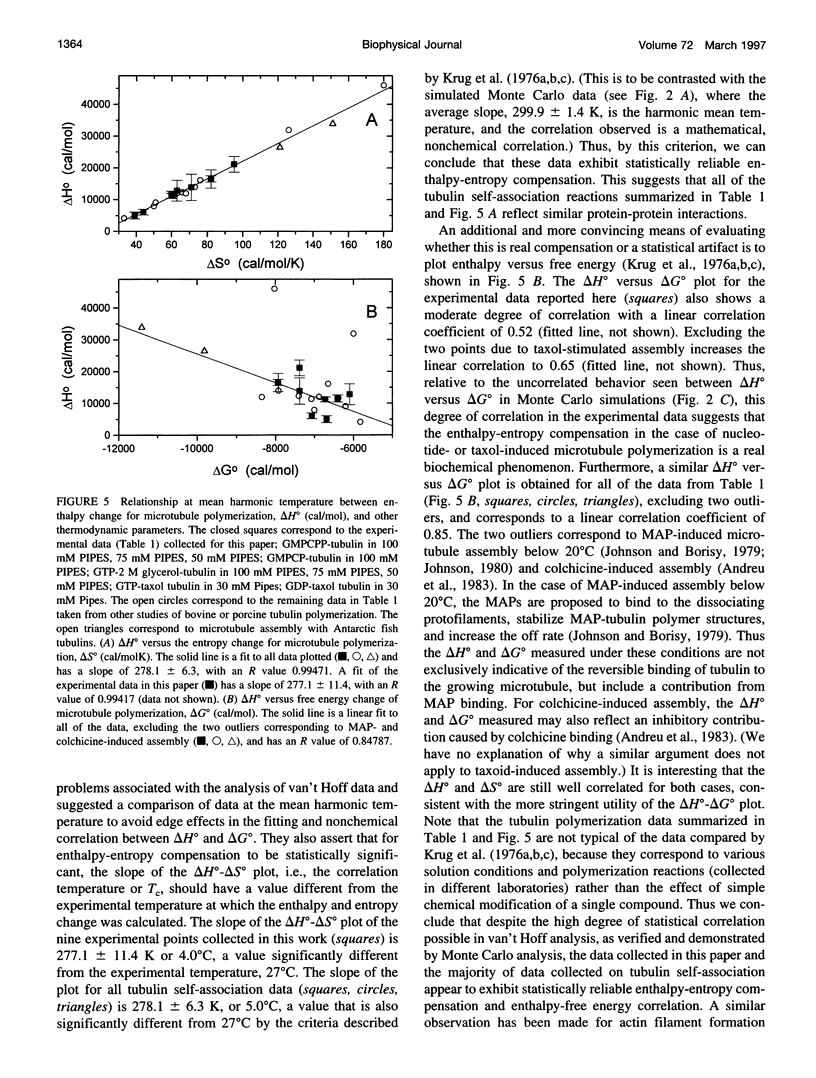
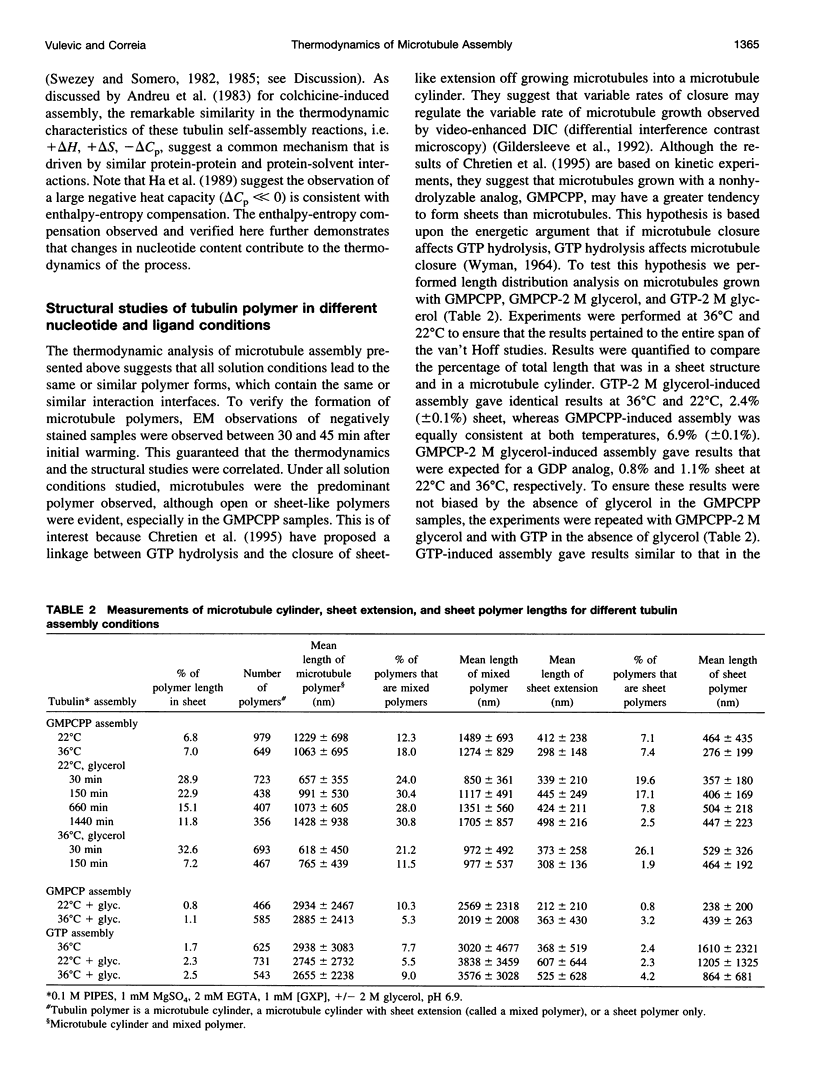
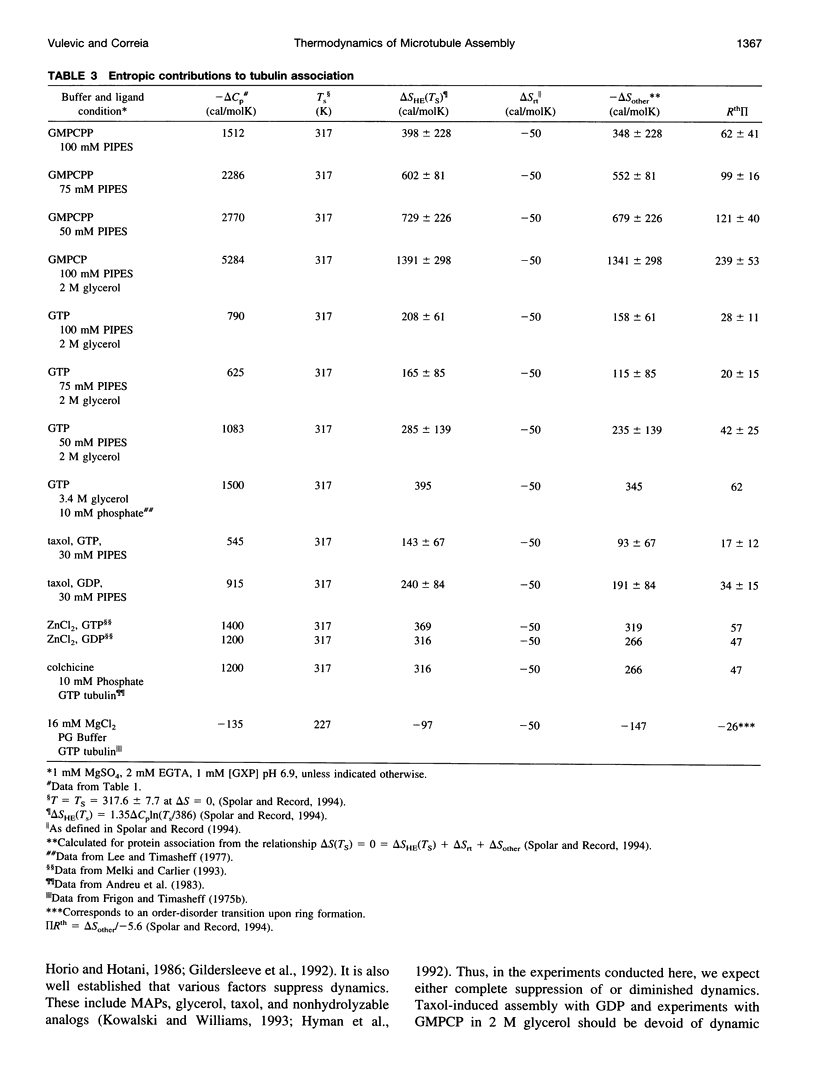
Abstract
Different models have been proposed that link the tubulin heterodimer nucleotide content and the role of GTP hydrolysis with microtubule assembly and dynamics. Here we compare the thermodynamics of microtubule assembly as a function of nucleotide content by van't Hoff analysis. The thermodynamic parameters of tubulin assembly in 30-100 mM piperazine-N,N'-bis(2-ethanesulfonic acid), 1 mM MgSO4, 2 mM EGTA, pH 6.9, in the presence of a weakly hydrolyzable analog, GMPCPP, the dinucleotide analog GMPCP plus 2 M glycerol, and GTP plus 2 M glycerol were obtained together with data for taxol-GTP/GDP tubulin assembly (GMPCPP and GMPCP are the GTP and GDP nucleotide analogs where the alpha beta oxygen has been replaced by a methylene, -CH2-). All of the processes studied are characterized by a positive enthalpy, a positive entropy, and a large, negative heat capacity change. GMPCP-induced assembly has the largest negative heat capacity change and GMPCPP has the second largest, whereas GTP/2 M glycerol- and taxol-induced assembly have more positive values, respectively. A large, negative heat capacity is most consistent with the burial of water-accessible hydrophobic surface area, which gives rise to the release of bound water. The heat capacity changes observed with GTP/2 M glycerol-induced and with taxol-induced assembly are very similar, -790 +/- 190 cal/mol/k, and correspond to the burial of 3330 +/- 820 A2 of nonpolar surface area. This value is shown to be very similar to an estimate of the buried nonpolar surface in a reconstructed microtubule lattice. Polymerization data from GMPCP- and GMPCPP-induced assembly are consistent with buried nonpolar surface areas that are 3 and 6 times larger. A linear enthalpy-entropy and enthalpy-free energy plot for tubulin polymerization reactions verifies that enthalpy-entropy compensation for this system is based upon true biochemical correlation, most likely corresponding to a dominant hydrophobic effect. Entropy analysis suggests that assembly with GTP/2 M glycerol and with taxol is consistent with conformational rearrangements in 3-6% of the total amino acids in the heterodimer. In addition, taxol binding contributes to the thermodynamics of the overall process by reducing the delta H degree and delta S degree for microtubule assembly. In the presence of GMPCPP or GMPCP, tubulin subunits associate with extensive conformational rearrangement, corresponding to 10% and 26% of the total amino acids in the heterodimer, respectively, which gives rise to a large loss of configurational entropy. An alternative, and probably preferable, interpretation of these data is that, especially with GMPCP-tubulin, additional isomerization or protonation events are induced by the presence of the methylene moiety and linked to microtubule assembly. Structural analysis shows that GTP hydrolysis is not required for sheet closure into a microtubule cylinder, but only increases the probability of this event occurring. Sheet extensions and sheet polymers appear to have a similar average length under various conditions, suggesting that the minimum cooperative unit for closure of sheets into a microtubule cylinder is approximately 400 nm long. Because of their low level of occurrence, sheets are not expected to significantly affect the thermodynamics of assembly.
Full text
PDF


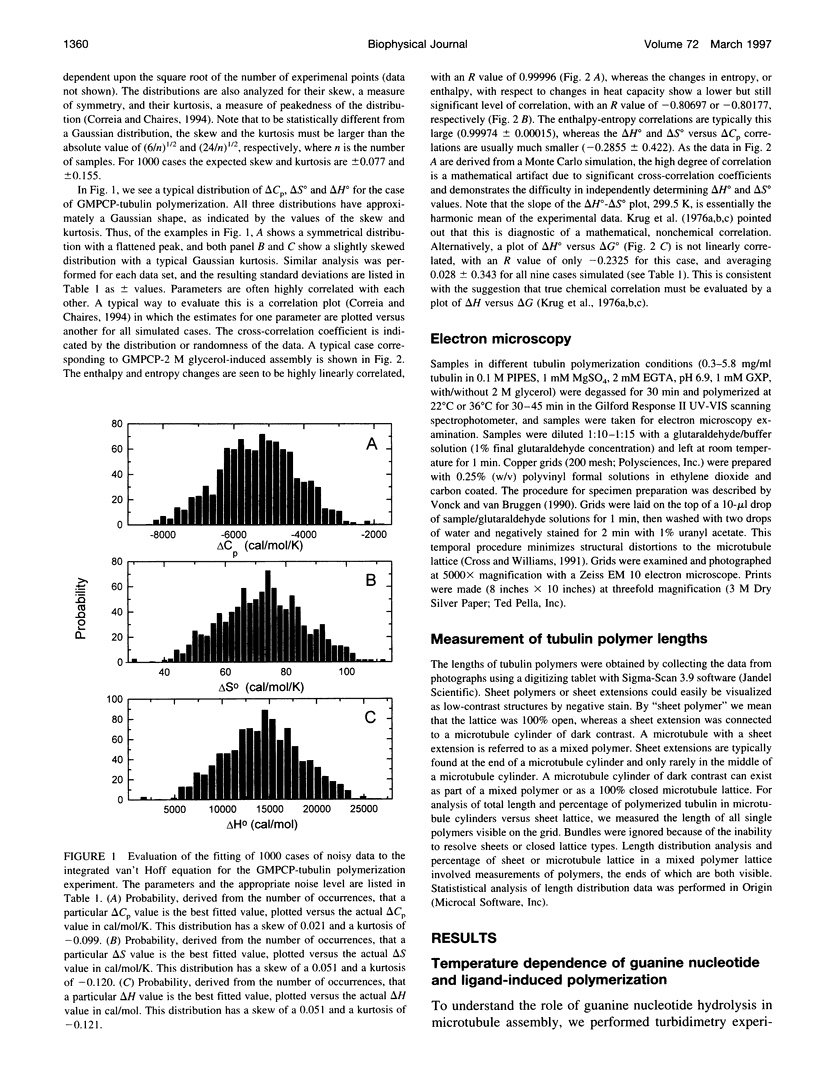
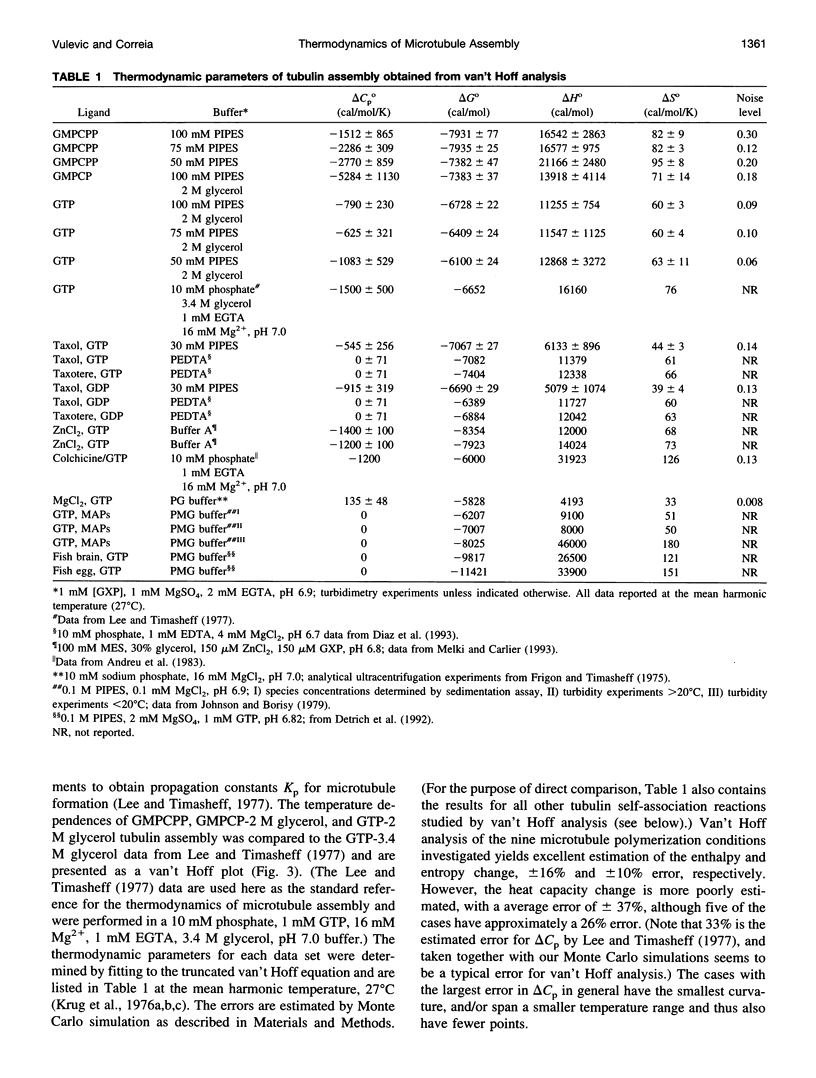
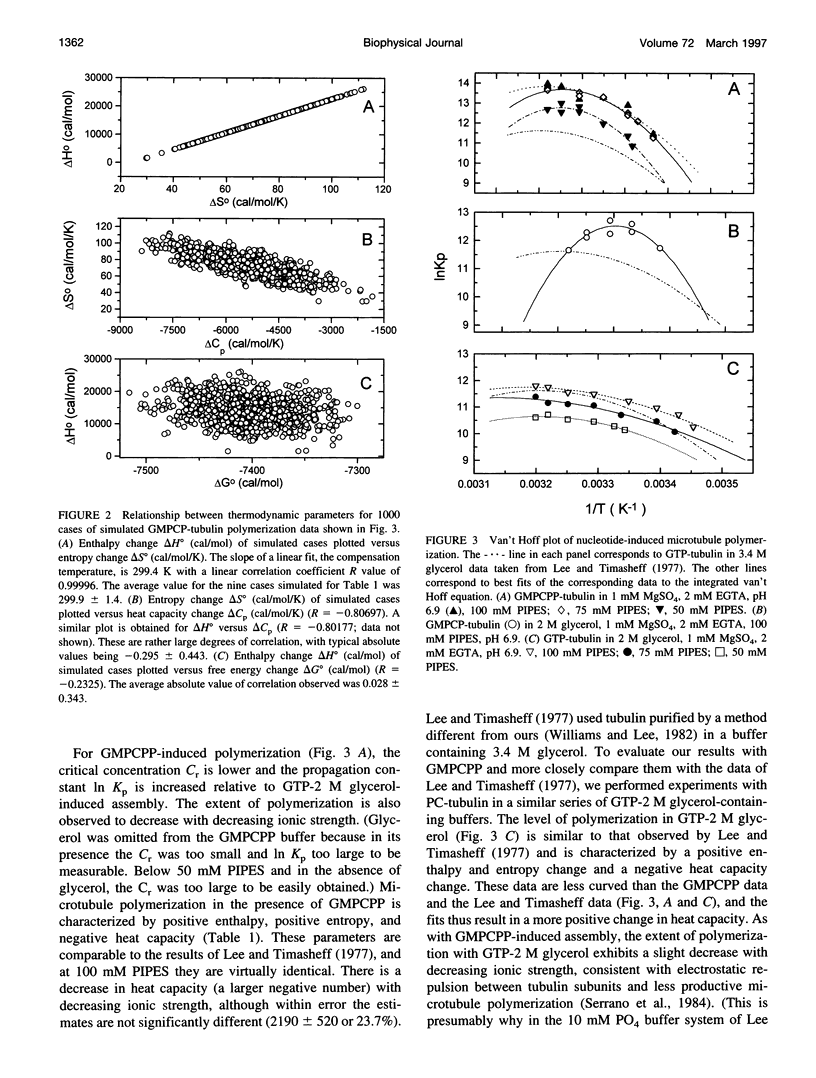
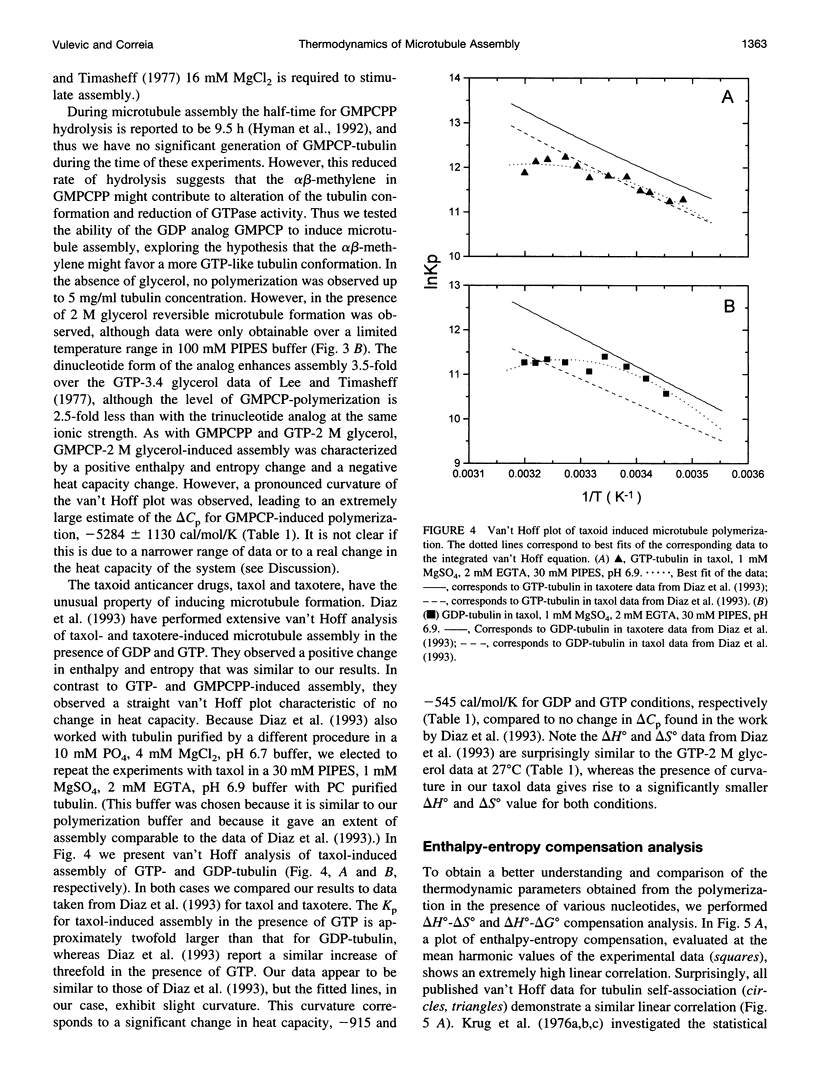









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu J. M., Wagenknecht T., Timasheff S. N. Polymerization of the tubulin-colchicine complex: relation to microtubule assembly. Biochemistry. 1983 Mar 29;22(7):1556–1566. doi: 10.1021/bi00276a006. [DOI] [PubMed] [Google Scholar]
- Audenaert R., Heremans L., Heremans K., Engelborghs Y. Secondary structure analysis of tubulin and microtubules with Raman spectroscopy. Biochim Biophys Acta. 1989 Jun 13;996(1-2):110–115. doi: 10.1016/0167-4838(89)90102-7. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L. Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8069–8072. doi: 10.1073/pnas.83.21.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S. A., Velicelebi G., Sutherland J. W., Sturtevant J. M. Observation of an exothermic process associated with the in vitro polymerization of brain tubulin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4425–4429. doi: 10.1073/pnas.77.8.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne B. J. Interpretation of the light scattering from long rods. J Mol Biol. 1974 Nov 15;89(4):755–758. doi: 10.1016/0022-2836(74)90049-7. [DOI] [PubMed] [Google Scholar]
- Caplow M. Microtubule dynamics. Curr Opin Cell Biol. 1992 Feb;4(1):58–65. doi: 10.1016/0955-0674(92)90059-l. [DOI] [PubMed] [Google Scholar]
- Caplow M., Ruhlen R. L., Shanks J. The free energy for hydrolysis of a microtubule-bound nucleotide triphosphate is near zero: all of the free energy for hydrolysis is stored in the microtubule lattice. J Cell Biol. 1994 Nov;127(3):779–788. doi: 10.1083/jcb.127.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplow M., Shanks J. Evidence that a single monolayer tubulin-GTP cap is both necessary and sufficient to stabilize microtubules. Mol Biol Cell. 1996 Apr;7(4):663–675. doi: 10.1091/mbc.7.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Didry D., Pantaloni D. Microtubule elongation and guanosine 5'-triphosphate hydrolysis. Role of guanine nucleotides in microtubule dynamics. Biochemistry. 1987 Jul 14;26(14):4428–4437. doi: 10.1021/bi00388a036. [DOI] [PubMed] [Google Scholar]
- Chrétien D., Fuller S. D., Karsenti E. Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. J Cell Biol. 1995 Jun;129(5):1311–1328. doi: 10.1083/jcb.129.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia J. J., Baty L. T., Williams R. C., Jr Mg2+ dependence of guanine nucleotide binding to tubulin. J Biol Chem. 1987 Dec 25;262(36):17278–17284. [PubMed] [Google Scholar]
- Correia J. J., Chaires J. B. Analysis of drug-DNA binding isotherms: a Monte Carlo approach. Methods Enzymol. 1994;240:593–614. doi: 10.1016/s0076-6879(94)40065-2. [DOI] [PubMed] [Google Scholar]
- Correia J. J., Williams R. C., Jr Mechanisms of assembly and disassembly of microtubules. Annu Rev Biophys Bioeng. 1983;12:211–235. doi: 10.1146/annurev.bb.12.060183.001235. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Williams R. C., Jr Kinky microtubules: bending and breaking induced by fixation in vitro with glutaraldehyde and formaldehyde. Cell Motil Cytoskeleton. 1991;20(4):272–278. doi: 10.1002/cm.970200403. [DOI] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Fitzgerald T. J., Dinsmore J. H., Marchese-Ragona S. P. Brain and egg tubulins from antarctic fishes are functionally and structurally distinct. J Biol Chem. 1992 Sep 15;267(26):18766–18775. [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Jordan M. A., Wilson L., Williams R. C., Jr Mechanism of microtubule assembly. Changes in polymer structure and organization during assembly of sea urchin egg tubulin. J Biol Chem. 1985 Aug 5;260(16):9479–9490. [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Williams R. C. Reversible dissociation of the alpha beta dimer of tubulin from bovine brain. Biochemistry. 1978 Sep 19;17(19):3900–3907. doi: 10.1021/bi00612a002. [DOI] [PubMed] [Google Scholar]
- Diaz J. F., Andreu J. M., Diakun G., Towns-Andrews E., Bordas J. Structural intermediates in the assembly of taxoid-induced microtubules and GDP-tubulin double rings: time-resolved X-ray scattering. Biophys J. 1996 May;70(5):2408–2420. doi: 10.1016/S0006-3495(96)79809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel D. N., Kirschner M. W. The minimum GTP cap required to stabilize microtubules. Curr Biol. 1994 Dec 1;4(12):1053–1061. doi: 10.1016/s0960-9822(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Dunitz J. D. Win some, lose some: enthalpy-entropy compensation in weak intermolecular interactions. Chem Biol. 1995 Nov;2(11):709–712. doi: 10.1016/1074-5521(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Dye R. B., Williams R. C., Jr Assembly of microtubules from tubulin bearing the nonhydrolyzable guanosine triphosphate analogue GMPPCP [guanylyl 5'-(beta, gamma-methylenediphosphonate)]: variability of growth rates and the hydrolysis of GTP. Biochemistry. 1996 Nov 12;35(45):14331–14339. doi: 10.1021/bi961070e. [DOI] [PubMed] [Google Scholar]
- Díaz J. F., Menéndez M., Andreu J. M. Thermodynamics of ligand-induced assembly of tubulin. Biochemistry. 1993 Sep 28;32(38):10067–10077. doi: 10.1021/bi00089a023. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Anusiem A. C., Biltonen R. L. Enthalpy-entropy compensation and heat capacity changes for protein-ligand interactions: general thermodynamic models and data for the binding of nucleotides to ribonuclease A. Biochemistry. 1983 Aug 2;22(16):3884–3896. doi: 10.1021/bi00285a025. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Co-operativity in protein-protein association. The structure and stability of the actin filament. J Mol Biol. 1989 Apr 5;206(3):465–474. doi: 10.1016/0022-2836(89)90494-4. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Pantaloni D. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophys J. 1981 May;34(2):293–309. doi: 10.1016/S0006-3495(81)84850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. I. Stoichiometry. Biochemistry. 1975 Oct 21;14(21):4559–4566. doi: 10.1021/bi00692a001. [DOI] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. II. Thermodynamics. Biochemistry. 1975 Oct 21;14(21):4567–4573. doi: 10.1021/bi00692a002. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Geahlen R. L., Haley B. E. Use of a GTP photoaffinity probe to resolve aspects of the mechanism of tubulin polymerization. J Biol Chem. 1979 Dec 10;254(23):11982–11987. [PubMed] [Google Scholar]
- Gildersleeve R. F., Cross A. R., Cullen K. E., Fagen A. P., Williams R. C., Jr Microtubules grow and shorten at intrinsically variable rates. J Biol Chem. 1992 Apr 25;267(12):7995–8006. [PubMed] [Google Scholar]
- Ha J. H., Spolar R. S., Record M. T., Jr Role of the hydrophobic effect in stability of site-specific protein-DNA complexes. J Mol Biol. 1989 Oct 20;209(4):801–816. doi: 10.1016/0022-2836(89)90608-6. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Gorbunoff M. J., Price B., Timasheff S. N. Heat capacity microcalorimetry of the in vitro reconstitution of calf brain microtubules. Biochemistry. 1979 Jul 10;18(14):3084–3089. doi: 10.1021/bi00581a027. [DOI] [PubMed] [Google Scholar]
- Horio T., Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986 Jun 5;321(6070):605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Horton N., Lewis M. Calculation of the free energy of association for protein complexes. Protein Sci. 1992 Jan;1(1):169–181. doi: 10.1002/pro.5560010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard W. D., Timasheff S. N. GDP state of tubulin: stabilization of double rings. Biochemistry. 1986 Dec 16;25(25):8292–8300. doi: 10.1021/bi00373a025. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Chrétien D., Arnal I., Wade R. H. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(alpha,beta)-methylene-diphosphonate. J Cell Biol. 1995 Jan;128(1-2):117–125. doi: 10.1083/jcb.128.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A. A., Salser S., Drechsel D. N., Unwin N., Mitchison T. J. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol Biol Cell. 1992 Oct;3(10):1155–1167. doi: 10.1091/mbc.3.10.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A., Borisy G. G. Thermodynamic analysis of microtubule self-assembly in vitro. J Mol Biol. 1979 Sep 15;133(2):199–216. doi: 10.1016/0022-2836(79)90530-8. [DOI] [PubMed] [Google Scholar]
- Johnson K. A. Thermodynamics of microtubule assembly. Biophys J. 1980 Oct;32(1):443–445. doi: 10.1016/S0006-3495(80)84975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W. Microtubule assembly and nucleation. Int Rev Cytol. 1978;54:1–71. doi: 10.1016/s0074-7696(08)60164-3. [DOI] [PubMed] [Google Scholar]
- Kowalski R. J., Williams R. C., Jr Microtubule-associated protein 2 alters the dynamic properties of microtubule assembly and disassembly. J Biol Chem. 1993 May 5;268(13):9847–9855. [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. In vitro reconstitution of calf brain microtubules: effects of solution variables. Biochemistry. 1977 Apr 19;16(8):1754–1764. doi: 10.1021/bi00627a037. [DOI] [PubMed] [Google Scholar]
- Livingstone J. R., Spolar R. S., Record M. T., Jr Contribution to the thermodynamics of protein folding from the reduction in water-accessible nonpolar surface area. Biochemistry. 1991 Apr 30;30(17):4237–4244. doi: 10.1021/bi00231a019. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E., Milligan R. A. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J Cell Biol. 1991 Sep;114(5):977–991. doi: 10.1083/jcb.114.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Martin S. R., Schilstra M. J., Bayley P. M. Dynamic instability of microtubules: Monte Carlo simulation and application to different types of microtubule lattice. Biophys J. 1993 Aug;65(2):578–596. doi: 10.1016/S0006-3495(93)81091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R., Carlier M. F., Pantaloni D., Timasheff S. N. Cold depolymerization of microtubules to double rings: geometric stabilization of assemblies. Biochemistry. 1989 Nov 14;28(23):9143–9152. doi: 10.1021/bi00449a028. [DOI] [PubMed] [Google Scholar]
- Melki R., Carlier M. F. Thermodynamics of tubulin polymerization into zinc sheets: assembly is not regulated by GTP hydrolysis. Biochemistry. 1993 Apr 6;32(13):3405–3413. doi: 10.1021/bi00064a026. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Privalov P. L., Gill S. J. Common features of protein unfolding and dissolution of hydrophobic compounds. Science. 1990 Feb 2;247(4942):559–561. doi: 10.1126/science.2300815. [DOI] [PubMed] [Google Scholar]
- Nogales E., Wolf S. G., Khan I. A., Ludueña R. F., Downing K. H. Structure of tubulin at 6.5 A and location of the taxol-binding site. Nature. 1995 Jun 1;375(6530):424–427. doi: 10.1038/375424a0. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry. 1981 May 26;20(11):3096–3102. doi: 10.1021/bi00514a017. [DOI] [PubMed] [Google Scholar]
- Seckler R., Wu G. M., Timasheff S. N. Interactions of tubulin with guanylyl-(beta-gamma-methylene)diphosphonate. Formation and assembly of a stoichiometric complex. J Biol Chem. 1990 May 5;265(13):7655–7661. [PubMed] [Google Scholar]
- Serrano L., de la Torre J., Maccioni R. B., Avila J. Involvement of the carboxyl-terminal domain of tubulin in the regulation of its assembly. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5989–5993. doi: 10.1073/pnas.81.19.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. R., Salmon E. D. The structure of microtubule ends during the elongation and shortening phases of dynamic instability examined by negative-stain electron microscopy. J Cell Sci. 1990 Aug;96(Pt 4):571–582. doi: 10.1242/jcs.96.4.571. [DOI] [PubMed] [Google Scholar]
- Spolar R. S., Ha J. H., Record M. T., Jr Hydrophobic effect in protein folding and other noncovalent processes involving proteins. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8382–8385. doi: 10.1073/pnas.86.21.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolar R. S., Livingstone J. R., Record M. T., Jr Use of liquid hydrocarbon and amide transfer data to estimate contributions to thermodynamic functions of protein folding from the removal of nonpolar and polar surface from water. Biochemistry. 1992 Apr 28;31(16):3947–3955. doi: 10.1021/bi00131a009. [DOI] [PubMed] [Google Scholar]
- Spolar R. S., Record M. T., Jr Coupling of local folding to site-specific binding of proteins to DNA. Science. 1994 Feb 11;263(5148):777–784. doi: 10.1126/science.8303294. [DOI] [PubMed] [Google Scholar]
- Stewart R. J., Farrell K. W., Wilson L. Role of GTP hydrolysis in microtubule polymerization: evidence for a coupled hydrolysis mechanism. Biochemistry. 1990 Jul 10;29(27):6489–6498. doi: 10.1021/bi00479a022. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. M. Heat capacity and entropy changes in processes involving proteins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. W. Disagreement between calorimetric and van't Hoff enthalpies of assembly of protein supramolecular structures. Proc Natl Acad Sci U S A. 1977 May;74(5):2002–2006. doi: 10.1073/pnas.74.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. W., Sturtevant J. M. Calorimetric studies of the in vitro polymerization of brain tubulin. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3565–3569. doi: 10.1073/pnas.73.10.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swezey R. R., Somero G. N. Polymerization thermodynamics and structural stabilities of skeletal muscle actins from vertebrates adapted to different temperatures and hydrostatic pressures. Biochemistry. 1982 Aug 31;21(18):4496–4503. doi: 10.1021/bi00261a047. [DOI] [PubMed] [Google Scholar]
- Swezey R. R., Somero G. N. Pressure effects on actin self-assembly: interspecific differences in the equilibrium and kinetics of the G to F transformation. Biochemistry. 1985 Feb 12;24(4):852–860. doi: 10.1021/bi00325a007. [DOI] [PubMed] [Google Scholar]
- Timasheff S. N., Grisham L. M. In vitro assembly of cytoplasmic microtubules. Annu Rev Biochem. 1980;49:565–591. doi: 10.1146/annurev.bi.49.070180.003025. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Coppin C. M., Malik F., Kull F. J., Milligan R. A. Tubulin GTP hydrolysis influences the structure, mechanical properties, and kinesin-driven transport of microtubules. J Biol Chem. 1994 Sep 23;269(38):23769–23775. [PubMed] [Google Scholar]
- Venier P., Maggs A. C., Carlier M. F., Pantaloni D. Analysis of microtubule rigidity using hydrodynamic flow and thermal fluctuations. J Biol Chem. 1994 May 6;269(18):13353–13360. [PubMed] [Google Scholar]
- Vonck J., van Bruggen E. F. Electron microscopy and image analysis of two-dimensional crystals and single molecules of alcohol oxidase from Hansenula polymorpha. Biochim Biophys Acta. 1990 Mar 29;1038(1):74–79. doi: 10.1016/0167-4838(90)90012-5. [DOI] [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lee J. C. Preparation of tubulin from brain. Methods Enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]


