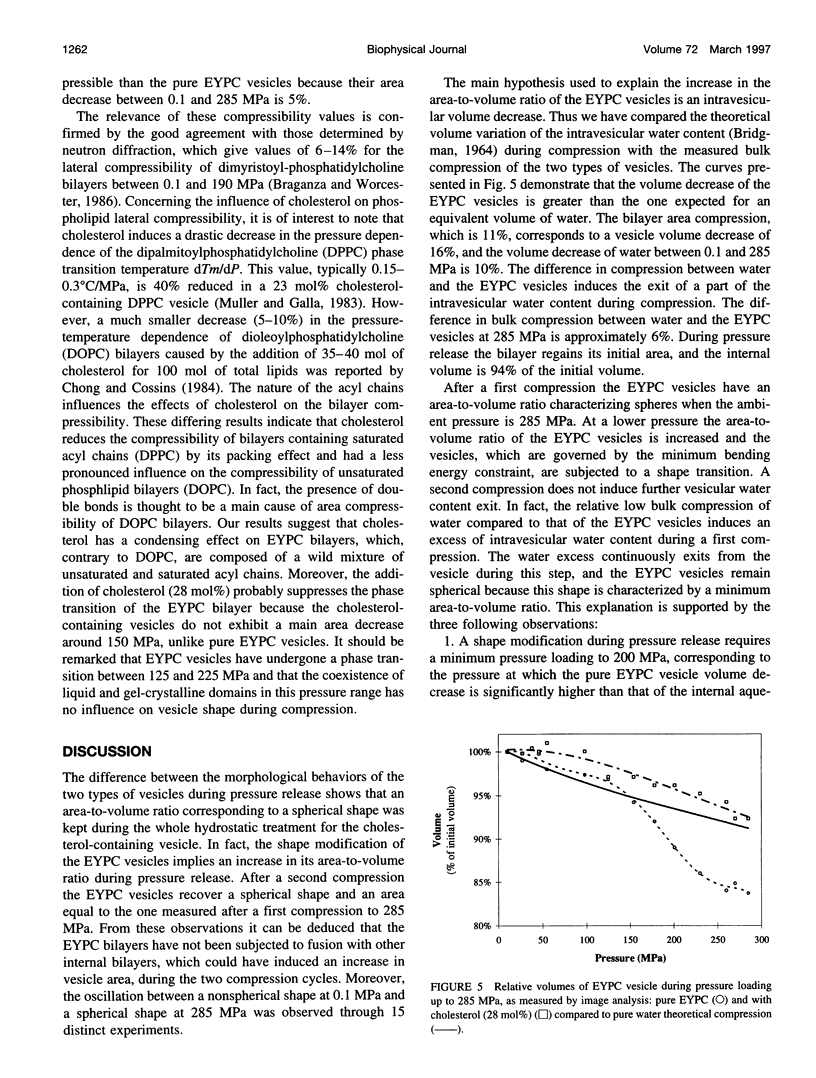
Giant vesicles composed of pure egg yolk phosphatidylcholine (EYPC) or containing cholesterol (28 mol%) have been studied during a high hydrostatic pressure treatment to 285 MPa by microscopic observation. During pressure loading the vesicles remain spherical. A shape transition consisting of budding only occurs on the cholesterol-free vesicles during pressure release. The decrease in the volume delimited by the pure EYPC bilayer between 0.1 and 285 MPa was found to be 16% of its initial volume, whereas the bulk compression of water in this pressure range is only 10%. So the compression at 285 MPa induced a water exit from the pure EYPC vesicle. The shape transition of the EYPC vesicle during pressure release is attributed to an increase in its area-to-volume ratio caused by the loss of its water content during compression. Because bulk compression of the cholesterol-containing vesicle is close to that of water, no water transfer would be induced across the bilayer and the vesicle remains spherical during the pressure release.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braganza L. F., Worcester D. L. Structural changes in lipid bilayers and biological membranes caused hydrostatic pressure. Biochemistry. 1986 Nov 18;25(23):7484–7488. doi: 10.1021/bi00371a034. [DOI] [PubMed] [Google Scholar]
- Chong P. L., Cossins A. R. Interacting effects of temperature, pressure and cholesterol content upon the molecular order of dioleoylphosphatidylcholine vesicles. Biochim Biophys Acta. 1984 May 16;772(2):197–201. doi: 10.1016/0005-2736(84)90044-0. [DOI] [PubMed] [Google Scholar]
- Döbereiner H. G., Käs J., Noppl D., Sprenger I., Sackmann E. Budding and fission of vesicles. Biophys J. 1993 Oct;65(4):1396–1403. doi: 10.1016/S0006-3495(93)81203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülicher F, Lipowsky R. Domain-induced budding of vesicles. Phys Rev Lett. 1993 May 10;70(19):2964–2967. doi: 10.1103/PhysRevLett.70.2964. [DOI] [PubMed] [Google Scholar]
- Käs J., Sackmann E. Shape transitions and shape stability of giant phospholipid vesicles in pure water induced by area-to-volume changes. Biophys J. 1991 Oct;60(4):825–844. doi: 10.1016/S0006-3495(91)82117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald A. G. The effects of pressure on the molecular structure and physiological functions of cell membranes. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 7;304(1118):47–68. doi: 10.1098/rstb.1984.0008. [DOI] [PubMed] [Google Scholar]
- Mui B. L., Döbereiner H. G., Madden T. D., Cullis P. R. Influence of transbilayer area asymmetry on the morphology of large unilamellar vesicles. Biophys J. 1995 Sep;69(3):930–941. doi: 10.1016/S0006-3495(95)79967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. J., Galla H. J. Pressure variation of the lateral diffusion in lipid bilayer membranes. Biochim Biophys Acta. 1983 Sep 7;733(2):291–294. doi: 10.1016/0005-2736(83)90535-7. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Reis O., Winter R., Zerda T. W. The effect of high external pressure on DPPC-cholesterol multilamellar vesicles: a pressure-tuning Fourier transform infrared spectroscopy study. Biochim Biophys Acta. 1996 Feb 21;1279(1):5–16. doi: 10.1016/0005-2736(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Seifert U, Berndl K, Lipowsky R. Shape transformations of vesicles: Phase diagram for spontaneous- curvature and bilayer-coupling models. Phys Rev A. 1991 Jul 15;44(2):1182–1202. doi: 10.1103/physreva.44.1182. [DOI] [PubMed] [Google Scholar]
- Seifert U. Curvature-induced lateral phase segregation in two-component vesicles. Phys Rev Lett. 1993 Mar 1;70(9):1335–1338. doi: 10.1103/PhysRevLett.70.1335. [DOI] [PubMed] [Google Scholar]
- Svetina S., Zeks B. Membrane bending energy and shape determination of phospholipid vesicles and red blood cells. Eur Biophys J. 1989;17(2):101–111. doi: 10.1007/BF00257107. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Siminovitch D. J., Mantsch H. H. Structure and properties of model membranes: new knowledge from high-pressure vibrational spectroscopy. Biochim Biophys Acta. 1988 Feb 24;947(1):139–171. doi: 10.1016/0304-4157(88)90023-8. [DOI] [PubMed] [Google Scholar]