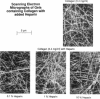
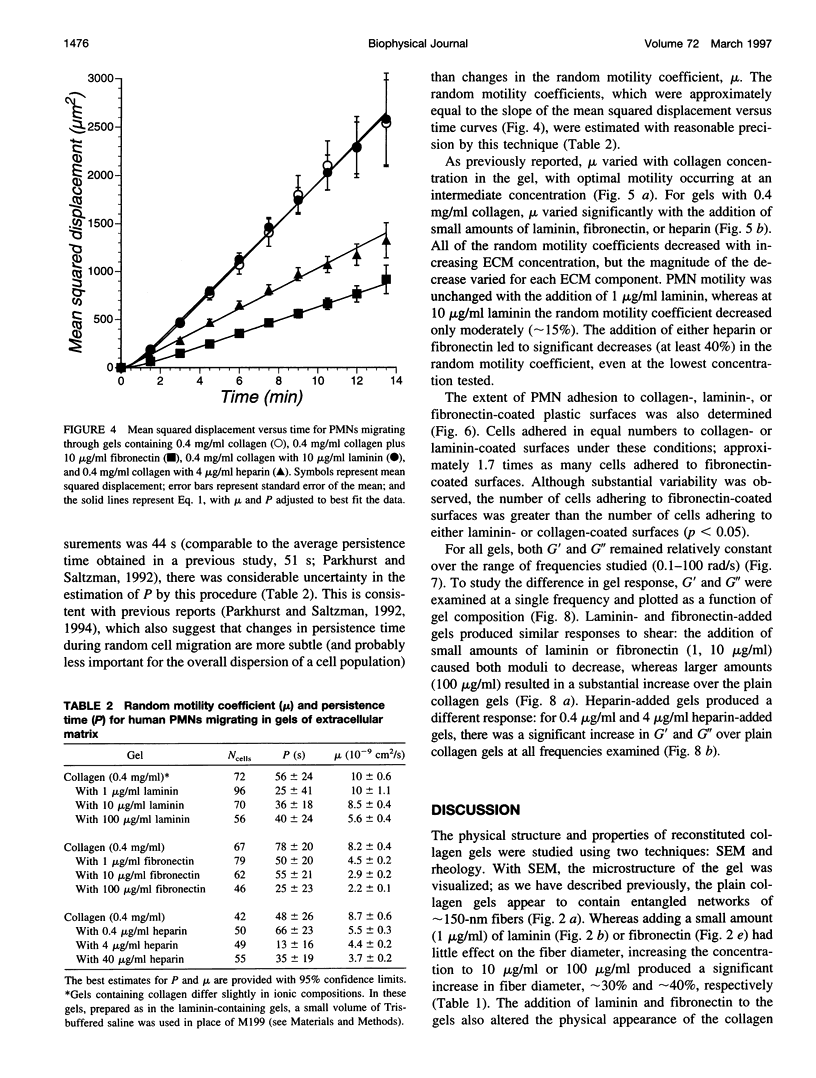
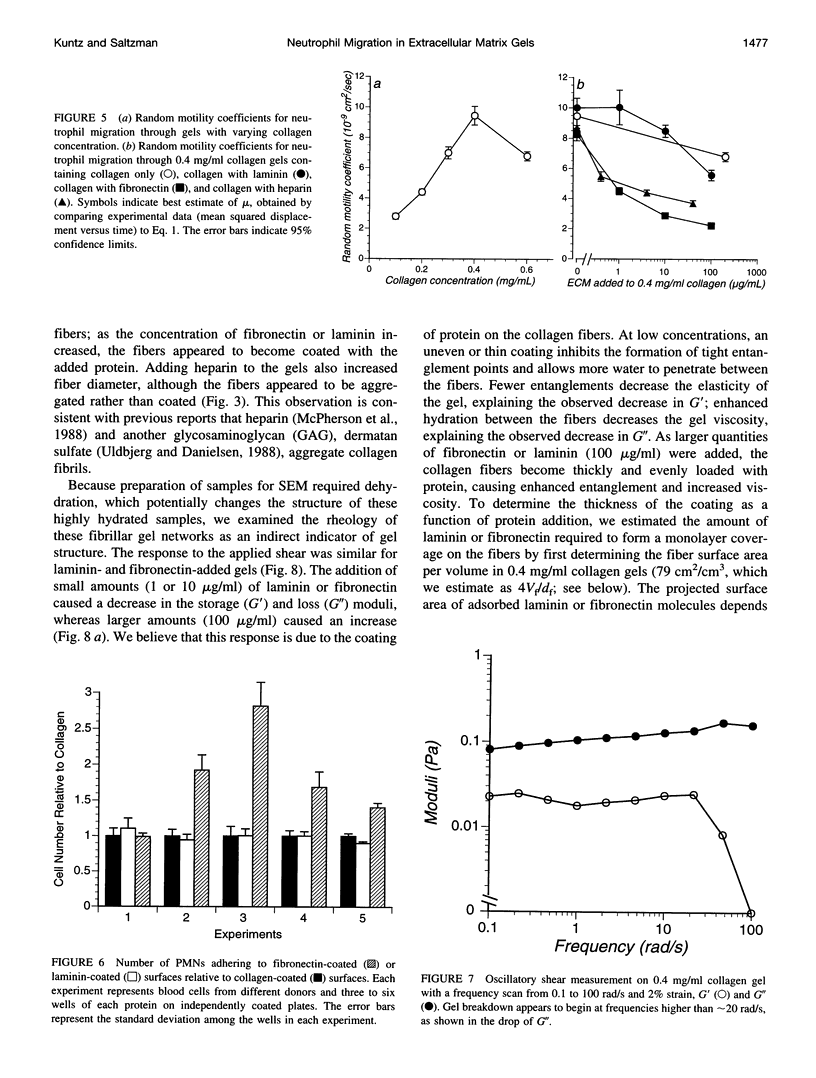
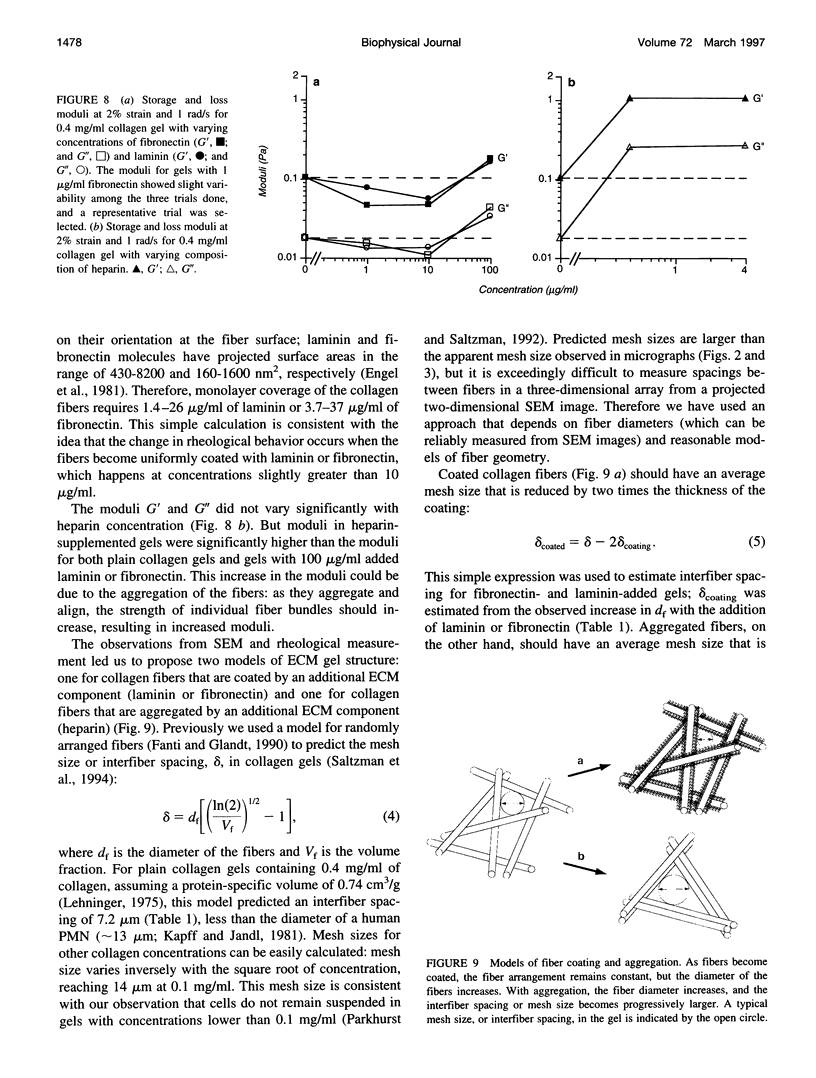
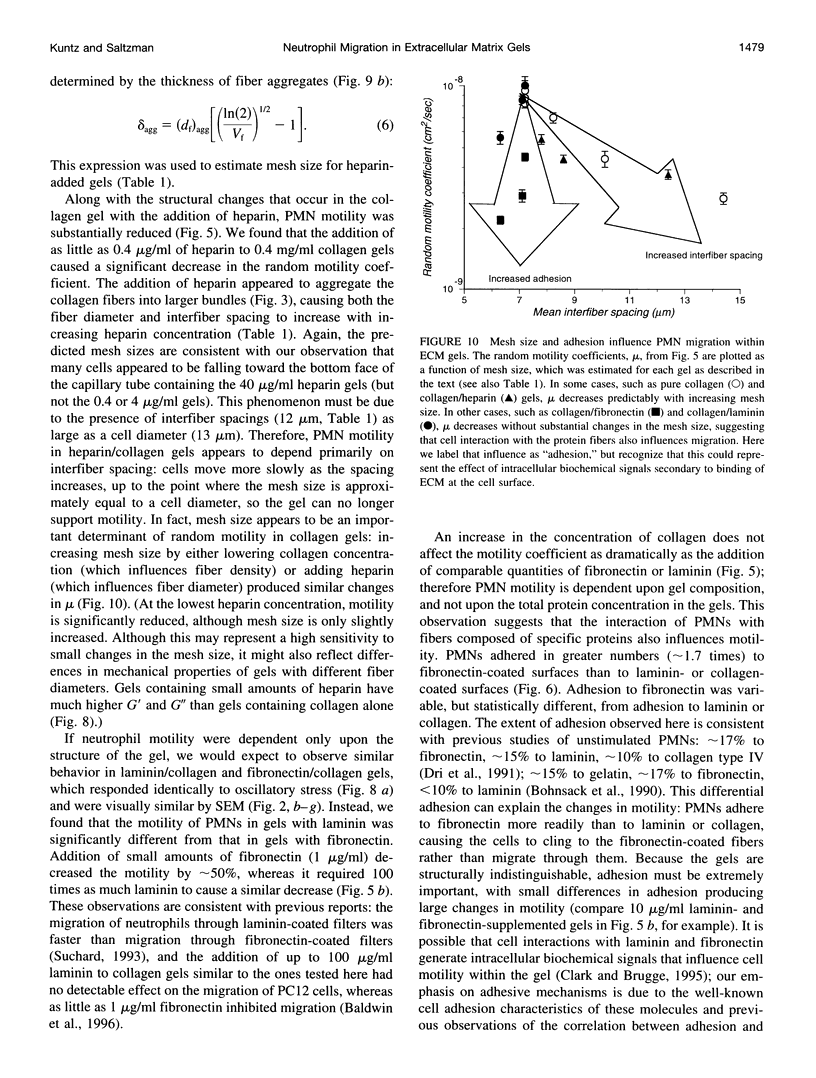
Abstract
Polymorphonuclear leukocyte (PMN) migration through tissue extracellular space is an essential step in the inflammatory response, but little is known about the factors influencing PMN migration through gels of extracellular matrix (ECM). In this study, PMN migration within reconstituted gels containing collagen type I or collagen type I supplemented with laminin, fibronectin, or heparin was measured by quantitative direct visualization, resulting in a random motility coefficient (mum a quantitative index for rate of cell dispersion) for the migrating cell population. The random motility coefficient in unsupplemented collagen (0.4 mg/ml) gels was approximately 9 x 10(-9) cm2/s. Supplementing gels with heparin or fibronectin produced a significant decrease in mu, even at the lowest concentrations studied (1 microgram/ml fibronectin or 0.4 microgram/ml heparin). At least 100 micrograms/ml of laminin, or 20% of the total gel protein, was required to produce a similar decrease in mu. Scanning electron microscopy revealed two different gel morphologies: laminin or fibronectin appeared to coat the 150-nm collagen fibers whereas heparin appeared to induce fiber bundle formation and, therefore, larger interstitial spaces. The decrease in mu observed in heparin-supplemented gels correlated with the increased mesh size of the fiber network, but the difference observed in mu for fibronectin- and laminin-supplemented gels did not correlate with either mesh size or the mechanical properties of the gel, as determined by rheological measurements. However, PMNs adhered to fibronectin-coated surfaces in greater numbers than to collagen- or laminin-coated surfaces, suggesting that changes in cell adhesion to protein fibers can also produce significant changes in cell motility within an ECM gel.
Full text
PDF
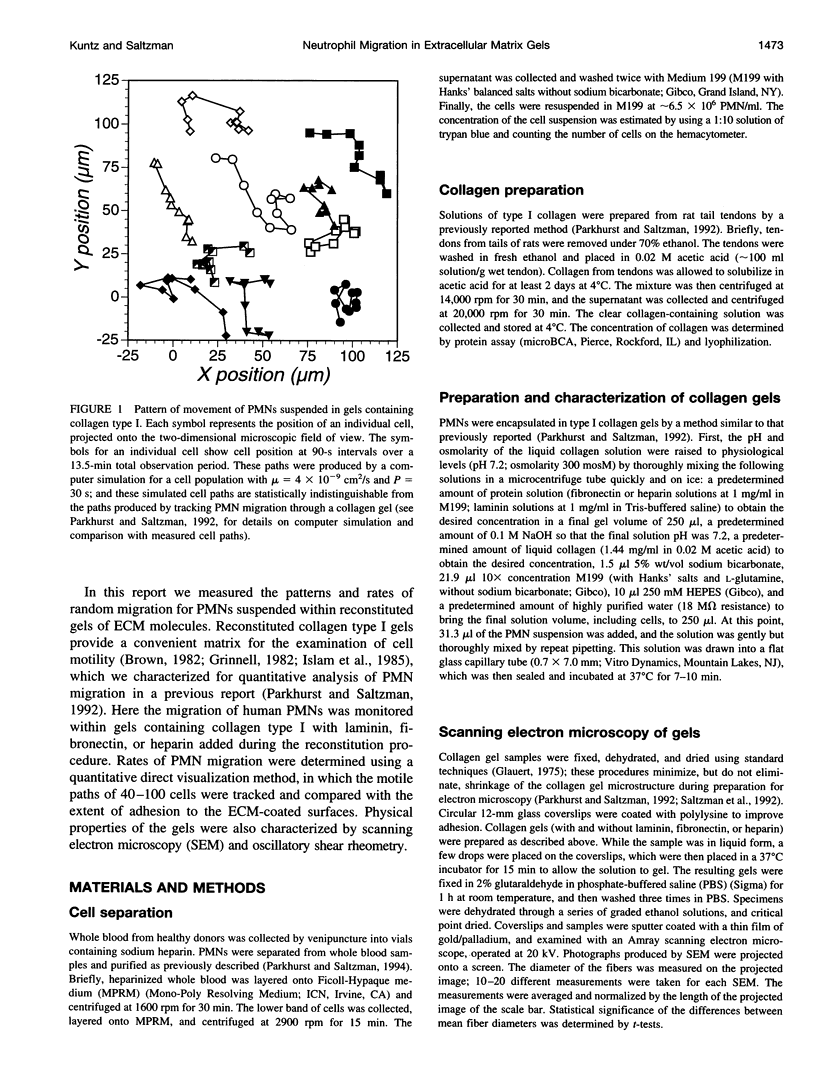

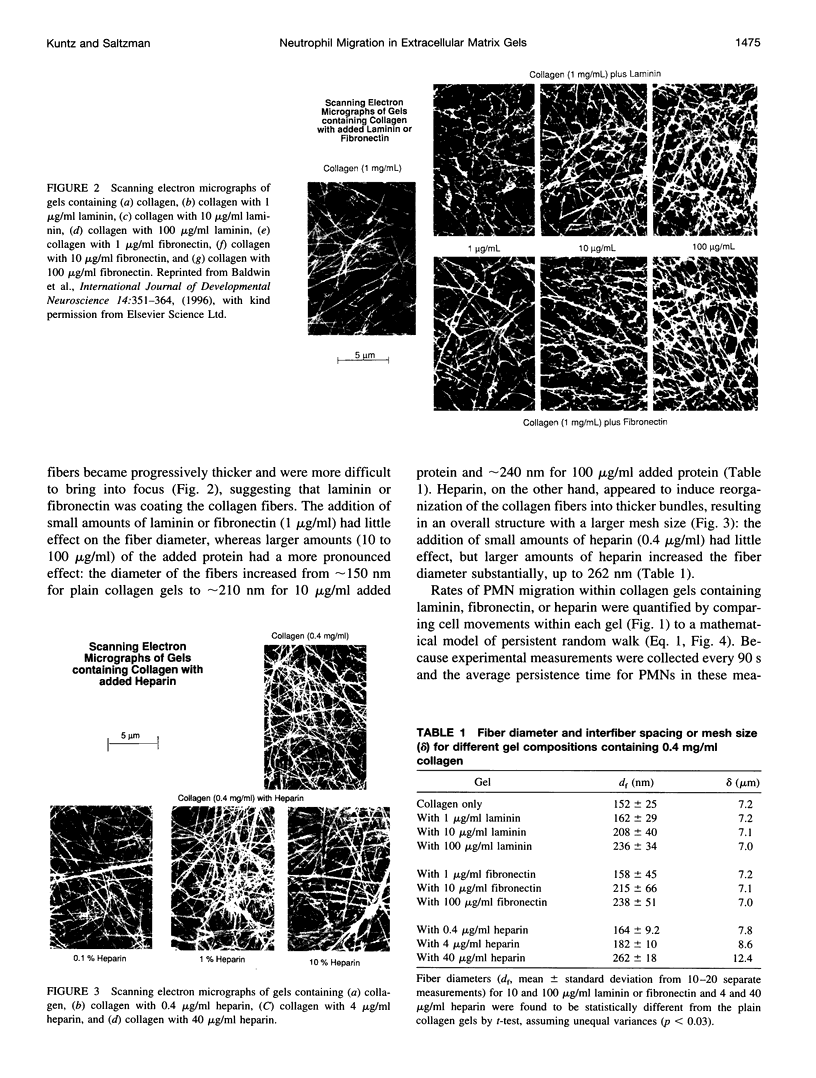

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin S. P., Krewson C. E., Saltzman W. M. PC12 cell aggregation and neurite growth in gels of collagen, laminin and fibronectin. Int J Dev Neurosci. 1996 Jun;14(3):351–364. doi: 10.1016/0736-5748(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Bohnsack J. F., Akiyama S. K., Damsky C. H., Knape W. A., Zimmerman G. A. Human neutrophil adherence to laminin in vitro. Evidence for a distinct neutrophil integrin receptor for laminin. J Exp Med. 1990 Apr 1;171(4):1221–1237. doi: 10.1084/jem.171.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. F. Neutrophil granulocytes: adhesion and locomotion on collagen substrata and in collagen matrices. J Cell Sci. 1982 Dec;58:455–467. doi: 10.1242/jcs.58.1.455. [DOI] [PubMed] [Google Scholar]
- Buettner H. M., Lauffenburger D. A., Zigmond S. H. Measurement of leukocyte motility and chemotaxis parameters with the Millipore filter assay. J Immunol Methods. 1989 Sep 29;123(1):25–37. doi: 10.1016/0022-1759(89)90026-4. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Brugge J. S. Integrins and signal transduction pathways: the road taken. Science. 1995 Apr 14;268(5208):233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- DiMilla P. A., Stone J. A., Quinn J. A., Albelda S. M., Lauffenburger D. A. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993 Aug;122(3):729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dri P., Cramer R., Romano M., Spessotto P., Patriarca P. Effect of biological surfaces on neutrophil O2- production and its relationship to the CD11b/CD18 integrin-dependent adherence. Int J Tissue React. 1991;13(4):193–201. [PubMed] [Google Scholar]
- Dunn G. A. Characterising a kinesis response: time averaged measures of cell speed and directional persistence. Agents Actions Suppl. 1983;12:14–33. doi: 10.1007/978-3-0348-9352-7_1. [DOI] [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J. A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981 Jul 25;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Gail M. H., Boone C. W. The locomotion of mouse fibroblasts in tissue culture. Biophys J. 1970 Oct;10(10):980–993. doi: 10.1016/S0006-3495(70)86347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Migration of human neutrophils in hydrated collagen lattices. J Cell Sci. 1982 Dec;58:95–108. doi: 10.1242/jcs.58.1.95. [DOI] [PubMed] [Google Scholar]
- Haston W. S., Wilkinson P. C. Visual methods for measuring leukocyte locomotion. Methods Enzymol. 1988;162:17–38. doi: 10.1016/0076-6879(88)62060-x. [DOI] [PubMed] [Google Scholar]
- Islam L. N., McKay I. C., Wilkinson P. C. The use of collagen or fibrin gels for the assay of human neutrophil chemotaxis. J Immunol Methods. 1985 Dec 17;85(1):137–151. doi: 10.1016/0022-1759(85)90282-0. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D., Rothman C., Zigmond S. H. Measurement of leukocyte motility and chemotaxis parameters with a linear under-agarose migration assay. J Immunol. 1983 Aug;131(2):940–947. [PubMed] [Google Scholar]
- Lundgren-Akerlund E., Olofsson A. M., Berger E., Arfors K. E. CD11b/CD18-dependent polymorphonuclear leucocyte interaction with matrix proteins in adhesion and migration. Scand J Immunol. 1993 May;37(5):569–574. doi: 10.1111/j.1365-3083.1993.tb02573.x. [DOI] [PubMed] [Google Scholar]
- McIntire L. V. 1992 ALZA Distinguished Lecture: bioengineering and vascular biology. Ann Biomed Eng. 1994 Jan-Feb;22(1):2–13. doi: 10.1007/BF02368217. [DOI] [PubMed] [Google Scholar]
- McPherson J. M., Sawamura S. J., Condell R. A., Rhee W., Wallace D. G. The effects of heparin on the physicochemical properties of reconstituted collagen. Coll Relat Res. 1988 Jan;8(1):65–82. doi: 10.1016/s0174-173x(88)80036-0. [DOI] [PubMed] [Google Scholar]
- Odekon L. E., Frewin M. B., Del Vecchio P., Saba T. M., Gudewicz P. W. Fibronectin fragments released from phorbol ester-stimulated pulmonary artery endothelial cell monolayers promote neutrophil chemotaxis. Immunology. 1991 Sep;74(1):114–120. [PMC free article] [PubMed] [Google Scholar]
- Parkhurst M. R., Saltzman W. M. Leukocytes migrate through three-dimensional gels of midcycle cervical mucus. Cell Immunol. 1994 Jun;156(1):77–94. doi: 10.1006/cimm.1994.1154. [DOI] [PubMed] [Google Scholar]
- Parkhurst M. R., Saltzman W. M. Quantification of human neutrophil motility in three-dimensional collagen gels. Effect of collagen concentration. Biophys J. 1992 Feb;61(2):306–315. doi: 10.1016/S0006-3495(92)81838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G. G., Lackie J. M., Gorham S. D. The behaviour of BHK cells and neutrophil leukocytes on collagen gels of defined mechanical strength. Cell Biol Int Rep. 1990 Nov;14(11):1033–1045. doi: 10.1016/0309-1651(90)90115-f. [DOI] [PubMed] [Google Scholar]
- Reid G. G., Newman I. Human leucocyte migration through collagen matrices containing other extracellular matrix components. Cell Biol Int Rep. 1991 Aug;15(8):711–720. doi: 10.1016/0309-1651(91)90071-p. [DOI] [PubMed] [Google Scholar]
- Saltzman W. M., Parkhurst M. R., Parsons-Wingerter P., Zhu W. H. Three-dimensional cell cultures mimic tissues. Ann N Y Acad Sci. 1992 Oct 13;665:259–273. doi: 10.1111/j.1749-6632.1992.tb42590.x. [DOI] [PubMed] [Google Scholar]
- Saltzman W. M., Radomsky M. L., Whaley K. J., Cone R. A. Antibody diffusion in human cervical mucus. Biophys J. 1994 Feb;66(2 Pt 1):508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Gresham H. D., Griffin G. L., Brown E. J., Chung A. E. Entactin stimulates neutrophil adhesion and chemotaxis through interactions between its Arg-Gly-Asp (RGD) domain and the leukocyte response integrin. J Clin Invest. 1992 Dec;90(6):2251–2257. doi: 10.1172/JCI116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Suchard S. J. Interaction of human neutrophils and HL-60 cells with the extracellular matrix. Blood Cells. 1993;19(2):197-221, discussion 221-3. [PubMed] [Google Scholar]
- Terranova V. P., DiFlorio R., Hujanen E. S., Lyall R. M., Liotta L. A., Thorgeirsson U., Siegal G. P., Schiffmann E. Laminin promotes rabbit neutrophil motility and attachment. J Clin Invest. 1986 Apr;77(4):1180–1186. doi: 10.1172/JCI112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldbjerg N., Danielsen C. C. A study of the interaction in vitro between type I collagen and a small dermatan sulphate proteoglycan. Biochem J. 1988 May 1;251(3):643–648. doi: 10.1042/bj2510643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Hoying J. B., Williams S. K., Kozikowski B. A., Lauffenburger D. A. Integrin-binding peptide in solution inhibits or enhances endothelial cell migration, predictably from cell adhesion. Ann Biomed Eng. 1994 Mar-Apr;22(2):144–152. doi: 10.1007/BF02390372. [DOI] [PubMed] [Google Scholar]