Abstract
We present our results on the manipulation of individual viruses using an advanced interface for atomic force microscopes (AFMs). We show that the viruses can be dissected, rotated, and translated with great facility. We interpret the behavior of tobacco mosaic virus with a mechanical model that makes explicit the competition between sample-substrate lateral friction and the flexural rigidity of the manipulated object. The manipulation behavior of tobacco mosaic virus on graphite is shown to be consistent with values of lateral friction observed on similar interfaces and the flexural rigidity expected for macromolecular assemblies. The ability to manipulate individual samples broadens the scope of possible studies by providing a means for positioning samples at specific binding sites or predefined measuring devices. The mechanical model provides a framework for interpreting quantitative measurements of virus binding and mechanical properties and for understanding the constraints on the successful, nondestructive AFM manipulation of delicate samples.
Full text
PDF


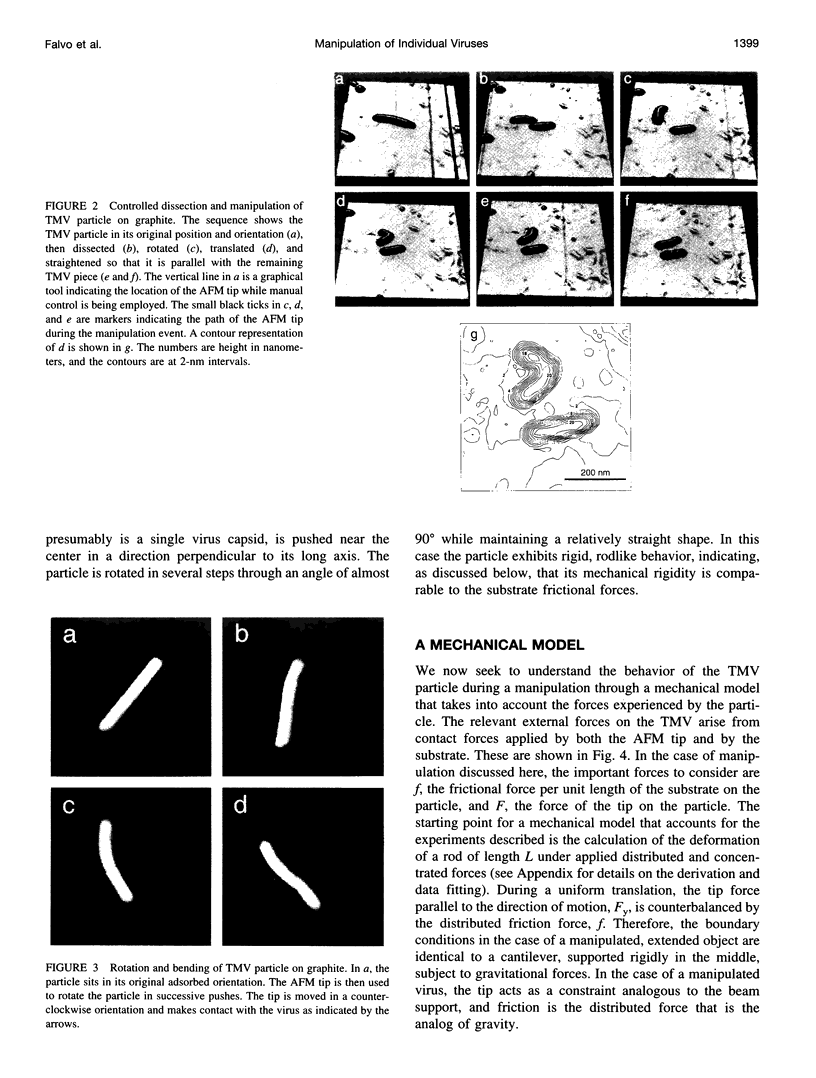
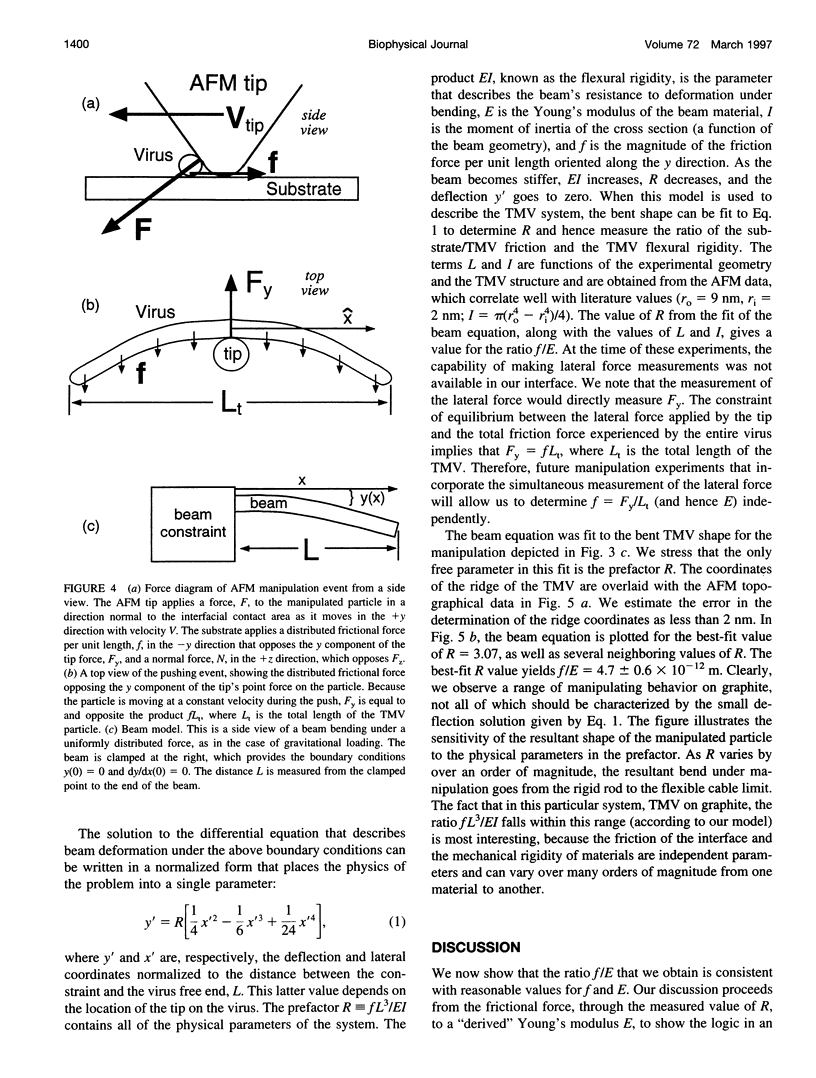
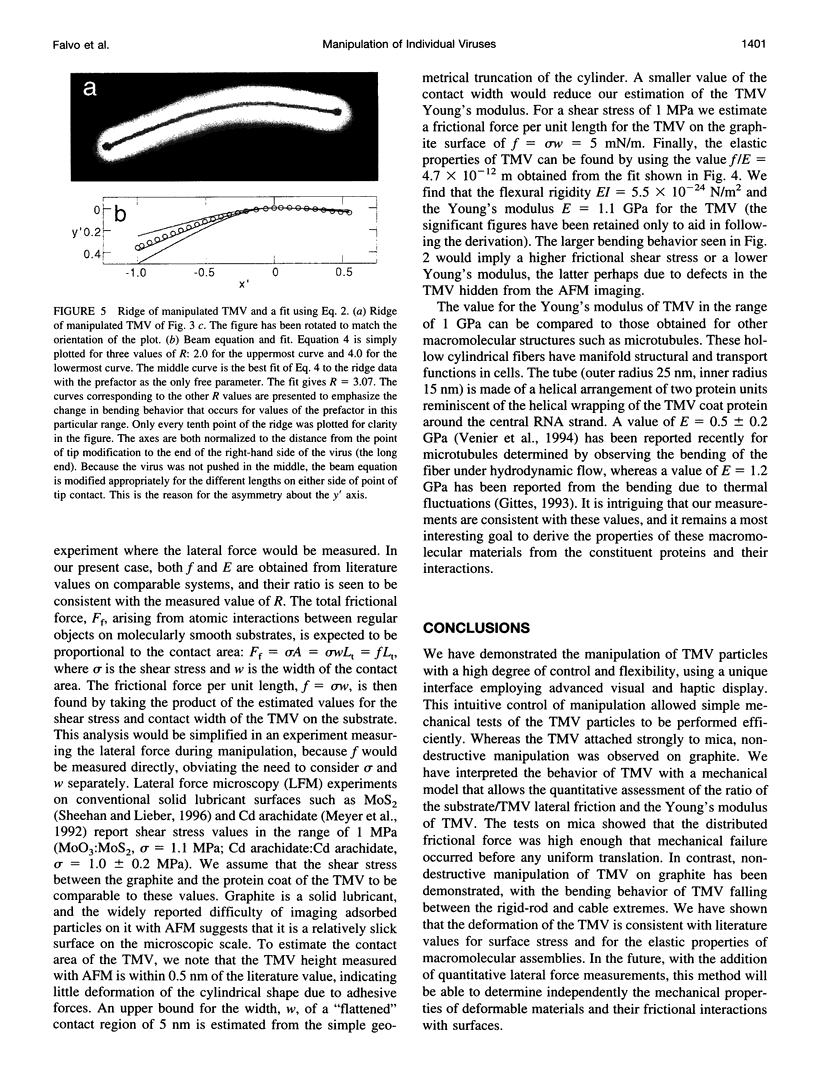


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensimon D, Simon AJ, Croquette V, V, Bensimon A. Stretching DNA with a receding meniscus: Experiments and models. Phys Rev Lett. 1995 Jun 5;74(23):4754–4757. doi: 10.1103/PhysRevLett.74.4754. [DOI] [PubMed] [Google Scholar]
- Block S. M., Blair D. F., Berg H. C. Compliance of bacterial flagella measured with optical tweezers. Nature. 1989 Apr 6;338(6215):514–518. doi: 10.1038/338514a0. [DOI] [PubMed] [Google Scholar]
- Boland T., Ratner B. D. Direct measurement of hydrogen bonding in DNA nucleotide bases by atomic force microscopy. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5297–5301. doi: 10.1073/pnas.92.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Elbaum M, Kuchnir Fygenson D, Libchaber A. Buckling microtubules in vesicles. Phys Rev Lett. 1996 May 20;76(21):4078–4081. doi: 10.1103/PhysRevLett.76.4078. [DOI] [PubMed] [Google Scholar]
- Florin E. L., Moy V. T., Gaub H. E. Adhesion forces between individual ligand-receptor pairs. Science. 1994 Apr 15;264(5157):415–417. doi: 10.1126/science.8153628. [DOI] [PubMed] [Google Scholar]
- Gittes F., Mickey B., Nettleton J., Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol. 1993 Feb;120(4):923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P. D., Soane D. S. Orientation effects on the electrophoretic mobility of rod-shaped molecules in free solution. Anal Chem. 1990 Aug 1;62(15):1592–1596. doi: 10.1021/ac00214a011. [DOI] [PubMed] [Google Scholar]
- Henderson E. Imaging and nanodissection of individual supercoiled plasmids by atomic force microscopy. Nucleic Acids Res. 1992 Feb 11;20(3):445–447. doi: 10.1093/nar/20.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Sheetz M. P. Force of single kinesin molecules measured with optical tweezers. Science. 1993 Apr 9;260(5105):232–234. doi: 10.1126/science.8469975. [DOI] [PubMed] [Google Scholar]
- Lee G. U., Chrisey L. A., Colton R. J. Direct measurement of the forces between complementary strands of DNA. Science. 1994 Nov 4;266(5186):771–773. doi: 10.1126/science.7973628. [DOI] [PubMed] [Google Scholar]
- Lüthi R., Meyer E., Haefke H., Howald L., Gutmannsbauer W., Güntherodt H. J. Sled-type motion on the nanometer scale: determination of dissipation and cohesive energies of c60. Science. 1994 Dec 23;266(5193):1979–1981. doi: 10.1126/science.266.5193.1979. [DOI] [PubMed] [Google Scholar]
- Meyer E, Overney R, Brodbeck D, Howald L, Lüthi R, Frommer J, Güntherodt H. Friction and wear of Langmuir-Blodgett films observed by friction force microscopy. Phys Rev Lett. 1992 Sep 21;69(12):1777–1780. doi: 10.1103/PhysRevLett.69.1777. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- Perkins T. T., Smith D. E., Chu S. Direct observation of tube-like motion of a single polymer chain. Science. 1994 May 6;264(5160):819–822. doi: 10.1126/science.8171335. [DOI] [PubMed] [Google Scholar]
- Putman C. A., van der Werf K. O., de Grooth B. G., van Hulst N. F., Greve J. Viscoelasticity of living cells allows high resolution imaging by tapping mode atomic force microscopy. Biophys J. 1994 Oct;67(4):1749–1753. doi: 10.1016/S0006-3495(94)80649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan PE, Lieber CM. Nanotribology and Nanofabrication of MoO3 Structures by Atomic Force Microscopy. Science. 1996 May 24;272(5265):1158–1161. doi: 10.1126/science.272.5265.1158. [DOI] [PubMed] [Google Scholar]
- Sirenko YM, Stroscio MA, Kim KW. Elastic vibrations of microtubules in a fluid. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1996 Jan;53(1):1003–1010. doi: 10.1103/physreve.53.1003. [DOI] [PubMed] [Google Scholar]
- Skibbens R. V., Rieder C. L., Salmon E. D. Kinetochore motility after severing between sister centromeres using laser microsurgery: evidence that kinetochore directional instability and position is regulated by tension. J Cell Sci. 1995 Jul;108(Pt 7):2537–2548. doi: 10.1242/jcs.108.7.2537. [DOI] [PubMed] [Google Scholar]
- Smith S. B., Cui Y., Bustamante C. Overstretching B-DNA: the elastic response of individual double-stranded and single-stranded DNA molecules. Science. 1996 Feb 9;271(5250):795–799. doi: 10.1126/science.271.5250.795. [DOI] [PubMed] [Google Scholar]
- Smith S. B., Finzi L., Bustamante C. Direct mechanical measurements of the elasticity of single DNA molecules by using magnetic beads. Science. 1992 Nov 13;258(5085):1122–1126. doi: 10.1126/science.1439819. [DOI] [PubMed] [Google Scholar]
- Strick T. R., Allemand J. F., Bensimon D., Bensimon A., Croquette V. The elasticity of a single supercoiled DNA molecule. Science. 1996 Mar 29;271(5257):1835–1837. doi: 10.1126/science.271.5257.1835. [DOI] [PubMed] [Google Scholar]
- Tao N. J., Lindsay S. M., Lees S. Measuring the microelastic properties of biological material. Biophys J. 1992 Oct;63(4):1165–1169. doi: 10.1016/S0006-3495(92)81692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venier P., Maggs A. C., Carlier M. F., Pantaloni D. Analysis of microtubule rigidity using hydrodynamic flow and thermal fluctuations. J Biol Chem. 1994 May 6;269(18):13353–13360. [PubMed] [Google Scholar]
- Weisenhorn A. L., Mac Dougall J. E., Gould S. A., Cox S. D., Wise W. S., Massie J., Maivald P., Elings V. B., Stucky G. D., Hansma P. K. Imaging and manipulating molecules on a zeolite surface with an atomic force microscope. Science. 1990 Mar 16;247(4948):1330–1333. doi: 10.1126/science.247.4948.1330. [DOI] [PubMed] [Google Scholar]