Abstract
A protein expressed in immune cells and muscle was detected in muscle extracts as a substrate for several SAPKs (stress-activated protein kinases). It interacted specifically with the F-actin capping protein CapZ in splenocytes, and was therefore termed ‘CapZIP’ (CapZ-interacting protein). Human CapZIP was phosphorylated at Ser-179 and Ser-244 by MAPKAP-K2 (mitogen-activated protein kinase-activated protein kinase 2) or MAPKAP-K3 in vitro. Anisomycin induced the phosphorylation of CapZIP at Ser-179 in Jurkat cells, which was prevented by SB 203580, consistent with phosphorylation by MAPKAP-K2 and/or MAPKAP-K3. However, osmotic shock-induced phosphorylation of Ser-179 was unaffected by SB 203580. These and other results suggest that CapZIP is phosphorylated at Ser-179 in cells by MAPKAP-K2/MAPKAP-K3, and at least one other protein kinase. Stress-activated MAP kinase family members phosphorylated human CapZIP at many sites, including Ser-68, Ser-83, Ser-108 and Ser-216. Ser-108 became phosphorylated when Jurkat cells were exposed to osmotic shock, which was unaffected by SB 203580 and/or PD 184352, or in splenocytes from mice that do not express either SAPK3/p38γ or SAPK4/p38δ. Our results suggest that CapZIP may be phosphorylated by JNK (c-Jun N-terminal kinase), which phosphorylates CapZIP to >5 mol/mol within minutes in vitro. Osmotic shock or anisomycin triggered the dissociation of CapZIP from CapZ in Jurkat cells, suggesting that phosphorylation of CapZIP may regulate the ability of CapZ to remodel actin filament assembly in vivo.
Keywords: actin, c-Jun N-terminal kinase (JNK), cytoskeleton, immune cell, muscle, p38 mitogen-activated protein kinase (p38 MAPK)
Abbreviations: CaMK, calcium/calmodulin-dependent protein kinase; CapZIP, CapZ-interacting protein; ERK, extracellular-signal-regulated kinase; EST, expressed sequence tag; GST, glutathione S-transferase; HOS, hyperosmotic shock; HSP27, heat-shock protein 27; HUCL, Human Universal cDNA Library; JNK, c-Jun N-terminal kinase; KESTREL, KinaseSubstrate Tracking and Elucidation; MALDI–TOF-MS, matrix-assisted laser-desorption ionization–time-of-flight MS; MAPK(AP-K2/3), mitogen-activated protein kinase (-activated protein kinase 2 or 3); PEG-6000, poly(ethylene glycol)-6000; SAPK, stress-activated protein kinase
INTRODUCTION
Identifying the physiological substrates of the 500-plus protein kinases encoded by the human genome is one of the major challenges in the post-genomic era, and powerful methodologies will be needed to tackle this problem effectively. A few years ago we introduced a new biochemical approach for the identification of protein kinase substrates, termed KESTREL (for Kinase Substrate Tracking and Elucidation), and exploited it to identify eEF2K (eukaryotic elongation factor 2 kinase) as a likely physiological target for SAPK4 (stress-activated protein kinase 4, also called p38δ) [1]. More recently, KESTREL has been used successfully to identify novel physiological substrates for several other protein kinases, including SGK1 (serum- and glucocorticoid-induced kinase 1) [2], PKB (protein kinase B) [3,4] and GSK3 (glycogen synthase kinase 3) [5]. In the present study, we have exploited KESTREL to identify a protein that is targeted by several SAPKs in vivo.
Exposing cells to cellular stresses, such as heat shock, osmotic shock or the protein synthesis inhibitor anisomycin, triggers the activation of several MAPKs (mitogen-activated protein kinases), including SAPK2a (also called p38α MAPK), SAPK2b/p38β, SAPK3/p38γ, SAPK4/p38δ, JNK1 (c-Jun N-terminal kinase-1) and JNK2 (reviewed in [6]). The physiological roles of SAPK2a/p38α include the activation of other protein kinases, such as MAPKAP-K2 (MAPK-activated protein kinase 2) and MAPKAP-K3, MSK1 (mitogen- and stress-activated protein kinase 1) and MSK2, and MNK1 (MAPK-integrating kinase 1) and MNK2 (reviewed in [7]). MAPKAP-K5 [also called PRAK (p38-regulated, activated kinase)] was originally identified as a protein kinase activated by SAPK2/p38α in vitro [8], but recent work has established that it is phosphorylated and activated in vivo by ERK3 (extracellular-signal-regulated kinase-3) [9,10], a distinct MAPK family member which does not appear to be activated by cellular stresses.
Identification of the physiological substrates of SAPK2a/p38α has been greatly facilitated by the availability of relatively specific inhibitors of this protein kinase, most notably SB 203580 [11]. This compound also inhibits SAPK2b/p38β, but not SAPK3/p38γ, SAPK4/p38δ or other MAPK family members [12]. However, the lack of potent and specific inhibitors for SAPK3/p38γ and SAPK4/p38δ has hampered progress in understanding the physiological roles of these enzymes.
The results presented in the present paper started as three separate projects, aimed at using KESTREL to identify new physiological substrates for MAPKAP-K2, SAPK3/p38γ and SAPK4/p38δ in skeletal muscle. Surprisingly, one of the most prominent substrates we detected in skeletal-muscle extracts with all three protein kinases turned out to be the same protein. Here, we identify this protein and demonstrate that it interacts specifically with CapZ, an actin-capping protein. We have therefore termed this substrate ‘CapZIP’ (CapZ-interacting protein). Cellular stresses trigger the dissociation of CapZIP from CapZ, suggesting that CapZIP phosphorylation may modulate the ability of CapZ to remodel actin filaments.
EXPERIMENTAL
Materials
Materials for protein purification, glutathione–Sepharose, PreScission protease and [γ-32P]ATP were purchased from Amersham Biosciences (Little Chalfont, Bucks, U.K.), the GC-rich PCR system and Complete™ protease inhibitor cocktail were from Roche Molecular Biochemicals (Lewes, East Sussex, U.K.) and Ni2+-nitrilotriacetate agarose was from Qiagen (Crawley, West Sussex, U.K.). The human marathon skeletal-muscle cDNA library and HUCL (Human Universal cDNA Library) Array Cloning System were both purchased from Stratagene (La Jolla, CA, U.S.A.), the multiple tissue Northern membrane was from ClonTech (Palo Alto, CA, U.S.A.), SYPRO-Orange stain was from Molecular Probes (Leiden, The Netherlands), and rabbit anti-sheep IgG conjugated to horseradish peroxidase was from Pierce (Tattenhall, Cheshire, U.K.). The sources of other reagents are given elsewhere [1,13].
Expression and purification of proteins
MAPKAP-K2, MAPKAP-K3, SAPK3/p38γ, SAPK4/p38δ, JNK1 and ERK2 were expressed as inactive forms in Escherichia coli strain BL21 as GST (glutathione S-transferase) fusion proteins, MAPKAP-K5 was expressed as a His6-tagged fusion protein in Sf21 cells, and these were converted into their phosphorylated, activated forms, as described previously [12]. ATF2(19–96) and HSP27 (heat-shock protein 27) were also expressed in E. coli as GST fusion proteins, and used as substrates for JNK1α1 and MAPKAP-K2/MAPKAP-K3 respectively.
Protein kinase assays
All protein kinases were assayed at 30 °C, as described previously [12]. One unit of protein kinase activity was that amount catalysing the phosphorylation of 1 nmol of the standard substrate in 1 min.
Purification of MAPKAP-K2 substrate of apparent molecular mass 70 kDa from rabbit skeletal-muscle extracts
The extracts were chromatographed on fast-flow Q-Sepharose, fractionated from 16–24% (w/v) PEG-6000 [poly(ethylene glycol)-600], and the redissolved 24% pellet was then chromatographed on Mono-Q, as described previously [13]. The column was developed with a 40 ml linear salt gradient in buffer A [30 mM Tris/HCl (pH 7.5)0.1 mM EGTA/0.1% (v/v) 2-mercaptoethanol/5% (v/v) glycerol/0.03% (w/v) Brij-35/0.1 mM PMSF/1 mM benzamidine] to 1 M NaCl at a flow rate of 1 ml/min. Fractions of 1 ml were collected, and those containing the MAPKAP-K2 substrate of apparent molecular mass 70 kDa (eluting at 0.20–0.25 M NaCl) were pooled and exchanged into buffer B [30 mM Mes/NaOH (pH 6.0)/0.1 mM EGTA/0.1% (v/v) 2-mercaptoethanol/5% (v/v) glycerol/0.03% (w/v) Brij-35] using a Vivascience spin column. The material was then chromatographed on a 1 ml Hi-Trap Heparin (HP) column, as described for Mono-Q. Fractions containing the 70 kDa protein (eluting at 0.85 M NaCl) were pooled and exchanged into buffer C [50 mM Bistris/HCl (pH 6.5)/0.1 mM EGTA/0.1% (v/v) 2-mercaptoethanol/5% (v/v) glycerol/0.03% (w/v) Brij-35]. Finally, the material was chromatographed on Mono-S equilibrated in buffer C (using a 40 ml linear gradient to 1 M NaCl in buffer C). Fractions containing the substrate (eluting at 0.5 M NaCl) were pooled and dialysed against buffer A.
Purification of a SAPK3/p38γ substrate of apparent molecular mass 70 kDa from rabbit skeletal-muscle extracts
The extracts were chromatographed on SP-Sepharose, fractionated from 16–24% (w/v) PEG-6000, and the redissolved 24% pellet was chromatographed on Source S, as described previously [14]. Fractions of 1 ml were collected, and those containing the substrate (peaking at 0.5 M NaCl) were pooled and chromatographed on Hi-Trap Heparin, as described previously [14]. Fractions containing the substrate (eluting between 0.5 and 0.6 M NaCl) were pooled, concentrated, dialysed against 30 mM Tris/HCl, pH 7.5, containing 0.1 mM EGTA and 0.1% (v/v) 2-mercaptoethanol, and stored in aliquots at −80 °C.
Cloning of full-length human CapZIP
An approx. 900 bp fragment of the cDNA encoding CapZIP was cloned from a human skeletal-muscle cDNA library using a GC-rich PCR system with the following primers: GGCGCCCTCCCTCCAGGCGATTCCG and CGTTTGGTGTGGAGGCCAG. The DNA fragment was then used to screen the HUCL array. Four positive clones were identified from the master membrane, and secondary membranes were obtained for the two most intense signals. A single positive clone was identified in each case, and bacterial cultures were obtained and sequenced. One clone revealed 5′ and 3′ sequence additional to that present in the NCBI database, including a stop codon at the 3′ end. The nucleotide sequence of full-length CapZIP has been deposited with GenBank® under the accession number AY530954.
The full-length cDNA was cloned into the bacterial expression vector pGEX-6P after introduction of EcoRI and Not1 sites. Protein expression was induced for 16 h at 30 °C with 200 μM isopropyl β-D-thiogalactoside. The expressed protein was purified using glutathione–Sepharose, and the GST tag was cleaved on the beads using PreScission protease.
Antibodies
The phosphopeptides RFRRSQpSDCGEL and ALLPGASPKpSPGLKA, corresponding to the sequences surrounding Ser-179 and Ser-108 in CapZIP respectively (where pS is phosphoserine), were synthesized by Dr G. Bloomberg (University of Bristol, U.K.), coupled separately to BSA and keyhole-limpet haemocyanin, then mixed together and injected into a sheep at Diagnostics Scotland (Penicuik, U.K.). The antisera were affinity-purified on a phosphopeptide antigen–Sepharose column and used at 1.0 μg/ml for immunoblotting in the presence of 10 μg/ml of the unphosphorylated peptide immunogen, to neutralize antibodies that recognize the unphosphorylated form of the proteins. Immunodetection by the phosphospecific antibodies was prevented by pre-incubation with the respective phosphopeptide immunogens, but not by pre-incubation with the unphosphorylated forms of these peptides (results not shown). These antibodies recognized as little as 10 ng of the expressed protein. An antibody that immunoprecipitates CapZIP and recognizes phosphorylated and unphosphorylated CapZIP protein equally well was generated by injecting sheep with GST-tagged CapZIP. The antibody was affinity-purified on GST–Sepharose to remove anti-GST antibodies, and then on GST–CapZIP–Sepharose. This antibody also recognized less than 10 ng of expressed CapZIP in immunoblotting experiments.
Antibodies that recognize SAPK2a/p38α and SAPK3/p38γ phosphorylated at their Thr-Gly-Tyr motifs, JNK isoforms phosphorylated at their Thr-Pro-Tyr motifs and MAPKAP-K2 phosphorylated at Thr-334 were purchased from New England Biolabs (Hitchin, Herts., U.K.). An antibody that immunoprecipitates SAPK3/p38γ has been described previously [14]. An anti-CapZα antibody was obtained from BD Biosciences (Oxford, U.K.). Rabbit anti-sheep IgG, goat anti-rabbit IgG and rabbit anti-mouse IgG horseradish peroxidase-conjugated antibodies were from Perbio Science UK (Tattenhall, Cheshire, U.K.).
Cell culture
Jurkat cells were grown in RPMI-1640 medium containing 10% foetal bovine serum in a 95% O2/5% CO2 atmosphere. Cells were incubated for 1 h without or with 10 μM SB 203580 and/or 2 μM PD 184352 before exposure of the cells to anisomycin (10 μg/ml) or an osmotic shock (0.5 M sorbitol). At the times indicated, the cells were lysed in 50 mM Tris/HCl, pH 7.5, containing 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 50 mM sodium β-glycerophosphate, 0.27 M sucrose, 1 μM microcystin-LR, 1% (v/v) Triton X-100, 0.1% (v/v) 2-mercaptoethanol, 0.1 mM PMSF and Complete™ proteinase inhibitor cocktail. Lysates were centrifuged at 13000 g for 15 min at 4 °C, and the supernatants (termed ‘cell extract’) were removed, quick-frozen in liquid nitrogen and stored at −80 °C.
Isolation of primary splenocytes from mice
Wild-type mice and mice that do not express SAPK3/p38γ and SAPK4/p38δ [15] were killed by cervical dislocation, and the spleens were rapidly removed and placed into ice-cold RPMI-1640 medium (Invitrogen). The spleens were cut into small pieces, ground and repeatedly passed through a 19G needle to disrupt the tissue. The suspension was then passed through a nylon-mesh filter and centrifuged for 5 min at 1000 g. Erythrocytes were lysed by incubation for 5 min at 21 °C in solution comprising 0.15 M NH4Cl, 10 mM KHCO3 and 0.1 mM EDTA, pH 7.2. Lysis was terminated by the addition of 4 vol. of PBS, and the splenocytes were collected by centrifugation and resuspended in RPMI-1640 medium containing 5% (v/v) foetal bovine serum. They were then plated into 30 mm culture dishes and incubated in RPMI-1640 medium containing 5% foetal bovine serum for 1 h prior to stimulation.
Immunoprecipitation of CapZIP from cell extracts and immunoblotting
CapZIP was immunoprecipitated from 0.5 mg of cell extract protein with 1 μg of anti-CapZIP antibody coupled with Protein G–Sepharose. SAPK3/p38γ was immunoprecipitated from 0.5 mg of cell extract protein as described previously [14]. After incubation for 1 h at 4 °C, the captured proteins were centrifuged for 5 min at 13000 g, the supernatants discarded and the beads washed twice in lysis buffer containing 0.5 M NaCl, then twice in lysis buffer alone. Samples were denatured in SDS, subjected to SDS/PAGE, transferred to nitrocellulose membranes and immunoblotted using the ECL® detection system (Amersham Biosciences).
RESULTS
Identification of a protein of apparent molecular mass 70 kDa that is phosphorylated efficiently by MAPKAP-K2
Rabbit skeletal-muscle extracts were fractionated as described in the Experimental section. After chromatography on Mono-Q, a protein of apparent molecular mass 70 kDa was identified, which was phosphorylated strongly by MAPKAP-K2, but not at all by the related MAPKAP-K5 (Figure 1A). The substrate was purified further by chromatography on Hi-Trap Heparin and Mono-S, and the peak fraction from the final column was subjected to SDS/PAGE. The major Coomassie Blue-staining band, which co-migrated with the 32P-labelled substrate, was excised, digested with trypsin and analysed by MS (mass spectrometry; Table 1a). Eight tryptic peptides obtained from the purified rabbit muscle protein could be matched to a novel hypothetical human protein in the NCBI database (accession number CAB70910), hereafter called ‘CapZIP’ (for ‘CapZ-interacting protein’).
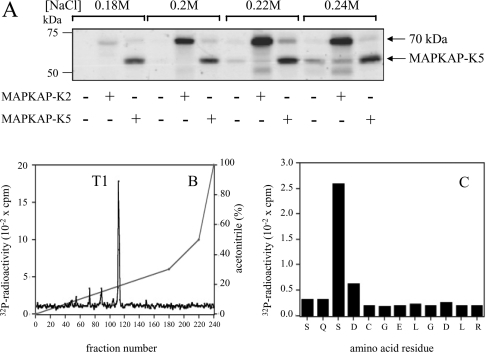
Figure 1. Identification of a 70 kDa protein in rabbit skeletal muscle extracts that is phosphorylated by MAPKAP-K2.
(A) The 16–24% PEG-6000 fraction was chromatographed on Mono-Q, as described in the Experimental section. Aliquots of each fraction were phosphorylated by incubation for 5 min at 30 °C with 10 mM magnesium acetate/20 nM Mg[γ-32P]ATP (4×106 c.p.m./pmol) in the presence (+) or absence (−) of 2 units/ml MAPKAP-K2 or MAPKAP-K5, then subjected to SDS/PAGE and autoradiographed. The 70 kDa substrate eluted between 0.18 and 0.24 M NaCl and the band corresponding to autophosphorylated MAPKAP-K5 is indicated. (B) The 70 kDa substrate from (A) was purified further by chromatography on heparin–Sepharose and Mono-S, maximally phosphorylated by incubation for 30 min at 30 °C with MAPKAP-K2 (2 units/ml) in 10 mM magnesium acetate and 0.1 mM [γ-32P]ATP, and denatured in SDS. Cysteine residues were carbamidomethylated with iodoacetamide, the sample was subjected to SDS/PAGE, and the 32P-labelled protein was excised, digested with trypsin and chromatographed on a Vydac C18 column equilibrated in 0.1% (v/v) trifluoroacetic acid. Columns were developed with an acetonitrile gradient (diagonal lines) at a flow rate of 0.8 ml/min and fractions of 0.4 ml were collected. The continuous (non-diagonal) black line in (B) shows the 32P-radioactivity. (C) Peptide T1 from (B) was subjected to Edman degradation to determine the amino acid sequence and, after coupling the peptide to a Sequalon-AA membrane, to solid-phase sequencing to identify the site (Ser-179) phosphorylated by MAPKAP-K2 [32].
Table 1. The 70 kDa substrates for MAPKAP-K2 and SAPK3/p38γ are the same protein.
Identification of the 70 kDa substrate phosphorylated by MAPKAP-K2 (a) or SAPK3/p38γ (b) is shown. Tryptic digests were analysed by MALDI–TOF-MS (matrix-assisted laser-desorption ionization–time-of-flight MS) using a DE-STR MALDI-TOF mass spectrometer (Applied Biosystems) with 5 mg/ml α-cyanocinnamic acid as the matrix. MALDI–TOF spectra were internally calibrated and peptide ion masses were searched using the MS-FIT program of Protein Prospector (UCSF) run on a local server against NCBInr and Swiss-Prot databases. The tryptic peptides derived from the rabbit 70 kDa substrate shown here matched the peptide masses for the human CapZIP protein (CAB70910; gene index 6807585). ‘m’ in the peptide sequences represents a methionine residue that has become oxidized to the sulphoxide derivative.
| (a) | ||||
|---|---|---|---|---|
| Residue position… | ||||
| Masses submitted | Masses matched | Start | End | Peptide sequence |
| 768.4420 | 768.4593 | 203 | 208 | RRPPSR |
| 768.4420 | 768.4593 | 204 | 209 | RPPSRR |
| 986.5390 | 986.5271 | 72 | 80 | ETPASKPTR |
| 1031.5179 | 1031.5274 | 104 | 113 | SPPNASHPPK |
| 1305.6461 | 1305.5923 | 285 | 295 | QEEDRATEEAK |
| 1924.0040 | 1924.0544 | 125 | 143 | LQANLTFDPAALLPGASPK |
| 1937.9106 | 1937.9544 | 149 | 167 | AMVSPFHSPPSTPSSPGVR |
| 1953.8941 | 1953.9493 | 149 | 167 | AmVSPFHSPPSTPSSPGVR |
| (b) | ||||
| Residue position… | ||||
| Masses submitted | Masses matched | Start | End | Peptide sequence |
| 612.3526 | 612.3582 | 204 | 208 | RPPSR |
| 768.4606 | 768.4593 | 203 | 208 | RRPPSR |
| 768.4606 | 768.4593 | 204 | 209 | RPPSRR |
| 986.5568 | 986.5271 | 72 | 80 | ETPASKPTR |
| 1031.5465 | 1031.5274 | 104 | 113 | SPPNASHPPK |
| 1924.0264 | 1924.0544 | 125 | 143 | LQANLTFDPAALLPGASPK |
| 1937.9213 | 1937.9544 | 149 | 167 | AMVSPFHSPPSTPSSPGVR |
| 1953.9130 | 1953.9493 | 149 | 167 | AmVSPFHSPPSTPSSPGVR |
To investigate whether the MAPKAP-K2 substrate was CapZIP or another protein that co-migrated with it, we maximally phosphorylated the purified rabbit muscle protein with MAPKAP-K2 and Mg[γ-32P]ATP and, after digestion with trypsin, chromatographed the digest on a Vydac C18 column. A single 32P-labelled peptide was detected (Figure 1B), whose sequence (SQSDCGELGDLR) was virtually identical with residues 177–188 of the human CapZIP, the leucine in boldface type being replaced by phenylalanine in the human sequence. The peptide was phosphorylated at the third residue, corresponding to Ser-179 of the human protein (Figure 1C). Two further tryptic peptides in the digest that were not 32P-labelled were subjected to Edman degradation. One of these (LQANLTFDPAALLPGASP) had a sequence identical with that found in the human orthologue of CapZIP, whereas the second (ASVDSSATPSVAQLAGR) was similar, with only the residues in boldface type differing from the human sequence. Taken together, these experiments established that the MAPKAP-K2 substrate and the major protein-staining band in the preparation were indeed the same protein (i.e. CapZIP).
Identification of CapZIP as a protein that is phosphorylated efficiently by SAPK3/p38γ and SAPK4/p38δ in vitro
While using the KESTREL method to identify substrates for SAPK3/p38γ, we identified a protein of apparent molecular mass 70 kDa in muscle extracts that was phosphorylated efficiently by this protein kinase. This substrate was purified (see the Experimental section) and eluted from the final Hi-Trap heparin column at 0.4–0.6 M NaCl. After this step, the substrate co-migrated with a major protein staining band (results not shown), which was excised and digested with trypsin. Analysis by MS revealed that the protein was also CapZIP (Table 1b).
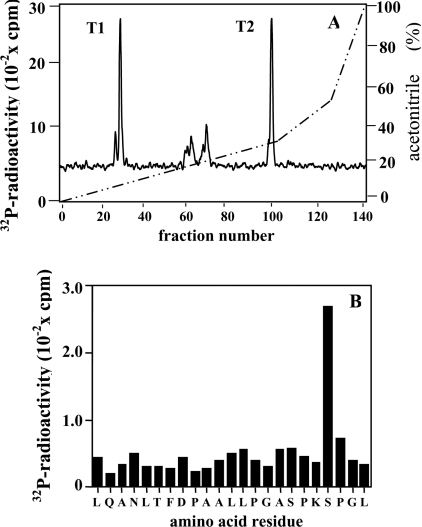
To investigate whether the SAPK3/p38γ substrate was really CapZIP, we maximally phosphorylated the purified rabbit protein with SAPK3/p38γ and Mg[γ-32P]ATP, digested this with trypsin and chromatographed the peptides on a Vydac C18 column. Two major 32P-labelled peptides were detected, termed T1 and T2 (Figure 2A). Peptide T2 was sequenced and shown to comprise residues 79–112 of CapZIP, phosphorylated at Ser-108 (Figure 2B). The identity of peptide T1 is unknown. These experiments established that the SAPK3/p38γ substrate was CapZIP.
Figure 2. Identification of sites on the 70 kDa substrate that are phosphorylated by SAPK3/p38γ.
(A) The 70 kDa substrate for SAPK3/p38γ was purified from rabbit skeletal muscle and phosphorylated as in Figure 1(B), except that SAPK3/p38γ (2 units/ml) replaced MAPKAP-K2. A tryptic digest was then prepared and chromatographed on a Vydac C18 column, as in Figure 1(B). The continuous black line shows the 32P-radioactivity, and the broken diagonal line shows the acetonitrile gradient. T1 and T2 represent the two major 32P-labelled peptides. (B) Peptide T2 from (A) was subjected to Edman degradation to determine the amino acid sequence and, after coupling the peptide to a Sequalon-AA membrane, to solid-phase sequencing to identify the site (Ser-108) phosphorylated by SAPK3/p38γ [32].
Using an analogous approach, we also identified CapZIP in a separate KESTREL screen aimed at identifying novel substrates for SAPK4/p38δ (A. Knebel, unpublished work).
Cloning of human CapZIP
The cDNA encoding human CapZIP was cloned as described in the Experimental section. Since the predicted molecular mass (44.5 kDa) was much lower than that estimated for the purified rabbit protein by SDS/PAGE (70 kDa), we expressed the human protein as a GST fusion protein. Following removal of the GST tag by digestion with PreScission protease, the cleaved protein migrated with an apparent molecular mass of 70 kDa (results not shown). Abnormally low binding of SDS may explain why CapZIP migrates atypically on SDS/polyacrylamide gels. The sequence of human CapZIP is compared with the murine sequence in Figure 3. The two proteins display 63% overall identity, with 90% identity over the first 180 residues and far less similarity thereafter. The murine protein is annotated in the NCBI database as ‘similar to neurofilament, heavy polypeptide’ (AAH25872), presumably because of the presence of two Lys-Ser-Pro sequences (residues 67–69 and residues 107–109). In the neurofilament polypeptide, the serine residues that lie within these sequences are phosphorylated by neuronal protein kinases [16,17]. CapZIP is also phosphorylated at these serines (Figure 2B, and see below), but there is no other obvious sequence homology with the neurofilament heavy subunit or other human proteins.
Figure 3. Comparison of the amino acid sequences of human and mouse CapZIP.
Identities and similarities are highlighted by reversed-out letters on a black or grey background respectively. Black asterisks denote sites identified after phosphorylation by SAPK3/p38γ; grey asterisks denote additional sites identified after phosphorylation by JNK1α1, whereas ‘open’ asterisks with white centres denote sites phosphorylated by MAPKAP-K2.
Phosphorylation of CapZIP by different SAPKs in vitro
We maximally phosphorylated human CapZIP with MAPKAP-K2, which revealed that it was phosphorylated at Ser-244, as well as Ser-179 (results not shown). Like Ser-179, Ser-244 of human CapZIP lies within a consensus sequence for phosphorylation by MAPKAP-K2, which is Hyd-Xaa-Arg-Xaa-Xaa-Ser- (where Hyd is a bulky hydrophobic residue and Xaa is any amino acid) [18]. However, Ser-244 is not conserved in murine CapZIP, being replaced by Asn (Figure 3). Lack of conservation of Ser-244, or lack of a MAPKAP-K2 consensus sequence surrounding this site, may explain why purified rabbit CapZIP was only phosphorylated by MAPKAP-K2 at Ser-179 in vitro (Figures 1B and 1C). In contrast, Ser-179 and the sequence surrounding it is highly conserved in human, mouse (Figure 3) and rabbit CapZIP.
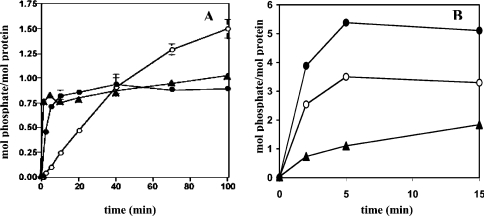
MAPKAP-K3 is a protein kinase that is closely related to MAPKAP-K2 [19]. Human CapZIP was a remarkably good substrate for MAPKAP-K3 as well as MAPKAP-K2, the initial rate of phosphorylation greatly exceeding that of HSP27 (Figure 4A), an established physiological substrate for MAPKAP-K2 [20–22].
Figure 4. Phosphorylation of CapZIP by different protein kinases in vitro.
Incubations were carried out at 30 °C in the presence of 10 mM magnesium acetate and 0.1 mM [γ-32P]ATP, as described in [12]. (A) Bacterially expressed human CapZIP (1 μM) was phosphorylated with 2 units/ml MAPKAP-K2 (closed circles) or MAPKAP-K3 (closed triangles) for the times indicated. For comparison, the phosphorylation of HSP27 (1 μM) by MAPKAP-K2 was examined in parallel (open circles). (B) Phosphorylation of CapZIP (1 μM) with 1 unit/ml JNK1α1 (closed circles), 1 unit/ml SAPK3/p38γ (open circles) or 1 unit/ml SAPK4/p38δ (closed triangles).
CapZIP was also phosphorylated rapidly by SAPK3/p38γ and SAPK4/p38δ, and even faster and more extensively by JNK1α1, these protein kinases phosphorylating CapZIP in vitro to >3, approx. 2 and >5 mol of phosphate/mol of protein respectively within a few minutes. Following tryptic digestion and C18 chromatography, further sites phosphorylated by JNK1α1 were identified as Ser-68, Ser-83 and Ser-216 (results not shown), and are highlighted in Figure 3.
Tissue distribution and chromosomal location of human CapZIP
Northern blot analysis showed that the mRNA encoding CapZIP was strongly expressed in skeletal muscle and at lower levels in cardiac muscle, but was barely detectable in the brain, placenta, lung, liver, kidney and pancreas. A major 3.4 kb and a minor 7 kb transcript were observed in both striated muscles (results not shown). ESTs (expressed sequence tags) have also been detected in smooth muscles. The human gene has been mapped to the short arm of chromosome 1 at position 24.
A more extensive analysis using a multiple-tissue expression array demonstrated that the mRNA encoding CapZIP was also expressed in multiple cells of the immune system, including the spleen, thymus, peripheral blood leucocytes, lymph node and bone marrow. Consistent with this, ESTs encoding CapZIP have been isolated from B cells and leukaemia and lymphoma cell lines (results not shown).
CapZIP is phosphorylated at Ser-179 in cells
A phosphospecific antibody was raised that only recognized CapZIP when it was phosphorylated at Ser-179. Using this antibody, we showed that CapZIP was phosphorylated at this residue by MAPKAP-K3 as well as by MAPKAP-K2 in vitro (results not shown).
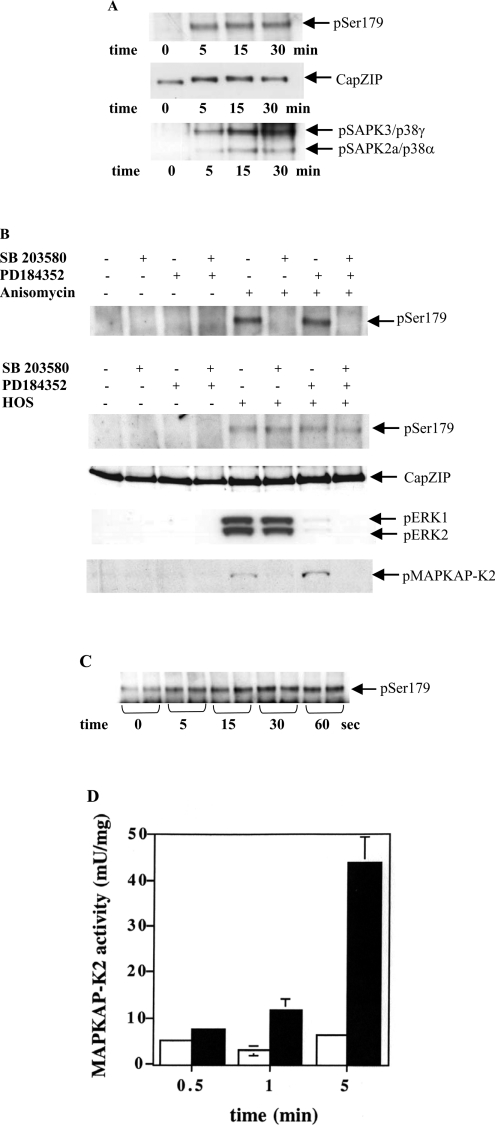
CapZIP is expressed in immune cells, as well as muscle, and we found that CapZIP was expressed at easily detectable levels in Jurkat cells. This T-cell line was therefore used to investigate the site-specific phosphorylation of CapZIP in cells. CapZIP became phosphorylated at Ser-179 when Jurkat cells were exposed to HOS (hyperosmotic shock). Phosphorylation was maximal after 5 min, maintained for at least 30 min, and this was associated with a decrease in its electrophoretic mobility (Figure 5A). The phosphorylation (activation) of SAPK2a/p38α and SAPK3/p38γ followed a similar time course. Ser-179 also became phosphorylated in response to the protein synthesis inhibitor anisomycin (Figure 5B) or sodium arsenite (results not shown). The same stimuli also induced the phosphorylation of Ser-179 in HL60 cells (results not shown).
Figure 5. Investigation of signalling pathways that mediate the phosphorylation of CapZIP at Ser-179.
(A) Jurkat T-cells were exposed to HOS (0.5 M sorbitol) for the times indicated. Following cell lysis, CapZIP was immunoprecipitated and immunoblotted (see the Experimental section) with a phosphospecific antibody that recognizes CapZIP phosphorylated at Ser-179 (pSer179), or with an antibody that recognizes the phosphorylated and unphosphorylated forms of CapZIP equally well (CapZIP). SAPK3/p38γ was immunoprecipitated [14], then probed with a phosphospecific antibody that detects SAPK3/p38γ and SAPK2a/p38α phosphorylated at the Thr-Gly-Tyr motifs. The anti-SAPK3/p38γ immunoprecipitates a small amount of the SAPK2a/p38α. (B) Jurkat cells were incubated for 1 h without (−) or with (+) 10 μM SB 203580 and/or 2 μM PD 184352, then left untreated (−) or exposed (+) for 15 min to either 10 μg/ml of the protein synthesis inhibitor anisomycin (uppermost panel) or HOS (bottom four panels). CapZIP was immunoprecipitated from the cell extracts and immunoblotted with the antibodies used in (A). The lysates (30 μg of protein) from osmotically shocked cells were also immunoblotted with phosphospecific antibodies that recognize ERK1/ERK2 phosphorylated at their Thr-Glu-Tyr motifs and MAPKAP-K2 phosphorylated at Thr-334. (C) Hind-limbs from adult male Wistar rats were stimulated at tetanic frequencies for the times indicated, and the muscle was frozen and extracted exactly as described previously [13]. CapZIP was immunoprecipitated from extracts and immunoblotted with an antibody that recognizes CapZIP phosphorylated at Ser-179. (D) The experiment was performed for the times indicated as in (C), except that MAPKAP-K2 was immunoprecipitated from 50 μg of muscle extract protein and assayed as described previously [13]. Results with electrically stimulated muscle are shown as black bars; results from the control unstimulated muscle are shown as white bars.
Exposure to HOS or anisomycin triggers the activation of several MAPK cascades, raising the question of which protein kinase(s) is/are responsible for the phosphorylation of Ser-179 in cells. We found that the anisomycin-induced phosphorylation of Ser-179 was prevented by SB 203580, consistent with phosphorylation by MAPKAP-K2 and/or MAPKAP-K3. However, HOS-induced phosphorylation of Ser-179 in Jurkat cells was unaffected by SB 203580, although the activity of SAPK2a/p38α was inhibited, as shown by complete suppression of the phosphorylation (activation) of MAPKAP-K2 (Figure 5B). The phosphorylation of Ser-179 was also unaffected by PD 184352 [23], a specific inhibitor of MAPK kinase-1 (MKK1, also called MEK), the ‘upstream’ activator of ERK1 and ERK2, either in the presence or absence of SB 203580 (Figure 5B). In these experiments, PD 184352 prevented the phosphorylation (activation) of ERK1 and ERK2, as expected (Figure 5B). These results demonstrate that MAPKAP-K2/MAPKAP-K3 are not rate-limiting for HOS-induced phosphorylation of Ser-179, and that at least one other protein kinase is involved that is independent of the SAPK2/p38 pathway.
We also showed that electrical excitation of rat skeletal muscle induced the phosphorylation of CapZIP at Ser-179 and that this was maximal after 30 s (Figure 5C), a time at which MAPKAP-K2 was not yet activated (Figure 5D; see also [13]). Thus contraction-induced phosphorylation of Ser-179 must also be mediated by a protein kinase distinct from MAPKAP-K2.
CapZIP is phosphorylated at Ser-108 in cells
To study the phosphorylation of Ser-108 in cells, we raised a phosphospecific antibody that only recognized CapZIP when it was phosphorylated at this residue. Using this antibody, we showed by immunoblotting that bacterially expressed CapZIP was phosphorylated at Ser-108 by SAPK4/p38δ, JNK1α1 and ERK2 in vitro, as well as by SAPK3/p38γ (results not shown).
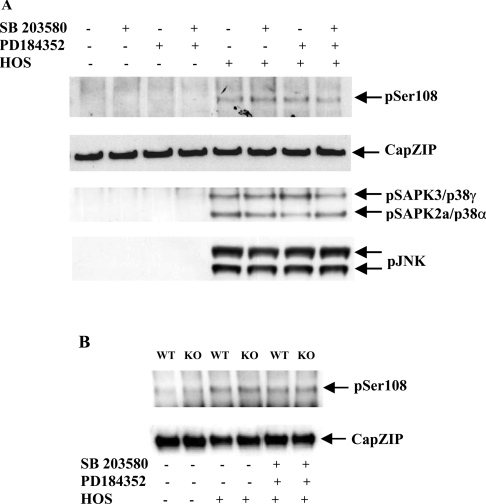
HOS induced phosphorylation of CapZIP at Ser-108 in Jurkat cells, and this was unaffected by SB 203580 and PD 184352, alone or in combination (Figure 6A), excluding the involvement of the MAPK family members SAPK2a/p38α, SAPK2b/p38β, ERK1 and ERK2 in the phosphorylation of Ser-108 under these conditions (Figure 6A).
Figure 6. Investigation of signalling pathways that mediate the phosphorylation of CapZIP at Ser-108.
(A) CapZIP and SAPK3/p38γ were immunoprecipitated from the same lysates used in Figure 5(B), and immunoblotted with an antibody that recognizes CapZIP phosphorylated at Ser-108 (pSer108), an antibody that recognizes the phosphorylated and dephosphorylated forms of CapZIP equally well (CapZIP) and an antibody that recognizes SAPK3/p38γ and SAPK2a/p38α, phosphorylated at their Thr-Gly-Tyr motifs (pSAPK3/p38γ, SAPK2a/p38α). Further aliquots of the lysates (30 μg of protein) were also immunoblotted with phosphospecific antibodies that recognize JNK isoforms phosphorylated at their Thr-Pro-Tyr motifs. (B) Primary splenocytes isolated from wild-type (WT) mice and mice that do not express SAPK3/p38γ and SAPK4/p38δ (knockout; KO) were incubated for 1 h without (−) or with (+) 10 μM SB 203580 plus 2 μM PD 184352, then exposed for 15 min to HOS (0.5 M sorbitol). Following cell lysis, CapZIP was immunoprecipitated from 2 mg of cell extract protein and immunoblotted with an antibody that recognizes CapZIP phosphorylated at Ser-108, and an antibody that recognizes both phosphorylated and unphosphorylated CapZIP equally well.
HOS induced the phosphorylation of CapZIP at Ser-108 similarly in primary splenocytes from both wild-type mice and those that do not express either SAPK3/p38γ or SAPK4/p38δ (Figure 6B), indicating that, under these conditions, neither of these protein kinases was rate-limiting for the phosphorylation of CapZIP at Ser-108. SB 203580 plus PD 184352 did not suppress HOS-induced phosphorylation of Ser-108 in primary murine splenocytes that do not express SAPK3/p38γ and SAPK4/p38δ (Figure 6B), demonstrating that SAPK2a/p38α, SAPK2b/p38β, ERK1 and ERK2 are not able to compensate for the lack of SAPK3/p38γ and SAPK4/p38δ.
CapZIP binds specifically to CapZ in spleen extracts
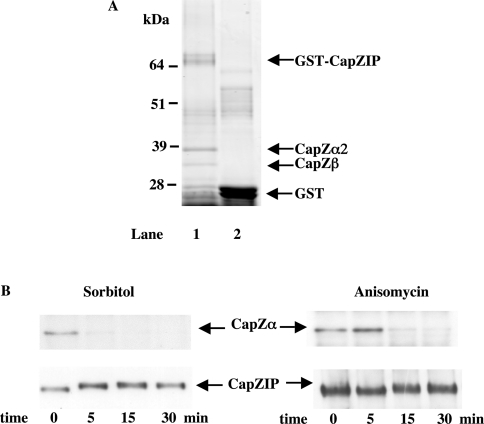
In order to identify proteins that interact specifically with CapZIP, we immobilized GST–CapZIP on glutathione–Sepharose and incubated these beads with mouse spleen extract. After extensive washing with 0.5 M NaCl, the beads were denatured in SDS and subjected to SDS/PAGE. These experiments identified two protein-staining bands that bound to GST–CapZIP, but not to GST (Figure 7A). They were excised from the gel, digested with trypsin and identified by MS. Fifteen peptides from the more slowly migrating band could be matched to the α-subunit of the actin-capping protein CapZ (Table 2a), whereas 15 peptides from the faster migrating band could be matched with the β subunit of CapZ [23,24] (Table 2b).
Figure 7. Effect of phosphorylation of CapZIP on its interaction with CapZ.
(A) A sample (5 μg) of bacterially expressed mouse GST–CapZIP (lane 1) or GST (lane 2) were coupled with 40 μl of a 1:1 slurry of glutathione–Sepharose 4B beads in cell-lysis buffer and incubated for 1 h at 4 °C with 10 mg of mouse spleen tissue extract. Beads were collected by centrifugation, and washed twice in lysis buffer containing 0.5 M NaCl, and once in lysis buffer alone. Samples were denatured, subjected to SDS/PAGE on 4–12% polyacrylamide gels and the gels were stained with colloidal Coomassie Blue. (B) Jurkat cells were exposed to osmotic stress (0.5 M sorbitol) or to anisomycin (10 μg/ml) for the times indicated. CapZIP was immunoprecipitated from the lysates, denatured in SDS, subjected to SDS/PAGE, transferred to nitrocellulose membranes and immunoblotted with an antibody that recognize CapZα and CapZIP.
Table 2. Identification of the subunits of CapZ as proteins that interact with CapZIP.
Identification of the more slowly migrating band in Figure 6(A) as CapZα2 (a) and of the more rapidly migrating band in Figure 6(A) as CapZβ (b) is shown. Tryptic digests were analysed by MALDI–TOF MS using a 4700 Proteomics analyser (Applied Biosystems) with 5 mg/ml α-cyannocinnamic acid as the matrix. MALDI–TOF spectra were internally calibrated and five peptide ions were automatically selected for MALDI–MS/MS fragmentation, and the combined MS and MS/MS data were searched using the Mascot (MatrixScience) search engine on a local server. Peptide sequences in boldface type were matched by both mass and MALDI MS/MS fragmentation. ‘m’ in the peptide sequences represents a methionine residue that has become oxidized to the sulphoxide derivative.
| (a) | ||||
|---|---|---|---|---|
| Residue position… | ||||
| Masses submitted | Masses calculated | Start | End | Peptide sequence |
| 869.5315 | 869.5230 | 260 | 266 | RQLPVTR |
| 876.4785 | 876.4791 | 122 | 129 | TSVETALR |
| 1197.6949 | 1197.6877 | 38 | 47 | LLLNNDNLLR |
| 1523.6947 | 1523.6729 | 134 | 146 | EHYPNGVCTVYGK |
| 1590.9100 | 1590.8888 | 179 | 193 | FTVTPSTTQVVGILK |
| 1747.8708 | 1747.8701 | 211 | 226 | DIQDSLTVSNEVQTAK |
| 2029.0137 | 2028.9944 | 194 | 210 | IQVHYYEDGNVQLVSHK |
| 2101.0500 | 2101.0271 | 20 | 37 | FIIHAPPGEFNEVFNDVR |
| 2107.0242 | 2107.0088 | 48 | 66 | EGAAHAFAQYNLDQFTPVK |
| 2133.9834 | 2133.9646 | 104 | 121 | EATDPRPYEAENAIESWR |
| 2173.0706 | 2173.0474 | 148 | 166 | VDGQQTIIACIESHQFQAK |
| 2187.0564 | 2187.0425 | 67 | 86 | IEGYEDQVLITEHGDLGNGK |
| 2301.1655 | 2301.1353 | 147 | 166 | KVDGQQTIIACIESHQFQAK |
| 2999.3462 | 2999.3450 | 231 | 256 | IVEAAENEYQTAISENYQTmSDTTFK |
| 3323.5735 | 3323.6116 | 231 | 259 | IVEAAENEYQTAISENYQTMSDTTFKALR |
| (b) | ||||
| Residue position… | ||||
| Masses submitted | Masses calculated | Start | End | Peptide sequence |
| 952.5461 | 952.5507 | 16 | 23 | LPPQQIEK |
| 1057.6000 | 1057.5936 | 260 | 268 | NDLVEALKR |
| 1108.6472 | 1108.6482 | 15 | 23 | RLPPQQIEK |
| 1171.5992 | 1171.5991 | 226 | 235 | STLNEIYFGK |
| 1231.5411 | 1231.5425 | 58 | 66 | DYLLCDYNR |
| 1353.6426 | 1353.6448 | 182 | 195 | SGSGTmNLGGSLTR |
| 1534.6842 | 1534.6857 | 79 | 92 | YDPPLEDGAmPSAR |
| 1550.8247 | 1550.8257 | 169 | 181 | LTSTVmLWLQTNK |
| 1568.7339 | 1568.7338 | 96 | 108 | LEVEANNAFDQYR |
| 1685.7952 | 1685.7943 | 146 | 159 | GCWDSIHVVEVQEK |
| 1696.8289 | 1696.8330 | 95 | 108 | KLEVEANNAFDQYR |
| 1770.8075 | 1770.8054 | 200 | 215 | DETVSDCSPHIANIGR |
| 2303.0391 | 2303.0808 | 196 | 215 | QmEKDETVSDCSPHIANIGR |
| 2783.4382 | 2783.4480 | 24 | 48 | NLSDLIDLVPSLCEDLLSSVDQPLK |
| 3013.5344 | 3013.5928 | 109 | 135 | DLYFEGGVSSVYLWDLDHGFAGVILIK |
To examine whether the endogenous CapZIP was bound to the endogenous CapZ in immune cells, we immunoprecipitated CapZIP from Jurkat cell extracts. After denaturation in SDS, SDS/PAGE and transfer to nitrocellulose, immunoblotting with a specific antibody revealed the presence of the α-subunit of CapZ. These experiments established that the endogenous CapZIP and CapZ do indeed interact (Figure 7B).
We then examined whether agonists that induce the phosphorylation of CapZIP affect its interaction with CapZ. Interestingly, exposure for 5 min to HOS, or for 15 min to anisomycin, caused CapZIP to dissociate from CapZα (Figure 7B).
DISCUSSION
In the present paper we have identified CapZIP as a protein that is phosphorylated exceptionally rapidly by several SAPKs in vitro (Figure 4), and which is expressed in muscles and immune cells. Both MAPKAP-K2 and MAPKAP-K3 phosphorylated CapZIP at Ser-179 in vitro, and this residue became phosphorylated when cells were exposed to several of the cellular stresses that induce the activation of these protein kinases (Figures 5A and 5B). The anisomycin-induced phosphorylation of CapZIP at Ser-179 was prevented by SB 203580 in Jurkat cells, consistent with phosphorylation of this residue being catalysed by MAPKAP-K2/K3, but the HOS-induced phosphorylation of Ser-179 was not. Thus an additional protein kinase(s) is clearly involved in mediating Ser-179 phosphorylation by this stimulus that is independent of the SAPK2/p38 pathway. Interestingly, CapZIP also became phosphorylated when skeletal muscle was made to contract by stimulating it electrically (Figure 5C). At the frequency of electrical stimulation employed, the phosphorylation of CapZIP at Ser-179 reached a maximum level after approx. 30 s, a time at which MAPKAP-K2 is not yet activated [13], again pointing to the involvement of another protein kinase, perhaps a CaMK (calcium/calmodulin-dependent protein kinase). MAPKAP-K2/K3 belong to the same protein kinase subfamily as the CaMKs, and CaMKII and MAPKAP-K2 are known to phosphorylate similar motifs when synthetic peptides are used as substrates, as shown previously [17]. HOS is known to elevate the intracellular concentration of calcium ions [24], raising the possibility that a CaMK phosphorylates Ser-179 in response to this stimulus.
CapZIP was also found to be a remarkably good substrate for several stress-activated MAPKs in vitro and, indeed, is far and away the best substrate for JNK that has so far been identified. MAPKs phosphorylate Ser/Thr-Pro sequences, 17 of which are present in human CapZIP, 11 being conserved in the mouse protein (Figure 3). There are also four Ser/Thr-Pro sequences in the C-terminal region of mouse CapZIP that are not conserved in human CapZIP. We have shown that at least four Ser-Pro sequences, conserved in human and murine CapZIP, can be phosphorylated by stress-activated MAPKs in vitro. So far, we have only examined the phosphorylation of one of these residues in cells, namely Ser-108. This residue became phosphorylated when Jurkat cells or primary splenocytes were exposed to HOS (Figure 6A). The phosphorylation of this residue was not prevented by SB 203580 and/or PD 184352, demonstrating that SAPK2a/p38α, SAPK2b/p38β, ERK1 and ERK2 are not rate-limiting for the phosphorylation of this residue under these conditions. HOS also induced a similar phosphorylation of Ser-108 in primary splenocytes from wild-type mice or mice that do not express SAPK3/p38γ or SAPK4/p38δ (Figure 6B). Thus, although we originally identified CapZIP as a protein in muscle extracts that is phosphorylated rapidly by SAPK3/p38γ or SAPK4/p38δ, these protein kinases are dispensable for the HOS-induced phosphorylation of Ser-108 in splenocytes. The protein kinase(s) that phosphorylate Ser-108 in response to HOS remain to be identified, but JNK isoforms are likely candidates, since CapZIP is such a good substrate for this protein kinase in vitro. However, the stress-activated MAPKs that phosphorylate Ser-108 may well vary with cell type and/or the type of stress to which they are exposed. For example, SAPK3/p38γ is expressed at extremely high levels in muscle, and might play a more important role in phosphorylating CapZIP in this tissue. Moreover, as CapZIP is likely to be phosphorylated at multiple Ser/Thr-Pro sequences in cells, different MAPKs may be involved in phosphorylating distinct residues.
An important clue to the function of CapZIP and its phosphorylation came from the finding that it binds to the actin-capping protein CapZ (Figure 7A), and that cellular stresses trigger the dissociation of these two proteins (Figure 7B). CapZ is a heterodimer composed of two subunits CapZα and CapZβ [25,26]. It is thought to regulate actin filament assembly and organization by binding to the barbed ends of the filaments. Here it works as a ‘cap’, preventing the addition and/or the loss of actin monomers at the ends, and can therefore reduce the length of actin filaments. One could therefore envisage that the interaction between CapZIP and CapZ affects the ability of the latter to remodel actin filaments. Such an effect is presumably lost when CapZIP is phosphorylated and dissociates from CapZ.
Although much further work is clearly needed to determine how CapZIP modulates the function of CapZ in the immune cells and muscles where it is expressed, it is intriguing that CapZIP is yet another example of an actin-binding protein that is a physiological substrate for MAPKAP-K2. For example MAPKAP-K2 phosphorylates HSP27 [20–22], a protein also known to act as an actin Cap-binding protein in vitro [27]. When phosphorylated, HSP27 contributes to enhanced actin polymerization and reorganization in vivo, a process thought to be involved in protecting the cytoskeleton from stress-induced damage, thereby aiding cell survival [28]. Interestingly, it has recently been reported that the amount of HSP27 and CapZ associated with actin increased and decreased, respectively, when smooth muscle from the saphenous vein was exposed to haemodynamic stress [29]. These changes would favour the generation of contractile stress fibres. An intriguing possibility is that these effects are both mediated by MAPKAP-K2 via the phosphorylation of HSP27 and CapZIP respectively. MAPKAP-K2 is also implicated in regulating another actin-dependent process, namely the chemotactic migration of cells to sites of inflammation. This appears to involve the phosphorylation of further MAPKAP-K2 substrates, such as LSP1 (lymphocyte-specific protein 1) [30,31] and NOGO-B (S. Rousseau, M. Peggie, D. G. Campbell, A. Nebreda and P. Cohen, unpublished work), but the possibility that CapZIP is also involved in this process merits investigation.
Finally, we note that the present study has again illustrated the power of the KESTREL method to identify interesting new physiological substrates of protein kinases.
Acknowledgments
We thank the UK Medical Research Council for providing a studentship (to C.E.E.), the European Molecular Biology Organization for a Long Term Fellowship (to A.K.) and Dr Simon Rousseau for helpful discussions. We thank the Protein Production and Antibody Production Teams in the Division of Signal Transduction Therapy at Dundee (co-ordinated by Hilary McLauchlan and James Hastie) for the protein kinases and antibodies used in this study, and the Sequencing Service (co-ordinated by Nick Helps) for DNA sequencing. The research was supported by the Medical Research Council, the Royal Society, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck and Co, Merck KGaA and Pfizer.
References
- 1.Knebel A., Haydon C. E., Morrice N., Cohen P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38δ. Biochem. J. 2002;367:525–532. [Google Scholar]
- 2.Murray J. T., Campbell D. G., Morrice N., Auld G. C., Shpiro N., Marquez R., Peggie M., Bain J., Bloomberg G. B., Grahammer F., et al. Exploitation of KESTREL to identify N-myc downstream regulated gene family members as physiological substrates for SGK1 and GSK3. Biochem. J. 2004;384:477–488. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray J. T., Campbell D. G., Peggie M., Mora A., Cohen P. The identification of Filamin C as a new physiological substrate of PKBα using KESTREL. Biochem. J. 2004;384:489–494. doi: 10.1042/BJ20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartlidge R. A., Knebel A., Peggie M., Alexandrov A., Phizicky E. M., Cohen P. The tRNA methylase METTL1 is phosphorylated and inactivated by protein kinase B and p90 ribosomal protein S6 kinase in vitro and in cells. EMBO J. 2005 doi: 10.1038/sj.emboj.7600648. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole A. R., Knebel A., Morrice N., Robertson L. S., Irving A. J., Connelly C. N., Sutherland C. GSK3 phosphorylation of the Alzheimer's epitope within collapsin response mediator proteins regulates axon elongation in primary neurones. J. Biol. Chem. 2004;279:50176–50180. doi: 10.1074/jbc.C400412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 7.Arthur S., Cohen P. Sigma-RBI Handbook, (4th edn), ‘MAPK-activated protein kinases’. 2001. pp. 174–175. [Google Scholar]
- 8.New L., Jiang Y., Zhao M., Liu K., Zhu W., Flood L. J., Kato Y., Parry G. C., Han J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher S., Laa K., Kant S., Shi Y., Visel A., Gruber A. D., Kotlyarov A., Gaestel M. Scaffolding by ERK3 regulates MK5 in development. EMBO J. 2004;23:4770–4779. doi: 10.1038/sj.emboj.7600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seternes O.-M., Mikalsen T., Johansen B., Michaelsen E., Armstrong C. G., Morrice N., Turgeon B., Meloche S., Moerns U., Keyse S. M. Activation of MK5/PRAK by the atypical MAP kinase ERK3 defines a novel signal transduction pathway. EMBO J. 2004;23:4780–4791. doi: 10.1038/sj.emboj.7600489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuenda A., Rouse J., Doza Y. N., Meier R., Cohen P., Gallagher T. F., Young P. R., Lee J. C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 12.Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haydon C. E., Watt P. W., Morrice N., Knebel A., Gaestel M., Cohen P. Identification of a phosphorylation site on skeletal muscle myosin light chain kinase that becomes phosphorylated during muscle contraction. Arch. Biochem. Biophys. 2002;397:224–231. doi: 10.1006/abbi.2001.2625. [DOI] [PubMed] [Google Scholar]
- 14.McNeil H., Knebel A., Arthur J. S. C., Cuenda A., Cohen P. A novel UBA and UBX domain protein that binds polyubiquitin and VCP and is a substrate for SAPKs. Biochem. J. 2004;384:391–400. doi: 10.1042/BJ20041498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabio G., Arthur J. S. C., Kuma Y., Peggie M., Carr J., Murray-Tait V., Centeno F., Goedert M., Morrice N., Cuenda A. p38γ regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005;24:1134–1145. doi: 10.1038/sj.emboj.7600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hisanaga S., Ishiguro K., Uchida T., Okumura E., Okano T., Kishimoto T. Tau protein kinase II has a similar characteristic to cdc2 kinase for phosphorylating neurofilament proteins. J. Biol. Chem. 1993;268:15056–15060. [PubMed] [Google Scholar]
- 17.Jayaraman D., Glasson B. I., Mushynski W. E. Increased phosphorylation of neurofilament subunits in PC 12 cells and rat dorsal root ganglion neurons treated with N-acetyl-Leu-Leu-norleucinal. Int. J. Dev. Neurosci. 1995;13:753–758. doi: 10.1016/0736-5748(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 18.Stokoe D., Caudwell B., Cohen P. T., Cohen P. The substrate specificity and structure of mitogen-activated protein (MAP) kinase-activated protein kinase-2. Biochem. J. 1993;296:843–849. doi: 10.1042/bj2960843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clifton A. D., Young P. R., Cohen P. A comparison of the substrate specificity of MAPKAP kinase-2 and MAPKAP kinase-3 and their activation by cytokines and cellular stress. FEBS Lett. 1996;392:209–214. doi: 10.1016/0014-5793(96)00816-2. [DOI] [PubMed] [Google Scholar]
- 20.Stokoe D., Engel K., Campbell D. G., Cohen P., Gaestel M. Identification of MAPKAP-kinase-2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- 21.Lavoie J. N., Hickey E., Weber L. A., Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J. Biol. Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- 22.Eyers P. A., van den IJssel P., Quinlan R. A., Goedert M., Cohen P. Use of a drug-resistant mutant of stress-activated protein kinase 2a/p38 to validate the in vivo specificity of SB 2–33580. FEBS Lett. 1999;451:191–196. doi: 10.1016/s0014-5793(99)00552-9. [DOI] [PubMed] [Google Scholar]
- 23.Seebolt-Leopold J. S., Dudley D. T., Herrera R., Becelaere K. V., Wiland A., Gowan R. C., Tecle H., Barrett S. D., Bridges A., Przbranowski A., et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat. Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 24.Qin S., Minami Y., Hibi M., Kurosaki T., Yamamura H. Syk-dependent and -independent signaling cascades in B-cells elicited by osmotic and oxidative stress. J. Biol. Chem. 1997;272:2098–2103. doi: 10.1074/jbc.272.4.2098. [DOI] [PubMed] [Google Scholar]
- 25.dos Remedios C. G., Chhabra D., Kekic M., Dedova I. V., Tsubakihara M., Berry D. A., Nosworthy N. J. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol. Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita A., Maeda K., Maeda Y. Crystal structure of CapZ: structural basis for actin filament barbed end capping. EMBO J. 2003;22:1529–1538. doi: 10.1093/emboj/cdg167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benndorf R., Hayess K., Ryazantsev S., Wieske M., Behlke J., Lutsch G. Phosphorylation and supramolecular organisation of murine small heat shock protein HSP25 abolish its actin polymerisation-inhibiting activity. J. Biol. Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- 28.Guay J., Lambert H., Gingras-Breton G., Lavoie J. N., Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J. Cell. Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 29.McGregor E., Kempster L., Wait R., Gosling M., Dunn M. J., Powell J. T. F-actin capping (CapZ) and other contractile saphenous vein smooth muscle proteins are altered by hemodynamic stress: a proteonomic approach. Mol. Cell Proteomics. 2004;3:115–124. doi: 10.1074/mcp.M300046-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Huang C. K., Zhan L., Ai Y., Jongstra J. LSP1 is the major substrate for mitogen-activated protein kinase-activated protein kinase 2 in human neutrophils. J. Biol. Chem. 1997;272:17–19. doi: 10.1074/jbc.272.1.17. [DOI] [PubMed] [Google Scholar]
- 31.Hannigan M. O., Zhan L., Ai Y., Kotlyarov A., Gaestel M., Huang C. K. Abnormal migration phenotype of mitogen-activated protein kinase 2−/− neutrophils in Zigmond chambers containing formyl-methionyl-leucyl-phenylalanine gradients. J. Immunol. 2001;167:3953–3961. doi: 10.4049/jimmunol.167.7.3953. [DOI] [PubMed] [Google Scholar]
- 32.Campbell D. G., Morrice N. A. Identification of protein phosphorylation sites by a combination of mass spectrometry and solid phase Edman sequencing. J. Biomol. Tech. 2002;13:119–130. [PMC free article] [PubMed] [Google Scholar]