Abstract
Glucose 6-phosphate transport has been well characterized in liver microsomes. The transport is required for the functioning of the glucose-6-phosphatase enzyme that is situated in the lumen of the hepatic endoplasmic reticulum. The genetic deficiency of the glucose 6-phosphate transport activity causes a severe metabolic disease termed type 1b glycogen storage disease. The cDNA encoding a liver transporter for glucose 6-phosphate was cloned and was found to be mutated in patients suffering from glycogen storage disease 1b. While related mRNAs have been described in liver and other tissues, the encoded protein(s) has not been immunologically characterized yet. In the present study, we report (using antibodies against three different peptides of the predicted amino acid sequence) that a major protein encoded by the glucose 6-phosphate transporter gene is expressed in the endoplasmic reticulum membranes of rat and human liver. The protein has an apparent molecular mass of approx. 33 kDa using SDS/PAGE, but several lines of evidence indicate that its real molecular mass is 46 kDa, as expected. The glucose 6-phosphate transporter protein was also immunodetected in kidney microsomes, but not in microsomes derived from human fibrocytes, rat spleen and lung, and a variety of cell lines. Moreover, little or no expression of the glucose 6-phosphate transporter protein was found in liver microsomes obtained from three glycogen storage disease 1b patients, even bearing mutations that do not directly interfere with protein translation, which can be explained by a (proteasome-mediated) degradation of the mutated transporter.
Keywords: endoplasmic reticulum, glucose-6-phosphatase, glucose 6-phosphate transporter, glycogen storage disease, liver, microsome
Abbreviations: CHO, Chinese-hamster ovary; ER, endoplasmic reticulum; G-6-P, glucose 6-phosphate; G-6-Pase, glucose-6-phosphatase; G-6-PT, G-6-P transporter; GSD, glycogen storage disease; HEK, human embryonic kidney; MMLV, Moloney murine leukaemia virus; P1, P2 and P3, peptide 1, 2 and 3
INTRODUCTION
Liver G-6-Pase (glucose-6-phosphatase) catalyses the common terminal reaction of gluconeogenesis and glycogenolysis, hence it plays a major role in the maintenance of blood glucose homoeostasis [1–3]. G-6-Pase1 is expressed mainly in the liver and in the kidney, where it is associated with the ER (endoplasmic reticulum) and functions as a multicomponent system [4]. The system consists of the enzyme protein with an intraluminal active site and transporters for the entry of the substrate G-6-P (glucose 6-phosphate) and for the exit of the products, phosphate and glucose [4–7]. The genetic deficiency of the G-6-Pase1 enzyme protein is termed type 1a glycogen storage disease (GSD1a) [2,8]. However, it has been known for a long time that a number of patients with the symptoms of GSD1 are not deficient in the G-6-Pase1 protein activity, and it has been hypothesized that the genetic deficiency of a putative ER G-6-PT (G-6-P transporter) can also cause a GSD1 subtype termed GSD1b (see [2] and references therein).
A cDNA encoding a liver putative G-6-PT has been cloned and found to be mutated in two GSD1b patients [9]. The predicted molecular mass of the encoded protein is 46 kDa [9]. We reported the structure of the gene and its mapping to human chromosome locus 11q23.3 by FISH (fluorescence in situ hybridization) analysis [10], where it was previously localized by linkage studies [11]. A variety of mutations in the G-6-PT gene have been subsequently found in the majority of the GSD1 non-a patients investigated [12–15]. Northern blot analysis revealed at least two mRNAs: a liver mRNA containing eight out of nine exons (without exon 7) and a brain mRNA containing all the nine exons [16]. The mRNA(s) are also present in a variety of extrahepatic cells [17]. Western blot analysis with an antibody against the 17-amino-acid N-terminus of G-6-PT revealed a liver microsomal protein, termed P46, but its apparent molecular mass was not reported [18]. A protein (over)expressed in COS-1 cells, transfected with the human liver cDNA coding G-6-PT, appeared to have a lower than predicted molecular mass that was approx. 37 kDa [19].
The present study is aimed at characterizing the protein products of the G-6-PT gene. To this aim, we employed antibodies raised against selected peptides of the liver G-6-PT protein. We show that a major protein is expressed in liver and kidney ER membranes, while it is virtually absent in microsomes from a variety of other tissues and cells. In addition, little or no expression of the identified G-6-PT protein was found in liver microsomes obtained from three GSD1b patients.
EXPERIMENTAL
Materials
Oligonucleotide primers and peptides were synthesized by Primm (Milan, Italy). MMLV (Moloney murine leukaemia virus) reverse transcriptase-RNase H minus was from Promega (Milan, Italy). Transfection reagent Lipofectamine™ 2000, TRIzol® reagent, pcDNA 3.1+ vector and cell-culture media were purchased from Invitrogen Life Technologies (San Giuliano Milanese, Mi, Italy). pShuttle vector was from BD Biosciences Clontech (Milan, Italy). Plasmid-purification columns and gel extraction kit were purchased from Qiagen (Milan, Italy). The ECL® (enhanced chemiluminescence) kit was from Amersham Biosciences (Milan, Italy). [14C]G-6-P was purchased from ICN Biomedicals (Segrate, Mi, Italy). All other chemicals were of analytical grade.
Tissue specimens
Human liver specimens were obtained in accordance with the guidelines of the Declaration of Helsinki. The control adult human liver samples were small portions of wedge or needle-biopsy samples obtained for the investigation of the original condition for which the patient was referred. All control liver samples were graded by a pathologist on routine histochemistry on a scale of 1–5, and only liver samples graded 1 (1 being apparently normal and 5 severely diseased) were used as controls in the present study. The Ethical Committee of the Semmelweis University approved the study on the G-6-Pase system in control human liver samples. The three GSD1b patients were initially diagnosed by kinetic analysis of the G-6-Pase system in microsomes isolated from liver biopsy samples. For two of these patients, the clinical–biochemical diagnosis was also confirmed by mutational analysis. The Ethics Committee of Tayside Health Board approved the study of the G-6-Pase system in GSD1b human liver samples.
Rat liver specimens were obtained from 24-h-fasted male Sprague–Dawley rats (180–230 g). Animals were given anaesthesia before being killed, and the study was approved by the University of Siena committee on animal care.
Preparation of microsomal fractions
Microsomes from rat liver, kidney and brain were prepared as reported in [6], except that, in the case of brain, 2 mM EDTA was included in the homogenization medium. Rat skeletal muscle microsomes were prepared as reported in [20]. Microsomes from human fibrocytes, as well as from the other cell lines (see below) were prepared as detailed in [21]. Microsomal fractions were resuspended in a buffer containing 100 mM KCl, 20 mM NaCl, 1 mM MgCl2 and 20 mM Mops, pH 7.2. The suspensions were frozen rapidly and maintained under liquid N2 until used. Microsomes from human liver biopsies were prepared as reported in [22].
Transient expression of G-6-PT–FLAG in HEK-293 (human embryonic kidney) and COS7 cells
A vector suitable to express human G-6-PT, containing a N-terminal FLAG peptide (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys) following the initial methionine residue, was constructed by PCR from the human G-6-PT cDNA template. The oligonucleotide primers derived from nucleotides 170–190 (sense, 5′-GCTCTAGAGCCGCCATGGACTACAAGGACGACGATGACAAGGCAGCCCAGGGCTATGGC-3′) and nucleotides 1439–1459 (antisense, 5′-GGGGTACCGTCACTCAGCCTTCTTGGACAC-3′) of the template [9] were used. The sense primer was modified to include 24 nucleotides encoding the FLAG epitope (underlined sequence) and a restriction site for XbaI, while the antisense primer included the restriction site for KpnI. The cDNA obtained was subjected to a double digestion with XbaI and KpnI and cloned in the expression vector pShuttle. The construct obtained was verified by DNA sequencing. HEK-293 and COS7 cells were grown in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) heat-inactivated foetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin and L-glutamine (2 mM) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
Preparation of CHO-K1 (Chinese-hamster ovary) cells stably expressing G-6-PT
RNA was isolated by the TRIzol® procedure. Total human liver RNA (1 μg) was reverse-transcribed according to the manufacturer's instructions using MMLV reverse transcriptase-RNase H minus. The following sense and antisense primers were used: sense, 5′-TCGTCTTTTCTACCATGGCA-3′, and antisense, 5′-ACCTGGACTCTCTTCACTCA-3′. PCR was performed as follows: 30 cycles of 95 °C (1 min), 55 °C (1 min) and 72 °C (1 min). The 1300 bp PCR product was analysed on a 1% (w/v) agarose gel and extracted using a Qiagen Gel Extraction kit. Gel-extracted PCR product was ligated into pcDNA 3.1+ at the EcoRV site and was used to transform XL1Blue cells. Colonies were grown up in LB (Luria–Bertani) broth with 50 μg/ml ampicillin, and plasmid DNA was purified using a Qiagen Plasmid Maxi kit. DNA was sequenced on an Applied Biosystems automated DNA sequencer. The CHO-K1 cells were transfected with G6PT-pcDNA 3.1 using the Lipofectamine™ reagent, according to the manufacturer's instructions. Transfected cells were subsequently transferred on to new plates before being tested. CHO-K1 cells were grown in F10 nutrient mixture supplemented with 10% (v/v) heat-inactivated foetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 1 mg/ml Geneticin.
Antibody production
We have produced polyclonal antibodies against three different synthetic peptides (referred to here as P1, P2 and P3) corresponding to three putative non-transmembrane amino acid sequences (P1, amino acids 40–49; P2, amino acids 122–134; P3, amino acids 415–429) of the predicted human hepatic G-6-PT protein [19]. Rat P1 differs from the human one in the amino acid residues 40 and 43, whereas P2 is identical except for the amino acid 124. P3 is identical with the human one [17]. Therefore we would expect that the antibodies could react both with the human and rat G-6-PT protein(s). Rabbits were immunized with the three peptides [containing an additional N-terminal cysteine residue to enable conjugation with the carrier protein KLH (keyhole-limpet haemocyanin)], and the antibodies against each peptide were purified by affinity-column chromatography. The antibodies against P2 and P3 were highly, while that against P1 was poorly, reactive towards the corresponding peptide, as verified by ELISA measurements.
Western blot analysis
Microsomal membrane proteins were resolved on 5–20% gradient or 11% polyacrylamide gels [23] and were blotted on to nitrocellulose. Immunoblots were probed with the different antibodies and were analysed by ECL®. For the determination of the G-6-Pase enzyme, membranes were re-probed with a sheep IgG shown previously to be monospecific for the G-6-Pase catalytic subunit [24].
G-6-P uptake
Microsomes prepared from COS7 cells (1 mg of protein/ml) were incubated in the KCl/Mops buffer (see above) in the presence of 1 mM G-6-P plus [14C]G-6-P (75 μCi/ml). To distinguish the intravesicular and the bound radioactivity, 0.1% deoxycholate was added to the incubation mixture [25]. The deoxycholate-releasable portion of radioactivity was regarded as intravesicular. At the indicated time intervals, aliquots (0.05 ml) were rapidly withdrawn and were filtered through cellulose acetate/nitrate filters (pore size 0.22 μm) and were washed with 4 ml of Hepes (20 mM) buffer, pH 7.2, containing 250 mM sucrose and 1 mM 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid. The radioactivity associated with microsomes retained by filters was measured by liquid-scintillation counting [6].
Immunohistochemistry
Specimens of rat liver and kidney were fixed with buffered (pH 7.0) formalin (5%) for 24 h and embedded in paraffin. Tissue sections of approx. 10 μm were cut and incubated for 40 min at 60 °C. Hydrated sections were permeabilized with Triton X-100 (0.2% in PBS) for 10 min, blocked with BSA (3%, in PBS) for 30 min, reacted with the (primary) antibodies against P1, P2 or P3 at 4 °C overnight, and, finally, with a fluorescein-conjugated secondary antibody for 1 h at room temperature (22 °C). Fluorescence images were taken with a Nikon Eclipse 300 inverted microscope coupled to a CCD (charge-coupled device) cooled camera and Metamorph® imaging software.
RESULTS
Western blot analysis of rat liver microsomes with antibodies raised against three different amino sequences of the G-6-PT protein
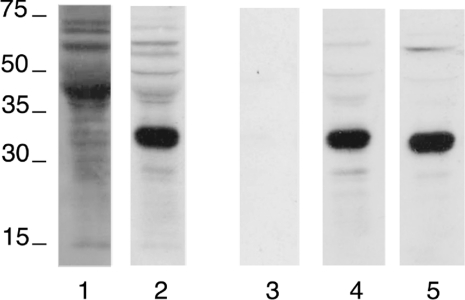
To immunodetect the protein(s) encoded by the human G-6-PT gene, we have produced a polyclonal serum by immunizing rabbits with a mixture of three different synthetic peptides corresponding to three predicted non-transmembrane amino acid sequences (see the Experimental section for details). First, we probed rat liver microsomal proteins using the whole immunoserum and the purified immunoglobulin fractions corresponding to each of the three peptides. The whole immunoserum and antibodies against P2 and P3 revealed a major band between 30 and 35 kDa (Figure 1, lanes 2, 4 and 5 respectively). In contrast, antibodies against P1 were not immunoreactive (Figure 1, lane 3). ELISAs confirmed that the antibodies against P1 were poorly reactive, while those against P2 and P3 were reactive towards the corresponding synthetic peptides (results not shown).
Figure 1. Immunodetection of G-6-PT protein in rat liver microsomes.
Rat liver microsomal proteins (40 μg) were separated by SDS/11% PAGE and blotted on to a nitrocellulose membrane. The blots were reacted either with the whole immunoserum against the three peptides P1, P2 and P3 (lane 2) or the purified antibodies against P1 (lane 3), P2 (lane 4) or P3 (lane 5) respectively. The blot on lane 1 was reacted with the pre-immunoserum. The immunoserum and the purified antibodies were produced as detailed in the Experimental section. The sizes of molecular mass markers (in kDa) are shown.
Antibodies against the liver G-6-PT protein recognize the recombinant protein expressed in heterologous cells
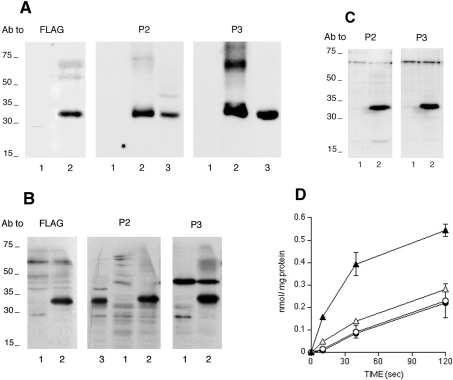
The apparent molecular mass of the visualized band in liver microsomes was smaller than the predicted molecular mass (46 kDa) of the protein encoded by the liver G-6-PT cDNA. We therefore expressed the human liver G-6-PT cDNA in three different mammalian cell lines to ascertain whether or not the expressed protein has the same apparent molecular mass as the band detected in liver microsomes. The human liver G-6-PT cDNA expressed both in HEK-293 and COS7 cells was FLAG-tagged at the N-terminus, thus allowing the visualization of the expressed protein with antibodies against FLAG. The anti-FLAG antibodies revealed a major band between 30 and 35 kDa in transfected cell lysates and microsomes (Figures 2A and 2B respectively). The same band was detected by antibodies against P2 and P3 (Figures 2A and 2B), but not by antibodies against P1 (results not shown). Microsomes obtained from CHO-K1 cells stably transfected with the human liver G-6-PT cDNA also contained a major band between 30 and 35 kDa, which could be visualized with antibodies against P2 and P3 (Figure 2C), but not with antibodies against P1 (results not shown). In additional experiments, we verified that the expression of the immunodetectable putative G-6-PT protein band was associated with an increase in the microsomal uptake of radiolabelled G-6-P. As demonstrated in Figure 2(D), the uptake of radiolabelled G-6-P was more than 2-fold higher in microsomes obtained from the COS7 transfected cells (as shown in Figure 2B), with respect to control (mock-transfected) COS7 cells. The increase in G-6-P uptake was sensitive to the selective inhibitor of G-6-PT, S3483 [26], while the basal uptake in control cells was not (Figure 2D).
Figure 2. Expression of the immunoreactive G-6-PT protein and G-6-P transport in microsomes from transfected cell lines.
The different cell lines were transfected for the expression of the G-6-PT protein as detailed in the Experimental section. (A) Cell lysates of HEK-293 (10 μg of protein) were run on an SDS/PAGE 5–20% gradient gel and blotted on to a nitrocellulose membrane. Blots were reacted with antibodies (Ab) against the indicated epitopes. Lanes 1, mock-transfected cells; lanes 2, G-6-PT–FLAG-transfected cells; lanes 3, rat liver microsomes. The sizes of molecular mass markers (in kDa) are shown. (B) Microsomal proteins (40 μg) from COS7 cells were analysed as in (A). Lanes 1, mock-transfected cells; lanes 2, G-6-PT–FLAG-transfected cells; lane 3, rat liver microsomes. The sizes of molecular mass markers (in kDa) are shown. (C) Microsomal proteins (25 μg) from CHO-K1 cell stable clones were analysed as in (A). Lanes 1, mock-transfected cells; lanes 2, G-6-PT-transfected cells. The sizes of molecular mass markers (in kDa) are shown. (D) Uptake of radiolabelled G-6-P by microsomes from G-6-PT-transfected COS7 cells measured in the presence (Δ) or absence (▲) of the G-6-PT inhibitor S3483. As a control, the uptake of G-6-P in mock-transfected COS7 cells was also measured with (○) or without (●) S3483.
Western blot analysis of microsomes from different tissues with antibodies against the G-6-PT protein
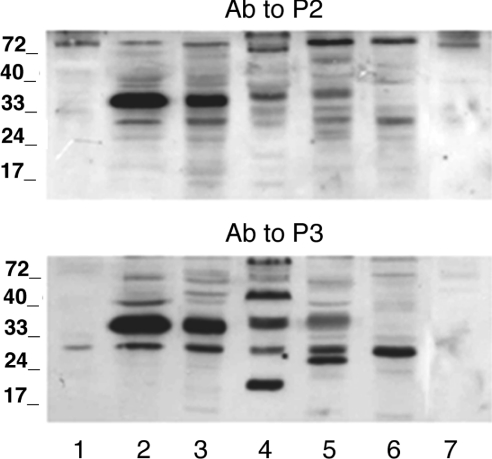
In further experiments, we performed immunoblot analysis of microsomal fractions derived from a variety of tissues (Figure 3). Rat kidney microsomes probed with anti-P2 and anti-P3 antibodies also contained an immunoreactive band with the same apparent molecular mass (Figure 3, lane 3). Antibodies against P1 did not detect immunoreactive bands in kidney microsomes (results not shown).
Figure 3. Immunoblot analysis of microsomes from human fibrocytes and different rat tissues.
Microsomal fractions were prepared as reported in the Experimental section. Microsomal proteins (20 μg) were separated by SDS/11% PAGE and blotted on to a nitrocellulose membrane. The blots were reacted with antibodies (Ab) against P2 (upper panel) or P3 (lower panel). Lanes 1, human fibrocyte; lanes 2, rat liver; lanes 3, rat kidney; lanes 4, rat skeletal muscle; lanes 5, rat brain; lanes 6, rat spleen; lanes 7, rat lung. The sizes of molecular mass markers (in kDa) are shown.
Microsomes obtained from human fibrocytes, rat spleen and rat lung (Figure 3, lanes 1, 6 and 7 respectively) did not contain the major immunoreactive band found in liver and kidney microsomes. It should be noted that microsomes from HEK-293, COS7, and CHO-K1 cells also did not contain the hepatic immunoreactive band (see Figures 2A, 2B and 2C).
Skeletal muscle microsomes contained a band at a slightly higher molecular mass than that of liver, which was visualized with both anti-P2 and anti-P3 antibodies (Figure 3, lane 4), but not with the antibody against P1 (results not shown). One faint immunoreactive band, at the same apparent molecular mass as the muscle band, was also present in brain microsomes immunoreacted with antibodies against either P2 or P3 (Figure 3, lane 5), but was not evident with anti-P1 (results not shown).
It should be noted that human liver microsomes revealed a band at the same apparent molecular mass as rat liver microsomes using both antibodies against P2 (results not shown) and P3 (see Figure 4, ‘contr’ lane).
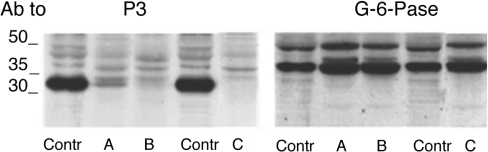
Figure 4. Expression of G-6-PT and G-6-Pase in liver microsomes from GSD1b patients and control subjects.
Microsomes from the different human liver biopsies were prepared as mentioned in the Experimental section. Microsomal proteins (20 μg) were separated by SDS/11% PAGE and blotted on to a nitrocellulose membrane. The blots were first probed with antibodies (Ab) against P3, and then re-probed with antibodies against the G-6Pase1 protein, as indicated. A, B and C are microsomal samples from three GSD1b patients; Contr are liver microsomes from a healthy subject (for details see the Experimental section). The sizes of molecular mass markers (in kDa) are shown.
Reduced expression of the G-6-PT protein in liver microsomes isolated from GSD1b patients
Liver microsomes isolated from liver biopsies from three patients who had the classic symptoms of GSD1b were analysed. The mutations of the G-6-PT gene in patient B consisted of a stop codon at Trp96 (G456A) and a four-amino acid repeat after Met311 (12 base insertion at 1103). Patient C has a homozygous mutation leading to deletion of Phe31 (260delTTC). Unfortunately, no mutational analysis of patient A is available, since the patient died. Western blot analysis with antibodies against P3 revealed a faint expression in patient A, low expression in patient B and no expression in patient C of the major liver band (Figure 4). Re-probing the same membrane with antibodies against the G-6-Pase1 enzyme revealed similar levels of expression of the enzyme in all patients and control liver microsomes (Figure 4). The same pattern of expression was seen with both the whole immunoserum and antibodies against P2 (results not shown).
Immunohistochemical localization of the G-6-PT protein in liver and kidney tissue
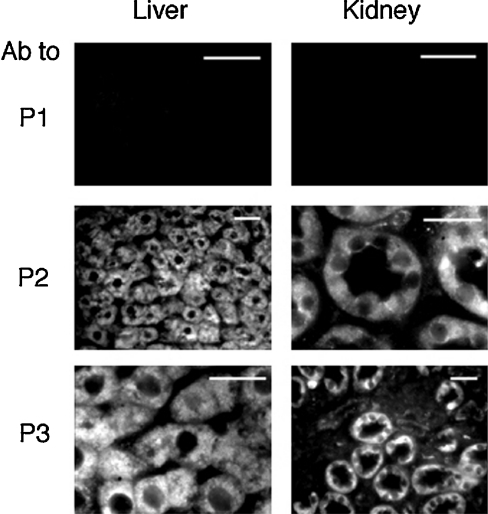
In an additional set of experiments, we probed rat liver and kidney microscopy sections with the antibodies against P1, P2 and P3. A fluorescein-labelled secondary antibody was used for the immunofluorescence microscopy analysis. Anti-P1 did not result in any appreciable fluorescence, while anti-P2 and anti-P3 reacted positively with hepatocytes and kidney tubule epithelial cells (Figure 5). Nuclei were immunonegative, and the fluorescence pattern was consistent with ER localization. Little or no immunoreaction was present in endothelial and Kupffer cells, or in the glomerula. These observations are consistent with the immunolocalization of the G-6-Pase1 enzyme protein [27,28].
Figure 5. Immunohistochemical detection of G-6-PT in rat liver and kidney.
Rat liver and kidney sections were prepared and immunoreacted with antibodies (Ab) against P1, P2 or P3, as reported in the Experimental section. Scale bars, 20 μm.
DISCUSSION
After the discovery of a cDNA encoding a human liver ER G-6-PT, several studies have characterized the gene and its mutations in patients with GSD type 1b, i.e. in the inherited deficiency of transport of G-6-P into the ER lumen [9,10,13–15]. Expression experiments have also demonstrated that the G-6-PT cDNA can encode a functional microsomal G-6-PT [12]. However, no clear-cut data are presently available about the G-6-PT protein(s) expressed in the (ER membranes of) liver and other tissues. Moreover, the microsomal G-6-PT expressed in cell lines upon the transfection with the G-6-PT cDNA appeared to have a different molecular mass from the expected 46 kDa.
In the present study, the identification of the G-6-PT protein(s) has been achieved by using antibodies against two different amino acid sequences (referred to as P2 and P3) of the predicted human (liver) G-6-PT protein. Neither of the two antibodies was entirely specific. However, the conclusions drawn on the presence of the G-6-PT protein(s) in tissues have been based on the concordance of the results obtained with the two antibodies. The predicted molecular mass of the liver G-6-PT is 46 kDa; however, a major protein band with an apparent molecular mass of approx. 33 kDa was revealed by both of the antibodies in rat and human liver microsomes. The cDNA coding for G-6-PT is very similar in rats and humans, with respect to the molecular mass and the sequence of the immunoreactive peptides (see the Experimental section for details). We concluded nonetheless that the immunoreactive band corresponded to the protein with the predicted G-6-PT sequence and that it has a real molecular mass of 46 kDa. Several lines of evidence support this.
First, a protein band, which migrates on SDS/PAGE as the band visualized in liver microsomes, was revealed by the two antibodies in microsomes prepared from cell lines transfected with the cDNA encoding human liver G-6-PT.
Secondly, the transfection of cell lines with a human liver, FLAG-tagged, G-6-PT cDNA resulted in the appearance of a protein band immunoreactive with both the antibodies against FLAG, and the antibodies against P2 and P3. It should be noted that the antibodies used probe the N-terminus (anti-FLAG), an internal peptide (anti-P2) and the C-terminus of the protein, which excludes proteolysis as the cause for the apparent size anomaly. These results therefore unequivocally demonstrate that the G-6-PT protein with a real molecular mass of 46 kDa migrates on SDS/PAGE at an apparent lower molecular mass. This discrepancy is possibly due to the high hydrophobicity of the protein. Consistently, other authors have observed that the human liver G-6-PT protein expressed in host cells always exhibits an apparent molecular mass lower than 46 kDa [12,29].
Thirdly, transfection resulted in a higher rate of microsomal G-6-P transport as demonstrated by rapid filtration experiments.
The present results indicate that a major, apparently unique, protein encoded by the G-6-PT gene is expressed in the liver and kidney ER membranes. This is consistent with the fact that both tissues are primarily involved in the pathology of GSD1b, as they express high levels of the G-6-Pase1 enzyme, whose activity depends on the functioning of the microsomal G-6-PT. Mutations in the gene of the hepatic G-6-PT not only cause glycogen deposition in hepatocytes and kidney tubule epithelial cells, but also impairment of neutrophils/monocyte function [30]. In these cells, G-6-PT is believed to play a role unrelated to the G-6-Pase1 enzyme (which is not present in these cells), but crucial for cell survival/differentiation [25]. In the present study, we did not get clear-cut evidence for the presence of a G-6-PT protein in neutrophil microsomes. A relatively low level of expression of the protein might account for the lack of visualization of the band. Moreover, neutrophil microsomal fractions are contaminated by non-reticular membranes because of the relatively low representation of the ER network in these cells [31].
In skeletal muscle and brain microsomes, a protein band at a slightly higher apparent molecular mass than that of liver was immunorevealed by either anti-P2 or anti-P3 (but not anti-P1) antibodies. As a logical interpretation, this band may correspond to a protein coded by the ‘brain’ mRNA [16], referred to also as vG-6-PT (variant G-6-PT) transcript [32]. Brain mRNA differs from liver mRNA, as it contains an additional 66 bp exon-7 sequence and encodes a protein with a predicted molecular mass of approx. 49 kDa, slightly higher than that the predicted ‘liver’ protein (46 kDa). The vG-6-PT transcript has been found exclusively in the brain, heart and skeletal muscle [32]. Moreover, knockout of the G-6-PT gene in mice resulted in a reduction of G-6-P transport in skeletal muscle microsomes [33].
The low or absent expression of the G-6-PT protein in GSD1b patients also confirms the identity of the major liver band. The lack of the protein in patients with mutations that do not directly interfere with protein translation, i.e. stop codons, can be explained by a proteasome-mediated degradation of the mutated transporter. Consistent with this interpretation, recent work from others shows that the expression in COS-1 cells of the cDNA encoding human G-6-PT, mutated at different levels, results in a detectable level of the ER G-6-PT protein only upon the pharmacological inhibition (with lactacystin) of the proteasome [34].
Acknowledgments
This work was supported by a grant from the Siena University (to R.F.) and by the Hungarian Scientific Research Fund (grant numbers F046740 and T038312). T.K. was a recipient of NATO-CNR Research Fellowship to Siena. The work carried out in Scotland was funded by grants from the Wellcome Trust, the Cunningham Trust, the Medical Research Council and Tenovus (Scotland). A Royal Society ESEP (European Science Exchange Programme) Award funded the collaboration between the laboratories in Siena and Dundee.
References
- 1.Cori G. T., Cori C. F. Glucose-6-phosphatase of the liver in glycogen storage disease. J. Biol. Chem. 1952;199:661–667. [PubMed] [Google Scholar]
- 2.Chen Y. T., Burchell A. Glycogen storage diseases. In: Scriver C. R., Beaudet A. L., Sly W. S., Valle D., editors. The Metabolic Basis of Inherited Disease. 7th edn. New York: McGraw-Hill; 1995. pp. 935–965. [Google Scholar]
- 3.Guionie O., Clottes E., Stafford K., Burchell A. Identification and characterisation of a new human glucose-6-phosphatase isoform. FEBS Lett. 2003;551:159–164. doi: 10.1016/s0014-5793(03)00903-7. [DOI] [PubMed] [Google Scholar]
- 4.Arion W. J., Lange A. J., Walls H. E., Ballas L. M. Evidence for the participation of independent translocases for phosphate and glucose 6-phosphate in the microsomal glucose-6-phosphatase system. J. Biol. Chem. 1980;255:10396–10406. [PubMed] [Google Scholar]
- 5.Benedetti A., Fulceri R., Ferro M., Comporti M. On a possible role for glucose-6-phosphatase in the regulation of liver cell cytosolic calcium concentration. Trends Biochem. Sci. 1986;11:284–285. [Google Scholar]
- 6.Bánhegyi G., Marcolongo P., Fulceri R., Hinds C., Burchell A., Benedetti A. Demonstration of a metabolically active glucose-6-phosphate pool in the lumen of liver microsomal vesicles. J. Biol. Chem. 1997;272:13584–13590. doi: 10.1074/jbc.272.21.13584. [DOI] [PubMed] [Google Scholar]
- 7.Fulceri R., Bellomo G., Gamberucci A., Scott H. M., Burchell A., Benedetti A. Permeability of rat liver microsomal membrane to glucose 6-phosphate. Biochem. J. 1992;286:813–817. doi: 10.1042/bj2860813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei K. J., Pan C. J., Shelly L. L., Liu J. L., Chou J. Y. Identification of mutations in the gene for glucose-6-phosphatase, the enzyme deficient in glycogen storage disease type 1a. J. Clin. Invest. 1994;93:1994–1999. doi: 10.1172/JCI117192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerin I., Veiga-da-Cunha M., Achouri Y., Collet J. F., Van Schaftingen E. Sequence of a putative glucose 6-phosphate translocase, mutated in glycogen storage disease type 1b. FEBS Lett. 1997;419:235–238. doi: 10.1016/s0014-5793(97)01463-4. [DOI] [PubMed] [Google Scholar]
- 10.Marcolongo P., Barone V., Priori G., Pirola B., Giglio S., Biasucci G., Zammarchi E., Parenti G., Burchell A., Benedetti A., Sorrentino V. Structure and mutation analysis of the glycogen storage disease type 1b gene. FEBS Lett. 1998;436:247–250. doi: 10.1016/s0014-5793(98)01129-6. [DOI] [PubMed] [Google Scholar]
- 11.Annabi B., Hiraiwa H., Mansfield B. C., Lei K. J., Ubagai T., Polymeropoulos M. H., Moses S. W., Parvari R., Hershkovitz E., Mandel H., et al. The gene for glycogen-storage disease type 1b maps to chromosome 11q23. Am. J. Hum. Gen. 1998;62:400–405. doi: 10.1086/301727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiraiwa H., Pan C. J., Lin B., Moses S. W., Chou J. Y. Inactivation of the glucose 6-phosphate transporter causes glycogen storage disease type 1b. J. Biol. Chem. 1999;274:5532–5536. doi: 10.1074/jbc.274.9.5532. [DOI] [PubMed] [Google Scholar]
- 13.Galli L., Orrico A., Marcolongo P., Fulceri R., Burchell A., Melis D., Parini R., Gatti R., Lam C. W., Benedetti A., Sorrentino V. Mutations in the glucose-6-phosphate transporter (G-6-PT) gene in patients with glycogen storage disease type 1b and 1c. FEBS Lett. 1999;459:255–258. doi: 10.1016/s0014-5793(99)01248-x. [DOI] [PubMed] [Google Scholar]
- 14.Veiga-da-Cunha M., Gerin I., Chen Y. T., Lee P. J., Leonard J. V., Maire I., Wendel U., Vikkula M., Van Schaftingen E. The putative glucose 6-phosphate translocase gene is mutated in essentially all cases of glycogen storage disease type 1 non-a. Eur. J. Hum. Gen. 1999;7:717–723. doi: 10.1038/sj.ejhg.5200366. [DOI] [PubMed] [Google Scholar]
- 15.Chou J. Y., Matern D., Mansfield B. C., Chen Y. T. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr. Mol. Med. 2002;2:121–143. doi: 10.2174/1566524024605798. [DOI] [PubMed] [Google Scholar]
- 16.Middleditch C., Clottes E., Burchell A. A different isoform of the transport protein mutated in the glycogen storage disease 1b is expressed in brain. FEBS Lett. 1998;433:33–36. doi: 10.1016/s0014-5793(98)00878-3. [DOI] [PubMed] [Google Scholar]
- 17.Lin B., Annabi B., Hiraiwa H., Pan C. J., Chou J. Y. Cloning and characterization of cDNAs encoding a candidate glycogen storage disease type 1b protein in rodents. J. Biol. Chem. 1998;273:31656–31660. doi: 10.1074/jbc.273.48.31656. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Mechin M. C., van de Werve G. Diabetes affects similarly the catalytic subunit and putative glucose-6-phosphate translocase of glucose-6-phosphatase. J. Biol. Chem. 1999;274:33866–33868. doi: 10.1074/jbc.274.48.33866. [DOI] [PubMed] [Google Scholar]
- 19.Pan C. J., Lin B., Chou J. Y. Transmembrane topology of human glucose 6-phosphate transporter. J. Biol. Chem. 1999;274:13865–13869. doi: 10.1074/jbc.274.20.13865. [DOI] [PubMed] [Google Scholar]
- 20.Gamberucci A., Marcolongo P., Fulceri R., Giunti R., Watkins S. L., Waddell I. D., Burchell A., Benedetti A. Low levels of glucose-6-phosphate hydrolysis in the sarcoplasmic reticulum of skeletal muscle: involvement of glucose-6-phosphatase. Mol. Membr. Biol. 1996;13:103–108. doi: 10.3109/09687689609160583. [DOI] [PubMed] [Google Scholar]
- 21.Leuzzi R., Fulceri R., Marcolongo P., Bánhegyi G., Zammarchi E., Stafford K., Burchell A., Benedetti A. Glucose 6-phosphate transport in fibroblast microsomes from glycogen storage disease type 1b patients: evidence for multiple glucose 6-phosphate transport systems. Biochem. J. 2001;357:557–562. doi: 10.1042/0264-6021:3570557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burchell A., Jung R. T., Lang C. C., Bennet W., Shepherd A. N. Diagnosis of type 1a and type 1c glycogen storage diseases in adults. Lancet. 1987;9:1059–1062. doi: 10.1016/s0140-6736(87)90484-3. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Burchell A., Waddell I. D., Countaway J. L., Arion W. J., Hume R. Identification of the human hepatic microsomal glucose-6-phosphatase enzyme. FEBS Lett. 1988;242:153–156. doi: 10.1016/0014-5793(88)81005-6. [DOI] [PubMed] [Google Scholar]
- 25.Leuzzi R., Bánhegyi G., Kardon T., Marcolongo P., Capecchi P. L., Burger H. J., Benedetti A., Fulceri R. Inhibition of microsomal glucose-6-phosphate transport in human neutrophils results in apoptosis: a potential explanation for neutrophil dysfunction in glycogen storage disease type 1b. Blood. 2003;101:2381–2387. doi: 10.1182/blood-2002-08-2576. [DOI] [PubMed] [Google Scholar]
- 26.Arion W. J., Canfield W. K., Ramos F. C., Su M. L., Burger H. J., Hemmerle H., Schubert G., Below P., Herling A. W. Chlorogenic acid analogue S3483: a potent competitive inhibitor of the hepatic and renal glucose-6-phosphatase systems. Arch. Biochem. Biophys. 1998;351:279–285. doi: 10.1006/abbi.1997.0563. [DOI] [PubMed] [Google Scholar]
- 27.Hume R., Bell J., Hallas A., Burchell A. Immunohistochemical localisation of glucose-6-phosphatase in developing human kidney. Histochemistry. 1994;101:413–417. doi: 10.1007/BF00269491. [DOI] [PubMed] [Google Scholar]
- 28.Burchell A., Hume R. The glucose-6-phosphatase system in human development. Histol. Histopathol. 1995;10:979–993. [PubMed] [Google Scholar]
- 29.Pan C. J., Chen L. Y., Mansfield B. C., Salani B., Varesio L., Chou J. Y. The signature motif in human glucose-6-phosphate transporter is essential for microsomal transport of glucose-6-phosphate. Hum. Genet. 2003;112:430–433. doi: 10.1007/s00439-002-0903-3. [DOI] [PubMed] [Google Scholar]
- 30.Van Schaftingen E., Gerin I. The glucose-6-phosphatase system. Biochem. J. 2002;362:513–532. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallett M. B., Pettit E. J., Davies E. V. Capacitative Ca2+ influx and a diffusible influx factor. Biochem. J. 1996;314:1054–1055. doi: 10.1042/bj3141054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin B., Pan C. J., Chou J. Y. Human variant of glucose-6-phosphate transporter is active in microsomal transport. Hum. Genet. 2000;107:526–529. doi: 10.1007/s004390000404. [DOI] [PubMed] [Google Scholar]
- 33.Shieh J. J., Pan C. J., Mansfield B. C., Chou J. Y. A potential new role for muscle in blood glucose homeostasis. J. Biol. Chem. 2004;279:26215–26219. doi: 10.1074/jbc.M402036200. [DOI] [PubMed] [Google Scholar]
- 34.Chen L. Y., Pan C. J., Shieh J. J., Chou J. Y. Structure-function analysis of the glucose-6-phosphate transporter deficient in glycogen storage disease type Ib. Hum. Mol. Genet. 2002;11:3199–3207. doi: 10.1093/hmg/11.25.3199. [DOI] [PubMed] [Google Scholar]