Abstract
ROS (reactive oxygen species) from mitochondrial and non-mitochondrial sources have been implicated in TNFα (tumour necrosis factor α)-mediated signalling. In the present study, a new class of specific mitochondria-targeted antioxidants were used to explore directly the role of mitochondrial ROS in TNF-induced apoptosis. MitoVit E {[2-(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)ethyl]triphenylphosphonium bromide} (vitamin E attached to a lipophilic cation that facilitates accumulation of the antioxidant in the mitochondrial matrix) enhanced TNF-induced apoptosis of U937 cells. In time course analyses, cleavage and activation of caspase 8 in response to TNF were not affected by MitoVit E, whereas the activation of caspase 3 was significantly increased. Furthermore, there was an increased cleavage of the proapoptotic Bcl-2 family member Bid and an increased release of cytochrome c from mitochondria, in cells treated with TNF in the presence of MitoVit E. We considered several mechanisms by which MitoVit E might accelerate TNF-induced apoptosis including mitochondrial integrity (ATP/ADP levels and permeability transition), alterations in calcium homoeostasis and transcription factor activation. Of these, only the transcription factor NF-κB (nuclear factor κB) was implicated. TNF caused maximal nuclear translocation of NF-κB within 15 min, compared with 1 h in cells pretreated with MitoVit E. Thus the accumulation of an antioxidant within the mitochondrial matrix enhances TNF-induced apoptosis by decreasing or delaying the expression of the protective antiapoptotic proteins. These results demonstrate that mitochondrial ROS production is a physiologically relevant component of the TNF signal-transduction pathway during apoptosis, and reveal a novel functional role for mitochondrial ROS as a temporal regulator of NF-κB activation and NF-κB-dependent antiapoptotic signalling.
Keywords: antioxidant, caspase, mitochondria, nuclear factor κB, reactive oxygen species, tumour necrosis factor
Abbreviations: AMC, aminomethylcoumarin; Ac-DEVD-AMC, N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin; AFC, aminotrifluoromethylcoumarin; AP-1, activator protein-1; BAPTA/AM, bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis; EMSA, electrophoretic mobility-shift assay; annexin V–FITC, FITC-conjugated annexin V; MitoPBN, [4-[4-[[(1,1-dimethylethyl)oxidoimino]methyl]phenoxy]butyl]triphenylphosphonium bromide; MitoQ, mixture of mito-quinol [10-(3,6-dihydroxy-4,5-dimethoxy-2-methylphenyl)decyl]triphenylphosphonium bromide and mito-quinone [10-(4,5-dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl)decyl]triphenylphosphonium bromide; MitoVit E, [2-(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)ethyl]triphenylphosphonium bromide; NAC, N-acetylcysteine; NF-κB, nuclear factor κB; NP40, Nonidet P40; PDTC, pyrrolidine dithiocarbamate; ROS, reactive oxygen species; TNF, tumour necrosis factor; hTNF, human TNF; TNFR, TNF receptor; TPMP, methyltriphenylphosphonium; Z-IETD-AFC, benzyloxycarbonyl-Ile-Glu-Thr-Asp-aminotrifluoromethylcoumarin
INTRODUCTION
Mitochondria are an important source of ROS (reactive oxygen species), since, under normal conditions, the respiratory chain continually releases ROS during oxidative phosphorylation. Uncontrolled ROS production by mitochondria causes oxidative damage to biological molecules and is associated with the pathophysiology of numerous disease processes and aging [1]. However, in apparent contradiction to the pathological role of ROS, a large body of evidence indicates that the regulated production of small amounts of ROS, from both mitochondrial and non-mitochondrial sources, functions as a physiological component of cytokine and growth factor-signalling pathways [2–4].
Much attention has been paid to the role of ROS in the signaltransduction pathways activated by the pro-inflammatory cytokine TNFα (tumour necrosis factor α) [5,6]. TNF exerts a broad spectrum of responses by binding to two cell-surface receptors, TNFR1 and TNFR2. These receptors activate pathways controlling cell proliferation, differentiation and death [6,7]. TNF induces cell death that is either apoptotic (caspase-dependent) or necrotic (caspase-independent) [6] and also supports survival pathways by activating transcription factors that increase the expression of antiapoptotic proteins [8]. TNF treatment has been reported to increase ROS production from mitochondria [6,9], plasma-membrane NADPH oxidase [10] and lipoxygenase [11]. Furthermore, the use of radical scavengers, antioxidants, anaerobic growth conditions, inhibitors of mitochondrial respiration and genetic deficiencies in mitochondrial respiration have implicated ROS in the regulation of TNF-mediated cytotoxicity and gene transcription [5,6,12–14]. However, there are conflicting data regarding ROS production in response to TNF [11,15], in part due to the lack of specific reagents to intervene in ROS production. For example, it has recently been shown that the antioxidants NAC (N-acetylcysteine) and PDTC (pyrrolidine dithiocarbamate) inhibit TNF action independently of their antioxidant action [16]. Similarly, although the NADPH oxidase inhibitor diphenyleneiodinium is often used to infer that the site of ROS production is a plasma-membrane NADPH oxidase, mitochondrial electron transport is also sensitive to this compound [17]. ROS production is most often assayed using oxidant-sensitive fluorescent probes, but the response of these probes to ROS production is often non-specific and may reflect other changes, including cytochrome c release, rather than an absolute increase in cellular ROS levels [17,18]. Furthermore, it is not currently possible to measure localized subcellular changes in ROS production [17].
In light of the doubts raised by these results, we have addressed the specific role of mitochondrial ROS and oxidative damage in TNF-induced apoptosis using mitochondria-targeted derivatives of vitamin E {MitoVit E; [2-(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)ethyl]triphenylphosphonium bromide}, ubiquinol (MitoQ, a mixture of mito-quinol [10-(3,6-dihydroxy-4,5-dimethoxy-2-methylphenyl)decyl]triphenylphosphonium bromide and mito-quinone [10-(4,5-dimethoxy-2-methyl-3, 6-dioxo-1, 4-cyclohexadien-1-yl) decyl]triphenylphosphonium bromide) and PBN {MitoPBN; [4-[4-[[(1,1-dimethylethyl)oxidoimino]methyl]phenoxy]butyl]triphenylphosphonium bromide} These targeted antioxidants, MitoVit E, MitoQ and MitoPBN, accumulate selectively in the mitochondrial matrix protecting mitochondria against oxidative damage [19,20]. MitoQ and MitoVit E protect cells from a variety of apoptotic stimuli, including 5-fluorouracil [21], growth factor deprivation [22] and glutathione depletion in frataxin-depleted cells [23], and also inhibit H2O2-induced growth factor receptor signalling [24]. These results have confirmed that mitochondrial ROS have a role in these processes and demonstrate that the mitochondria-targeted antioxidants are useful tools for determining the role of mitochondrial ROS in signal transduction. In the present study, we found that mitochondrial ROS were critical modulators of TNF-induced apoptosis and that this was mediated at least in part by a delay in the activation of NF-κB (nuclear factor κB). This suggests that mitochondrial ROS are produced in response to TNF treatment, and these ROS prevent a full apoptotic response to TNF by enhancing NF-κB-mediated expression of antiapoptotic proteins.
EXPERIMENTAL
Cells and reagents
The human monocytic cell line U937 and the human T cell line Jurkat were maintained in RPMI 1640, supplemented with 10% (v/v) foetal bovine serum, 1% glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. Recombinant hTNF (human TNF) was obtained from R & D Systems (Abingdon, Oxfordshire, U.K.) and annexin V–FITC (FITC-conjugated annexin V) was from Molecular Probes (Eugene, OR, U.S.A.). Anti-cytochrome c antibody (clone 7H8.2C12) was from Pharmingen (San Diego, CA, U.S.A.) and anti-Bid polyclonal antibody was from BioVision (Mountain View, CA, U.S.A.). Secondary antibodies were from Bio-Rad Laboratories (Hemel Hempstead, Herts., U.K.). The caspase 3 [Ac-DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin)] and caspase 8 [Z-IETD-AFC (benzyl-oxycarbonyl-Ile-Glu-Thr-Asp-aminotrifluoromethylcoumarin)] substrates were from Calbiochem (Merck, Palmerston North, New Zealand). The mitochondria-targeted antioxidants MitoVit E, MitoQ and MitoPBN were synthesized as described previously [19,20,25]. BAPTA/AM [bis-(o-aminophenoxy)ethane-N, N, N′, N′-tetra-acetic acid tetrakis] was obtained from Molecular Probes and all other reagents were from Sigma.
Analysis of cell death by flow cytometry
U937 and Jurkat cells were seeded at 4×105 cells/ml in a 24-well plate (0.5 ml/well) and incubated overnight. Cells were preincubated in the presence or absence of mitochondria-targeted antioxidant for 30 min before the addition of the apoptosis inducer. After various time periods, the cells were harvested, washed and stained with annexin V–FITC according to the manufacturer's instructions. Analysis was performed on a FACScan (Becton Dickinson, Cowley, Oxford, U.K.) with CellQuest software. Cells were gated on forward scatter versus side scatter to eliminate cellular debris, and annexin V–FITC-stained cells were defined as apoptotic.
Analysis of caspase activity
U937 cells (2×106 cells/ml) were incubated in the presence or absence of MitoVit E for 30 min before the addition of 5 ng/ml hTNF. After various time periods, the cells were harvested, washed and the cell pellets were snap-frozen and stored at −80 °C. To measure caspase 3 or caspase 8 activity, the frozen cell pellets were rapidly thawed by the addition of 100 μl of 100 mM Hepes, 10% (w/v) sucrose, 5 mM dithiothreitol and 0.1% CHAPS (pH 7.25), supplemented with 50 μM Ac-DEVD-AMC (for caspase 3) or 50 μM Z-IETD-AFC (for caspase 8). The release of AMC or AFC was measured in a PolarStar fluorescence plate reader (AMC, λEX=390 nm and λEM=490 nm; AFC, λEX=390 nm and λEM=510 nm) at 37 °C, calibrated with an AMC or AFC standard curve.
Isolation of cell extracts and Western blotting
For analysis of cytochrome c release, U937 cells were seeded at 2×106 cells/ml in a 12-well plate (1 ml/well) and incubated overnight. Cells were preincubated in the presence or absence of MitoVit E for 30 min before the addition of 5 ng/ml hTNF. After various time periods, the cells were harvested, washed and resuspended in 50 μl of STE buffer (0.25 M sucrose, 5 mM Tris and 1 mM EGTA), supplemented with Complete™ EDTA-free protease inhibitor tablets (Roche Diagnostics, Auckland, New Zealand). Digitonin was added to give 30 μg/mg of protein and, after a 15 min incubation on ice, the samples were microfuged for 5 min. The supernatant (cytosolic fraction) was precipitated with acetone and resuspended in SDS/PAGE sample buffer for analysis. For analysis of Bid cleavage, cells were seeded and treated as described above and whole cell lysates were prepared using RIPA buffer [50 mM Tris, pH 7.4, 1% NP40 (Nonidet P40), 0.25% sodium deoxycholate, 150 mM NaCl and 1 mM EGTA], supplemented with Complete™ EDTA-free protease inhibitor tablets. Proteins were separated by SDS/PAGE (15% polyacrylamide), transferred to a nitrocellulose membrane, blocked with 5% milk powder in TBST (50 mM Tris, 150 mM NaCl and 0.05% Tween 20, pH 7.4) and probed with the appropriate antibody. Blots were developed by the chemiluminescence method (Amersham Biosciences).
EMSA (electrophoretic mobility-shift assay)
Nuclear extracts were prepared from U937 cells (2×106) essentially as described in [26]; 2.5 μg (for NF-κB) or 5 μg [for AP-1 (activator protein-1)] of the nuclear extract was used for EMSAs. NF-κB binding reactions were performed in a total volume of 20 μl containing 25 mM Hepes (pH 7.9), 50 mM NaCl, 0.5 mM EDTA, 0.5 mM dithiothreitol, 5% (v/v) glycerol, 1% NP40, 3 μg of poly(dI-dC)·(dI-dC) and 16 fmol of 32P-labelled consensus NF-κB oligonucleotide probe (Promega, Chilworth, Southampton, U.K.). AP-1 binding reactions were performed in the same reaction mixture containing 16 fmol of 32P-labelled AP-1 oligonucleotide probe (Promega). Reactions mixtures were incubated at 37 °C for 15 min and loaded on to a non-denaturing 4% (w/v) polyacrylamide gel and run in 0.25×TBE buffer. The specificity of binding was determined routinely using a 50 times molar excess of unlabelled oligonucleotide for competition. A Molecular Imager FX (Bio-Rad Laboratories) with Quantity One software was used to visualize and quantify the radioactive bands.
ATP and ADP determination
To measure the cellular ATP/ADP ratio, U937 cells were seeded at 4×105 cells/ml in a 24-well plate (0.5 ml/well). Cells were preincubated in the presence of 2 μM MitoVit E for 15 min before the addition of 5 ng/ml hTNF. After incubation, the cells were mixed rapidly with 6% (v/v) HClO4, snap-frozen and stored at −80 °C. ATP and ADP concentrations were measured by the luciferase/luciferin chemiluminescence technique using a PolarStar with luminescence optics as described previously [27].
Statistics
ANOVA with Tukey–Kramer post-test was performed using GraphPad InStat version 3.0 b for MacIntosh (GraphPad Software, San Diego, CA, U.S.A.). P<0.05 was considered to be statistically significant.
RESULTS
Mitochondria-targeted antioxidants protect cells and isolated mitochondria from oxidative damage
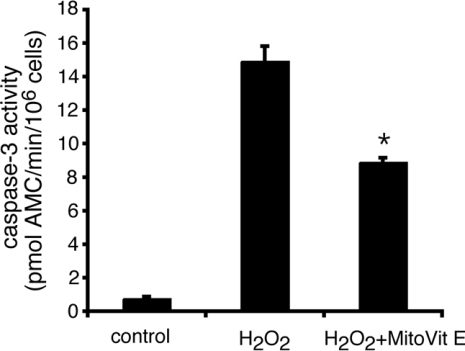
To investigate the role of mitochondria-derived ROS in cell signalling, we synthesized a series of mitochondria-targeted antioxidants that are rapidly taken up into isolated mitochondria and cultured cells, where they protect against oxidative damage [19,20]. MitoVit E (Figure 1) and MitoQ [20,28] effectively inhibit H2O2-induced apoptosis of Jurkat cells, but have no effect on apoptosis induced by either staurosporine, Fas ligation or UV (G. Hughes and E. C. Ledgerwood, unpublished work) [24,28], consistent with the proposed involvement of mitochondrial ROS in apoptosis induced by H2O2, but not the other stimuli.
Figure 1. MitoVit E inhibits H2O2-induced apoptosis of Jurkat cells.
Jurkat cells were incubated with 150 μM H2O2 to induce apoptosis in the presence or absence of 5 μM MitoVit E. After 6 h, caspase 3-like activity was measured in cell extracts with Ac-DEVD-AMC. *P<0.01 compared with H2O2. Results are expressed as means±S.E.M. (n=4).
Mitochondria-targeted antioxidants enhance TNF-induced apoptosis
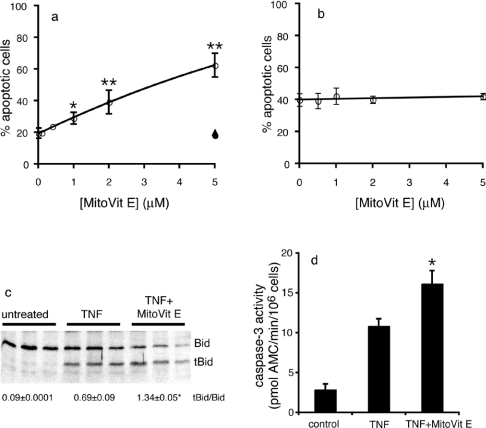
To determine whether mitochondrial ROS are important in TNF-induced apoptosis, we used the well-characterized U937 monocytic cell line [29]. Often, TNF induces apoptosis only when transcriptional or translational inhibitors are used to prevent the TNF-induced expression of antiapoptotic proteins. In the present study, TNF treatment resulted in apoptotic death of U937 cells in the absence of protein synthesis inhibitors, and the onset of death was enhanced in the presence of the protein synthesis inhibitor cycloheximide (Figures 2a and 2b). ROS have been implicated in TNF-induced apoptosis in both the experimental settings [29,30]; therefore the effect of mitochondria-targeted antioxidants on TNF-induced apoptosis of U937 cells in the presence and absence of cycloheximide was determined (Figure 2). Surprisingly, MitoVit E significantly increased TNF-induced apoptosis in a dosedependent manner in the absence (Figure 2a) but not in the presence (Figure 2b) of cycloheximide. MitoVit E (2 μM) doubled the percentage of apoptotic cells after 16 h and, importantly, the control compounds BromoVitE (the cell-permeable non-targeted vitamin E analogue) and TPMP cation (methyltriphenylphosphonium cation; the targeting cation) had no effect on TNF-induced apoptosis (Figure 2a). This confirms that the effect of MitoVit E requires mitochondrial accumulation of the antioxidant and is not due to non-specific mitochondrial damage. MitoQ also caused a significant dose-dependent increase in TNF-induced apoptosis (results not shown), but MitoQ had non-specific cytostatic effects (probably due to its greater hydrophobicity [19,20]) and was not used in subsequent experiments. A third mitochondria-targeted antioxidant, MitoPBN, which reacts rapidly with carbon-centred radicals, but is unreactive with superoxide and most of the lipid peroxidation intermediates [25], had no effect on TNF-induced apoptosis of U937 cells at concentrations up to 5 μM.
Figure 2. Effect of MitoVit E on TNF-induced apoptosis.
(a) U937 cells were incubated with 0–5 μM MitoVit E in the presence of 5 ng/ml hTNF (○). After a 16 h incubation, cells were stained with annexin V–FITC and analysed by flow cytometry. Treatment of cells with the control compounds TPMP (▲) or BromoVit E (●) had no effect on TNF-induced apoptosis. *P<0.05, **P<0.001 compared with TNF alone. (b) U937 cells were incubated with 0–5 μM MitoVit E in the presence of 5 ng/ml hTNF+0.5 μg/ml cycloheximide. After a 90 min incubation, cells were stained with annexin V–FITC and analysed by flow cytometry. (c) U937 cells were left untreated or treated with 5 ng/ml hTNF±2 μM MitoVit E in triplicate for 4 h. Cell lysates were analysed by immunoblotting with an anti-Bid polyclonal antibody. The average tBid/Bid ratio is shown below the blot. *P<0.01 compared with TNF. (d) U937 cells were incubated with 5 ng/ml hTNF±2 μM MitoVit E for 6 h. Caspase 3-like activity was measured in cell extracts with Ac-DEVD-AMC. *P<0.05 compared with TNF. Results are expressed as means±S.E.M. (n=3).
Agents that sensitize cells to TNF-induced death often do so by causing a switch from apoptosis to necrosis [31]. We evaluated whether MitoVit E was causing increased apoptosis or a switch to necrosis by monitoring Bid cleavage, cytochrome c release and caspase activity (Figures 2c and 2d). TNF-induced Bid cleavage, as indicated by increased tBid/Bid ratio (Figure 2c), cytochrome c release (see below) and caspase 3 activity (Figure 2d), were all significantly increased in the presence of MitoVit E. Thus the mitochondria-targeted antioxidant MitoVit E causes increased apoptotic death in cells treated with TNF.
MitoVit E acts by neutralizing mitochondrial ROS. Inhibition of ROS-mediated signalling can sometimes be alleviated by the addition of exogenous ROS [32,33]. The apoptosis-enhancing effect of MitoVit E was not overcome by the addition of either superoxide (by the addition of paraquat) or H2O2 (either as a bolus or by continuous generation with glucose oxidase), suggesting that the timing, location and concentration of TNF-induced mitochondrial ROS production is critical to its apparent antiapoptotic effect.
MitoVit E increases the rate of TNF-induced apoptosis without affecting the initiation phase
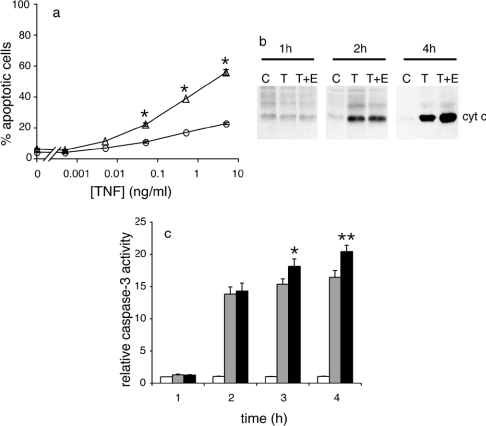
To explore the mechanism of action of MitoVit E, dose–response and time course experiments were performed (Figure 3). The minimum apoptosis-inducing TNF concentration did not decrease when MitoVit E was present (Figure 3a), suggesting that MitoVit E was altering downstream signalling events rather than affecting receptor binding. TNF caused a 10% increase in caspase 8 activity, which was not altered by MitoVit E (results not shown). In time course experiments, Bid cleavage was first observed 2 h after the addition of TNF, and MitoVit E significantly increased the tBid/Bid ratio after 4 h (Figure 2c). Paralleling this result, cytochrome c was first detected in the cytosol 2 h after TNF treatment and MitoVit E enhanced TNF-induced cytochrome c release after 4 h (Figure 3b). Caspase 3 activity had increased 12-fold 2 h after TNF treatment, and MitoVit E significantly increased caspase 3 activity when compared with TNF alone after 3 and 4 h (Figure 3c). Taken together, these results suggest that MitoVit E strengthens the commitment to apoptosis downstream of receptor binding and caspase 8 activation.
Figure 3. MitoVit E increases the rate of TNF-induced apoptosis.
(a) U937 cells were incubated in the presence (Δ) or absence (○) of 2 μM MitoVit E and treated with 0–10 ng/ml hTNF for 16 h. Cells were stained with annexin V–FITC and analysed by flow cytometry. *P<0.01 compared with TNF. Results are expressed as means±S.E.M. (n=3–6). (b) U937 cells were left untreated (C) or treated with 5 ng/ml hTNF (T) or 5 ng/ml hTNF+2 μM MitoVit E (T+E). Cytosolic fractions were analysed by immunoblotting using an anti-cytochrome c antibody. Equal protein loadings were confirmed by reprobing blots with anti-actin. (c) U937 cells were left untreated (white bars) or treated with 5 ng/ml hTNF (grey bars) or 5 ng/ml hTNF+2 μM MitoVit E (black bars) for various time periods. Caspase 3-like activity was measured in cell extracts with Ac-DEVD-AMC and results are expressed relative to control at 1 h. *P<0.05, **P<0.001 compared with TNF. Results are expressed as means±S.E.M. (n=5).
The apoptosis-accelerating effect of MitoVit E is not mediated by changes in ATP
Apoptosis is an energy-dependent process and, while large decreases in either absolute ATP or the ATP/ADP ratio cause necrosis, ATP concentration may be a limiting factor in allowing apoptosis to proceed [34]. Low levels of ROS produced after TNF treatment have been proposed to damage mitochondrial function, thereby decreasing ATP production and slowing down apoptosis [35]. Thus MitoVit E could protect mitochondrial function from ROS-mediated damage and maintain ATP and the ATP/ADP ratio at levels that would allow apoptosis to proceed at a faster rate. The U937 cells had an average ATP concentration of 8.3 nmol/106 cells and an ATP/ADP ratio of 9.8 at the beginning of the experiments, and there was no significant change in either the absolute concentration of ATP or the ATP/ADP ratio within the first 2 h of treatment with TNF or TNF+MitoVit E. Consistent with this result, the apoptosis-enhancing effect of MitoVit E was not overcome by incubation with oligomycin, an ATP synthase inhibitor, which decreases mitochondrial ATP production (results not shown). Thus, in U937 cells, ATP levels and the ATP/ADP ratio do not limit the ability of cells to perform the apoptotic programme in response to TNF stimulation.
Mitochondrial ROS modulate TNF-induced NF-κB activation
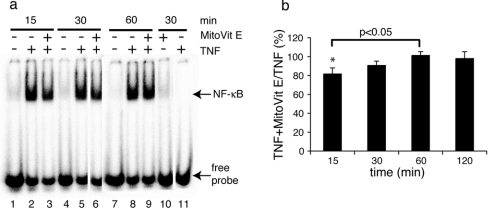
Sensitization to TNF-induced apoptosis is most often attributed to a failure to activate NF-κB and/or AP-1-dependent survival pathways [8]. Since MitoVit E accelerated the rate of TNF-induced apoptosis in the absence but not in the presence of a protein synthesis inhibitor, we reasoned that MitoVit E could be limiting the transcription factor activation. To determine whether this was the case, the binding of the transcription factors NF-κB and AP-1 to their cognate DNA-binding sequences was examined by EMSA (Figure 4). TNF stimulation caused maximal induction of NF-κB DNA-binding activity within 15 min and this was sustained for 2 h (Figures 4a and 4b). In the presence of MitoVit E, maximum DNA binding was not achieved until 60 min (Figure 4b). AP-1 was constitutively activated under our experimental conditions (culture in 10% serum) and TNF did not cause additional activation (results not shown).
Figure 4. MitoVit E delays TNF-induced NF-κB activation.
U937 cells were stimulated with 50 ng/ml hTNF in the presence or absence of 2 μM MitoVit E and nuclei were harvested at the time points indicated. (a) Cells were left unstimulated (lanes 1, 4, 7), stimulated with TNF for 15 min (lane 2), 30 min (lane 5) or 60 min (lane 8), stimulated with TNF+MitoVit E for 15 min (lane 3), 30 min (lane 6) or 60 min (lane 9) and stimulated with MitoVit E for 30 min (lane 10). In lane 11, a 100 times molar excess of unlabelled probe was incubated with the 30 min TNF-stimulated reaction mixture. Results shown are from one representative experiment of six. (b) Relative TNF+MitoVit E NF-κB gel shift expressed as a percentage of the TNF gel shift at 15, 30, 60 and 120 min. *P<0.05 compared with 60 min. Results are expressed as means±S.E.M. (n=3–6).
To investigate how mitochondria might sense increased mitochondrial ROS production and translate this into a cytosolic signal influencing NF-κB activation, two mechanisms were considered, namely changes in mitochondrial membrane potential and intracellular calcium. There was no change in mitochondrial membrane potential for the first 2 h of treatment with either TNF or TNF+MitoVit E, and consistent with this, the mitochondrial permeability transition inhibitors cyclosporin A and bongkrekic acid did not inhibit cell death. The cell-permeable calcium chelator BAPTA/AM also had no effect on TNF-induced apoptosis and did not prevent the MitoVit E-mediated increase in TNF-induced apoptosis.
DISCUSSION
ROS play a dual role in determining cell fate. On the one hand, excess ROS are associated with cell death, and blocking ROS often prevents cell death. On the other hand, low levels of ROS may function as second messengers activating pathways that protect cells against apoptotic stimuli [36]. It is therefore clear that the timing, location and magnitude of ROS production are important factors in determining the fate of cells. There are numerous reports that ROS contribute to TNF signalling, both as pro-death and pro-survival agents. However, the specific source(s) and target(s) of these ROS remain elusive. To determine the role of mitochondrial ROS in TNF-induced apoptosis, we made use of targeted antioxidants that specifically neutralize mitochondrial ROS. These reagents have advantages over alternative approaches such as the use of inhibitors of respiratory chain complexes or cells depleted of mitochondrial DNA (rho0 cells), which have multiple effects on mitochondrial function rather than solely blocking ROS production.
Incubation with TNF induces apoptosis in the U937 monocytic cell line, and ROS have been implicated in the apoptotic process. Interestingly, rather than having a protective effect, the mitochondria-targeted antioxidant MitoVit E significantly increased the amount of cell death after TNF treatment. We determined that pretreatment of U937 cells with MitoVit E accelerated the rate of TNF-induced apoptosis, downstream of caspase 8 activation. These effects required both mitochondrial targeting (provided by the targeting cation TPMP) and antioxidant activity (provided by the vitamin E moiety), since the effects were not seen with either TPMP or BromoVit E. The lack of effect of mitoPBN, which also accumulates in the mitochondrial matrix but targets different radical species, implies that specific mitochondrial oxidant species (superoxide and/or lipid peroxidation products) mediate the MitoVit E effect. Thus, in U937 cells, TNF treatment causes mitochondrial ROS production, and these ROS act as a brake on the apoptotic signalling pathway.
These results are consistent with previous reports that resistance to TNF-induced death is associated with the down-regulation or inhibition of antioxidant enzymes [35]. Moreover, Bai et al. [33,37] have determined the effect of mitochondrial overexpression of antioxidant enzymes on oxidative stress- and TNF-induced apoptosis. Completely in agreement with our observations, the cells overexpressing either catalase or magnesium superoxide dismutase acquired resistance to oxidative stress-induced apoptosis [37], but were sensitized to TNF-induced apoptosis [33]. Taken together, our results obtained by using targeted small molecule antioxidants and the results of Bai et al. [33,37] obtained using targeted antioxidant enzymes demonstrate that mitochondrial ROS have distinct functions in response to different apoptotic stimuli and that they can be involved in both pro- and antiapoptotic signalling.
Currently, there is much interest in understanding the mechanisms that underlie the regulation of NF-κB and, in particular, in reconciling the complexity of responses in which NF-κB family members play important roles. Recent investigations show that the timing and duration of NF-κB activation significantly influence outcomes (including apoptotic outcomes) in a stimulus-dependent manner [38–41]. It is well established that the activation of the transcription factor NF-κB is crucial to preventing or limiting TNF-induced apoptosis in most cell types [8]. The relationship between NF-κB and ROS is controversial. Since exogenous oxidants, such as H2O2, can activate NF-κB in some cell lines (including U937 cells [42]), and high concentrations of antioxidants can block NF-κB activation, NF-κB is often considered to be a ROS-responsive transcription factor [43]. However, the relevance of these pharmacological studies to cytokine-induced cell signalling is unclear. In general, the oxidants are administered at supraphysiological concentrations that do not reflect the kinetics, magnitude or subcellular compartmentalization of ROS produced as a consequence of receptor activation [16,44,45]. Furthermore, the role of ROS in TNF-induced NF-κB activation is primarily implicated from the use of the antioxidants NAC and PDTC [14,46]. The recent demonstration that both these antioxidants act independent of their antioxidant activity, NAC by decreasing the affinity of cell-surface TNFRs for TNF, and PDTC by inhibiting the IκB (inhibitory κB)–ubiquitin ligase [16], makes the role of ROS in TNF-induced NF-κB activation uncertain. Our results provide direct evidence that mitochondrial ROS, at least in part, help define apoptotic sensitivity by controlling the timing of TNF-induced NF-κB activation. This is probably mediated by changes in the pattern of expression of antiapoptotic and/or proapoptotic proteins.
Although our studies preclude the involvement of the other major TNF-responsive transcription factor AP-1, it is possible that other TNF-responsive transcription factors may be regulated by mitochondrial ROS and contribute to the MitoVit E effect. One possibility is the protein kinase B/Akt-regulated FOXO subfamily of forkhead transcription factors, which are important in controlling cell survival and protecting cells against the damaging effects of oxidative stress [47]. Recent results suggest that TNF-induced activation of FOXO4 involves ROS [48], although the non-specific antioxidant NAC was used to implicate ROS. Studies currently underway to compare TNF-induced gene expression in the presence and absence of MitoVit E will enable us to identify the downstream targets, provide insights into the effect of the delayed NF-κB activation on transcription and potentially implicate additional transcription factors.
The mechanism by which mitochondrial ROS influences NF-κB activation is unclear. Interferon γ sensitizes cells to TNF-induced apoptosis, and this is proposed to be due to a ROS-mediated mechanism involving the production of nitric oxide. Nitric oxide reacts with superoxide, decreasing H2O2 formation and decreasing NF-κB activation [32]. It is unlikely that H2O2, formed in mitochondria in response to TNF treatment, diffuses into the cytoplasm to affect directly the NF-κB pathway since untargeted antioxidants were ineffective. Instead, we favour the recently proposed model in which mitochondria are a ‘ROS sensor’ capable of translating changes in mitochondrial ROS production into a signal that is relayed to the cytoplasm [24]. Indeed, mitochondria-derived ROS have recently been proposed to contribute to TNF-induced NF-κB activation by triggering mitochondrial calcium release [13]. However, it is unlikely that this is a universal mechanism since the calcium chelator BAPTA/AM had no effect on TNF- or TNF+MitoVit E-induced apoptosis in U937 cells.
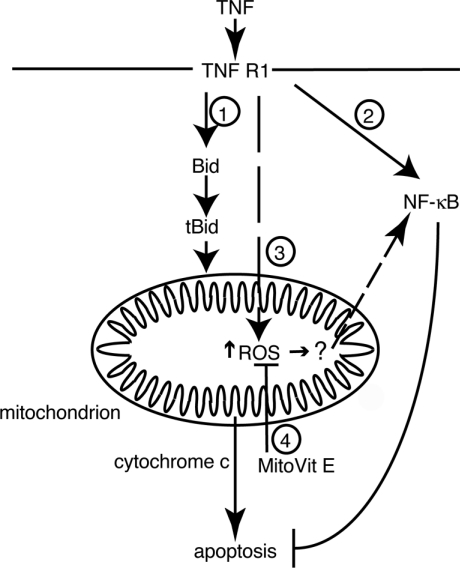
The findings presented here are summarized in Scheme 1. TNF binding leads to the activation of apoptotic (indicated by 1) and transcription factor (2) signalling cascades. In addition to causing the release of cytochrome c and caspase activation (1), TNF also acts on mitochondria through an unknown mechanism to alter the mitochondrial redox balance (3). The increased ROS production is ‘sensed’ by mitochondria that respond by releasing a signal into the cytoplasm to enhance TNF-induced NF-κB activation. In the presence of MitoVit E, this signal is blocked (4), delaying NF-κB activation and thereby enhancing the apoptotic rate. The mechanism by which an increase in mitochondrial ROS is translated into a signal capable of acting on the NF-κB activation pathway remains undefined, but does not require a change in mitochondrial membrane potential, induction of the mitochondrial permeability transition or changes in intracellular calcium.
Scheme 1. Mode of action of MitoVit E in modulating TNF-induced apoptosis.
TNF induces apoptosis (1), gene expression (2) and mitochondrial ROS production (3). By neutralizing TNF-dependent mitochondrial ROS production (4), MitoVit E decreases NF-κB activation, thereby enhancing the apoptotic rate.
Our results add to the growing evidence showing that mitochondria are both a source and a target for ROS, and demonstrate that the mitochondrial redox balance is a critical factor in modulating TNF-induced apoptosis by controlling the timing of TNF-induced NF-κB activation. Furthermore, they provide an experimental basis for future studies on the effect of differences in the temporal activation of NF-κB on gene expression. Unravelling the components of the signal-transduction pathway that are sensitive to ROS may enable more selective therapeutic approaches to disorders involving dysregulation of NF-κB signalling, thus avoiding the occurrence of side effects that would arise from complete deactivation of the TNF signal-transduction cascade.
Acknowledgments
This work was conducted during the tenure of a Repatriation Fellowship from the Health Research Council of New Zealand (to E.C.L.) and supported by project grants from the Otago Medical Research Foundation (to E.C.L.), the Health Research Council of New Zealand (to M.P.M.) and an institutional grant from the University of Otago (to E.C.L.). We thank Dr C. Day and Dr M. Hampton for a critical reading of this paper.
References
- 1.Raha S., Robinson B. H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 3.Haddad J. J. Pharmaco-redox regulation of cytokine-related pathways: from receptor signaling to pharmacogenetics. Free Radical Biol. Med. 2002;33:907–926. doi: 10.1016/s0891-5849(02)00985-1. [DOI] [PubMed] [Google Scholar]
- 4.Brookes P. S., Levonen A.-L., Shiva S., Sarti P., Darley-Usmar V. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radical Biol. Med. 2002;33:755–764. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 5.Garg A. K., Aggarwal B. B. Reactive oxygen intermediates in TNF signaling. Mol. Immunol. 2002;39:509–517. doi: 10.1016/s0161-5890(02)00207-9. [DOI] [PubMed] [Google Scholar]
- 6.Fiers W., Beyaert R., Declercq W., Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 7.Chen G., Goeddel D. V. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 8.Baud V., Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 9.Hennet T., Richter C., Peterhans E. Tumour necrosis factor-α induces superoxide anion generation in mitochondria of L929 cells. Biochem. J. 1993;289:587–592. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem. J. 1989;263:539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Donnell V. B., Spycher S., Azzi A. Involvement of oxidants and oxidant-generating enzyme(s) in tumour-necrosis-factor-α-mediated apoptosis: role for lipoxygenase pathway but not mitochondrial respiratory chain. Biochem. J. 1995;310:133–141. doi: 10.1042/bj3100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higuchi M., Aggarwal B. B., Yeh E. T. H. Activation of CPP32-like protease in tumor necrosis factor-induced apoptosis is dependent on mitochondrial function. J. Clin. Invest. 1997;99:1751–1758. doi: 10.1172/JCI119339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azevedo-Martins A. K., Lortz S., Lenzen S., Curi R., Eizirik D. L., Tiedge M. Improvement of the mitochondrial antioxidant defense status prevents cytokine-induced nuclear factor-kappaB activation in insulin-producing cells. Diabetes. 2003;52:93–101. doi: 10.2337/diabetes.52.1.93. [DOI] [PubMed] [Google Scholar]
- 14.Manna S. K., Zhang H. J., Yan T., Oberley L. W., Aggarwal B. B. Overexpression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-κB and activated protein-1. J. Biol. Chem. 1998;273:13245–13254. doi: 10.1074/jbc.273.21.13245. [DOI] [PubMed] [Google Scholar]
- 15.Gardner P. R., White C. W. Failure of tumor necrosis factor and interleukin-1 to elicit superoxide production in the mitochondrial matrices of mammalian cells. Arch. Biochem. Biophys. 1996;334:158–162. doi: 10.1006/abbi.1996.0441. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa M., Miyashita H., Sakamoto I., Kitagawa M., Tanaka H., Yasuda H., Karin M., Kikugawa K. Evidence that reactive oxygen species do not mediate NF-κB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikkelsen R. B., Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 18.Burkitt M. J., Wardman P. Cytochrome C is a potent catalyst of dichlorofluorescin oxidation: implications for the role of reactive oxygen species in apoptosis. Biochem. Biophys. Res. Commun. 2001;282:329–333. doi: 10.1006/bbrc.2001.4578. [DOI] [PubMed] [Google Scholar]
- 19.Smith R. A. J., Porteous C. M., Coulter C. V., Murphy M. P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 20.Kelso G. F., Porteous C. M., Coulter C. V., Hughes G., Porteous W. K., Ledgerwood E. C., Smith R. A. J., Murphy M. P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 21.Hwang P. M., Bunz F., Yu J., Rago C., Chan T. A., Murphy M. P., Kelso G. F., Smith R. A. J., Kinzler K. W., Vogelstein B. Ferredoxin reductase affects the p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat. Med. 2001;7:1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedogni B., Pani G., Colavitti R., Riccio A., Borrello S., Murphy M., Smith R., Eboli M. L., Galeotti T. Redox regulation of cAMP-responsive element-binding protein and induction of manganous superoxide dismutase in nerve growth factor-dependent cell survival. J. Biol. Chem. 2003;278:16510–16519. doi: 10.1074/jbc.M301089200. [DOI] [PubMed] [Google Scholar]
- 23.Jauslin M. L., Meier T., Smith R. A. J., Murphy M. P. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 24.Chen K., Thomas S. R., Albano A., Murphy M. P., Keaney J. F., Jr Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J. Biol. Chem. 2004;279:35079–35086. doi: 10.1074/jbc.M404859200. [DOI] [PubMed] [Google Scholar]
- 25.Murphy M. P., Echtay K. S., Blaikie F. H., Asin-Cayuela J., Cochemé H. M., Green K., Buckingham J., Taylor E. R., Hurrell F., Hughes G., et al. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation. J. Biol. Chem. 2003;278:48534–48545. doi: 10.1074/jbc.M308529200. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi M. M., Mukhopadhyay A., Aggarwal B. B. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 27.Scarlett J. L., Sheard P. W., Hughes G., Ledgerwood E. C., Ku H.-H., Murphy M. P. Changes in mitochondrial membrane potential during staurosporine-induced apoptosis in Jurkat cells. FEBS Lett. 2000;475:267–272. doi: 10.1016/s0014-5793(00)01681-1. [DOI] [PubMed] [Google Scholar]
- 28.Alleva R., Tomasetti M., Andera L., Gellert N., Borhgi B., Weber C., Murphy M. P., Neuzil J. Coenzyme Q blocks biochemical but not receptor-mediated apoptosis by increasing mitochondrial antioxidant protection. FEBS Lett. 2001;503:46–50. doi: 10.1016/s0014-5793(01)02694-1. [DOI] [PubMed] [Google Scholar]
- 29.Larrick J. W., Wright S. C. Cytotoxic mechanism of tumor necrosis factor-α. FASEB J. 1990;4:3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- 30.Cossarizza A., Franceschi C., Monti D., Salvioli S., Bellesia E., Rivabene R., Biondo L., Rainaldi G., Tinari A., Malorni W. Protective effect of N-acetylcysteine in tumor necrosis factor-α-induced apoptosis in U937 cells: the role of mitochondria. Exp. Cell Res. 1995;220:232–240. doi: 10.1006/excr.1995.1311. [DOI] [PubMed] [Google Scholar]
- 31.Vercammen D., Beyaert R., Denecker G., Goossens V., Van Loo G., Declercq W., Grooten J., Fiers W., Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garbán H. J., Bonavida B. Nitric oxide disrupts H2O2-dependent activation of nuclear factor κB. J. Biol. Chem. 2001;276:8918–8923. doi: 10.1074/jbc.M008471200. [DOI] [PubMed] [Google Scholar]
- 33.Bai J., Cederbaum A. I. Overexpression of catalase in mitochondrial or cytosolic compartment increases sensitivity of HepG2 cells to TNF-α induced apoptosis. J. Biol. Chem. 2000;275:19241–19249. doi: 10.1074/jbc.M000438200. [DOI] [PubMed] [Google Scholar]
- 34.Salvioli S., Barbi C., Dobrucki J., Moretti L., Pinti M., Pedrazzi J., Pazienza T. L., Bobyleva V., Franceschi C., Cossarizza A. Opposite role of changes in mitochondrial membrane potential in different apoptotic processes. FEBS Lett. 2000;469:186–190. doi: 10.1016/s0014-5793(00)01266-7. [DOI] [PubMed] [Google Scholar]
- 35.Chovolou Y., Watjen W., Kampkotter A., Kahl R. Resistance to tumor necrosis factor-alpha (TNF-alpha)-induced apoptosis in rat hepatoma cells expressing TNF-alpha is linked to low antioxidant enzyme expression. J. Biol. Chem. 2003;278:29626–29632. doi: 10.1074/jbc.M208665200. [DOI] [PubMed] [Google Scholar]
- 36.Chandra J., Samali A., Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000;29:323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 37.Bai J., Rodriguez A. M., Melendez J. A., Cederbaum A. I. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J. Biol. Chem. 1999;274:26217–26224. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann A., Levchenko A., Scott M. L., Baltimore D. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 39.Jiang B., Xu S., Hou X., Pimentel D. R., Brecher P., Cohen R. A. Temporal control of NF-kappaB activation by ERK differentially regulates interleukin-1beta-induced gene expression. J. Biol. Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- 40.Bose S., Kar N., Maitra R., DiDonato J. A., Banerjee A. K. Temporal activation of NF-kappaB regulates an interferon-independent innate antiviral response against cytoplasmic RNA viruses. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10890–10895. doi: 10.1073/pnas.1832775100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan C., Yang J., Engelhardt J. F. Temporal pattern of NFkappaB activation influences apoptotic cell fate in a stimuli-dependent fashion. J. Cell Sci. 2002;115:4843–4853. doi: 10.1242/jcs.00151. [DOI] [PubMed] [Google Scholar]
- 42.Kim D. K., Cho E. S., Lee B. R., Um H.-D. NF-κB mediates the adaptation of human U937 cells to hydrogen peroxide. Free Radical Biol. Med. 2001;30:563–571. doi: 10.1016/s0891-5849(00)00504-9. [DOI] [PubMed] [Google Scholar]
- 43.Janssen-Heininger Y. M. W., Poynter M. E., Baeuerle P. A. Recent advances towards understanding redox mechanisms in the activation of nuclear factor κB. Free Radical Biol. Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 44.Bowie A., O'Neill L. A. J. Oxidative stress and nuclear factor-κB activation. A reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 1999;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 45.Stone J. R. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch. Biochem. Biophys. 2004;422:119–124. doi: 10.1016/j.abb.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 46.Quillet-Mary A., Jaffrézou J.-P., Mansat V., Bordier C., Naval J., Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J. Biol. Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- 47.Burgering B. M. T., Medema R. H. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J. Leukoc. Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- 48.Essers M. A. G., Weijzen S., de Vries-Smits A. M. M., Saarloos I., de Ruiter N. D., Bos J. L., Burgering B. M. T. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]