Abstract
PLC (phospholipase C) plays an important role in intracellular signal transduction by hydrolysing phosphatidylinositol 4,5-bisphosphate, a membrane phospholipid. To date, 12 members of the mammalian PLC isoforms have been identified and classified into five isotypes β, γ, δ, ε and ζ, which are regulated by distinct mechanisms. In the present study, we describe the identification of a novel PLC isoform in the brains of human and mouse, named PLC-η, which contains the conserved pleckstrin homology domain, X and Y domains for catalytic activity and the C2 domain. The first identified gene encoded 1002 (human) or 1003 (mouse) amino acids with an estimated molecular mass of 115 kDa. The purified recombinant PLC-η exhibited Ca2+-dependent catalytic activity on phosphatidylinositol 4,5-bisphosphate. Furthermore, molecular biological analysis revealed that the PLC-η gene was transcribed to several splicing variants. Although some transcripts were detected in most of the tissues we examined, the transcript encoding 115 kDa was restricted to the brain and lung. In addition, the expression of the 115 kDa protein was defined in only nerve tissues such as the brain and spinal cord. In situ hybridization analysis with brain revealed that PLC-η was abundantly expressed in various regions including cerebral cortex, hippocampus, zona incerta and cerebellar Purkinje cell layer, which are neuronal cell-enriched regions. These results suggest that PLC-η may perform fundamental roles in the brain.
Keywords: Ca2+; inositol 1,4,5-trisphosphate; molecular cloning; phosphatidylinositol 4,5-bisphosphate; PLC-η; phospholipase
Abbreviations: DIG, digoxigenin; HEK-293 cells, human embryonic kidney 293 cells; ORF, open reading frame; PH, pleckstrin homology; PLC, phospholipase C; RT, reverse transcriptase
INTRODUCTION
Phosphoinositide-specific PLC (phospholipase C) takes part in early events in the regulation of various cellular functions. This enzyme catalyses the hydrolysis of PtdIns(4,5)P2 and thereby generates second messengers, Ins(1,4,5)P3 and diacylglycerol [1,2]. So far, 12 PLC isoenzymes have been identified in mammals. On the basis of their structure, they can be divided into five isotypes, four β, two γ, four δ, one ε and one ζ types [3–5]. In addition, two PLC-like proteins, PLC-L1 and -L2, have been identified. In spite of carrying all the domains that are common in PLCs, they do not have catalytic activity because the residues involved in catalytic activity are replaced with other amino acids [6,7].
All PLCs contain catalytic domains with high sequence homology (40–60% identity), and are designated as X and Y. A C2 domain, which mediates the Ca2+-dependent binding to lipid vesicles, is also shared by all the mammalian PLC isoforms [1–3]. A PH (pleckstrin homology) domain, which binds to phosphoinositides in the plasma membrane, is located in the N-terminal region of PLC-β, PLC-γ and PLC-δ, preceding the X domain. Three isoforms except PLC-δ and PLC-ζ contain additional regulatory regions. The C-terminal region (∼400 residues) of PLC-β isoenzymes might contribute to the tethering of an enzyme to the membrane, given that truncation of this region blocked membrane association and enzymatic activation by G-proteins of PLC-β1 [8–10]. PLC-γ isoenzymes have Src homology domains between the X and Y domains, which play a critical role in mitogenic signalling independent of enzymatic activity [11,12]. PLC-ε may be involved in ras-mediated signalling pathways through the RasGEF domain in the N-terminal and two ras-associating domains in the C-terminal region [4]. PLC isotypes are regulated by distinct mechanisms. PLC-β isoenzymes are activated by the Gαq/11 family of heterotrimeric G-proteins and βγ subunits are released from the Gαi/o family. PLC-γ isoenzymes are activated by both receptor and non-receptor tyrosine kinases through tyrosine phosphorylation [13]. PLC-ε is activated by Gα12 and ras through membrane translocation [4]. Most PLCs are expressed in various tissues and play diverse roles in triggering cellular responses. However, PLC-ζ is specifically expressed in mammalian spermatozoa and is considered to trigger egg activation in fertilization [5].
In the present study, we identified a new PLC (PLC-η) with a molecular mass of 115 kDa. PLC-η hydrolyses PtdIns(4,5)P2 in a Ca2+-dependant manner. It is mainly expressed in nerve tissues, suggesting that it may be involved in neuronal functions.
EXPERIMENTAL
cDNA cloning and plasmid construction
A 5.5 kb KIAA1069 cDNA containing a large ORF (open reading frame) was obtained from the Kazusa DNA Research Institute (Chiba, Japan) and cloned into SalI/NotI of pBluescript II SK(+). To identify the 5′-end of the gene, Marathon-ready cDNAs from human brain were amplified using Marathon adapter primers AP1 and AP2 (Clontech Laboratories, Palo Alto, CA, U.S.A.). Antisense gene-specific primers, KI1069-NR1 (5′-CTGCTGGATACTGCAGTGATTCTCG-3′) and the nested primer KI1069-NR2 (5′-GTAGTAGTTGCAGAGGGGCTGATCC-3′), were designed from the 5′-region of the KIAA1069 gene. The PCR products were subsequently ligated into pGEM-T easy vector (Promega, Madison, WI, U.S.A.) and sequenced. Since the gene fragment contained a stop codon followed by ATG residues and its 3′-region was overlapped with the 5′-region of KIAA1069, we designated it as a partial fragment of KIAA1069. We called the whole KIAA1069 gene PLC-η, because the gene product has common domains identified in all PLC enzymes. The partial gene was amplified using sense primer NPLC-F (5′-ATAAGAATGCGGCCGCGATGGCAGACCTTGAAGTGTAT-3′) and antisense primer NPLC-NR (5′-GAGCCAGTTCTTCCACAGTTAGGTG-3′). KIAA1069 cDNA was also amplified using sense primer NPLC-NF (5′-CACCTAACTGTGGAAGAACTGGCTC-3′) and antisense primer NPLC-R (5′-TCCCCCGGGTCAGATTTGTACTAGAGAGT-3′). Full-length cDNA of PLC-η was obtained by a second PCR using both PCR products as templates, with sense primer NPLC-F and antisense primer NPLC-R. A 3 kb PCR product was digested with NotI and SmaI, and then ligated into the NotI/SmaI site of pCMV2-FLAG to create the mammalian expression vector pCMV2-FLAG/PLC-η. To get the lipase-inactive mutant of PLC-η, His359 was mutated to glutamine, and confirmed by DNA sequencing. For expression in Sf9 cells, full-length cDNA including the gene of FLAG-epitope was amplified with PCR using the sense primer FLAG–Xba (5′-CGTCTAGAATGGACTACAAAGACGATGAC-3′) and antisense primer NPLC–R, and then digested with XbaI and SmaI to be ligated into XbaI/SmaI site of pVL1392 (Invitrogen).
A mouse gene of PLC-η was identified by using the NCBI BLAST program with the human gene. There were several alternative spliced forms in the mouse gene. We cloned the mouse gene from the cDNA library of mouse strain C57BL/6 by RT (reverse transcriptase)–PCR with sense primer (5′-CGGAATTCATGGCAGACCTTGAAGTG-3′) and antisense primer (5′-ACTGGTCGACTCAGATCTGTACCAGACAG-3′). The PCR products were digested with EcoRI and SalI, and then inserted into EcoRI/XhoI sites of pCDNA3.1 (neo). All genes were confirmed by sequencing with appropriate primers. To examine the localization of PLCs, each cDNA of PLC-β1, -γ1, -δ1 and -η was inserted into pEGFP-C1 (Clontech Laboratories) by PCR and digested with appropriate restriction enzymes.
Northern blotting and PCR analysis
To make the probe, 500 bp of PLC-η cDNA ranging over positions 691–1200 was amplified by PCR using primers KI1069-PF (5′-CTAACTGTGGAAGAACTGGCTCAG-3′) and KI1069-NR1. The PCR products were labelled by using rediprime DNA labelling system (Amersham Biosciences, Piscataway, NJ, U.S.A.) with [32P]dCTP. Multiple Northern blots of human (Clontech Laboratories) were hybridized with 32P-labelled probes as described previously [14]. Hybridization was performed in 5×SSC (where 1×SSC is 0.15 M NaCl and 0.015 M sodium citrate) and 5×Denhardt's solution (where 1×Denhardt's is 0.02% Ficoll 400, 0.02% polyvinylpyrrolidone and 0.02% BSA) containing 0.1 mg/ml salmon spermatozoa DNA, 0.5% SDS and 50% (v/v) formamide at 42 °C for 16–24 h. The membrane was then washed twice at room temperature (25 °C) in 2×SSC containing 0.1% SDS and once at 68 °C in 1×SSC containing 0.1% SDS for 2–3 h and then exposed to X-ray film.
PCR amplification using 30 cycles was performed with sense primer (5′-AAGAAGTTGCCGTATCACCTTGG-3′) and antisense primer (5′-GGTTTGGGGAGTTGCTGTCCACTG-3′) which define a 1 kb region with PLC-η, using cDNAs prepared from mouse tissues. In addition, the other PCR amplification was performed with sense primer (5′-GAACAGACAGCTCCAAGG CCTG-3′) and antisense primer (5′-TGCTTGCGCTGCTTCCTATTC-3′) to examine the expression of a splicing form that is compatible with the human gene. The antisense primer covers a part of the 23rd exon.
Generation of antibodies to PLC-η
Sf9 cells were co-transfected with recombinant PVL1392/FLAG-PLC-η and BaculoGold (Pharmingen, San Diego, CA, U.S.A.), using the FuGENE™ transfection reagent. Recombinant baculovirus was amplified using a standard procedure as described in the instruction manual (Pharmingen). Monolayers of Sf9 cells (2×107 cells/150 mm dish) were lysed with lysis buffer (20 mM Hepes, pH 7.5, 150 mM NaCl, 0.2 mM dithiothreitol, 1% Triton X-100, and protease inhibitor cocktail). A majority of the recombinant PLC-η proteins were expressed as Triton X-100-insoluble forms. The insoluble proteins were separated by SDS/PAGE and then PLC-η was electroeluted from the gel and used for immunization of the mice. After immunization four times, serum was extracted from immunized mice.
Western-blot analysis
For SDS/PAGE, samples containing 1×SDS sample buffer were boiled for 5 min and electrophoresed on 8% (w/v) SDS gel. Separated proteins were transferred on to nitrocellulose membrane (Schleicher and Schuell, Keene, NH, U.S.A.) and probed with appropriate antibodies. Immunoreactivity was visualized with enhanced chemiluminescence (ECL®; Amersham Biosciences).
Subcellular fractionation
C57BL/6 mouse was ether-anesthetized and killed by decapitation and whole brain was homogenized with 15 Dounce strokes in 5 vol. of homogenization buffer (20 mM Hepes, pH 7.5, 3 mM MgCl2 and 10 mM KCl) containing protease inhibitors. Homogenates were centrifuged twice at 1000 g for 10 min to remove unbroken cells and nuclei. The postnuclear fraction was subjected to ultracentrifugation at 100000 g for 1 h to produce a cytosolic fraction. The precipitate was washed with the same buffer twice and resuspended with 1% Triton X-100 buffer (150 mM NaCl, 20 mM Hepes, pH 7.5, and protease inhibitors), and then centrifuged at 100000 g for 1 h to separate a detergent-soluble membrane fraction. The precipitate was resuspended with RIPA buffer (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.1% SDS and 1% sodium deoxycholate) and kept as a detergent-insoluble membrane fraction. Each fraction of 20 μg was then used for SDS/PAGE, followed by Western blotting. All procedures were performed at 4 °C.
Generation of viruses
All adenoviral systems were provided by Dr B. Vogelstein from Johns Hopkins University [15]. Briefly, cDNA of wild-type and mutant PLC-η were inserted into pShuttle-CMV, a shuttle vector. For homologous recombination, 0.5 μg of the plasmids linearized with PmeI were mixed with 0.1 μg of pAdEasy-1, an adenoviral plasmid. Electrocompetent Escherichia coli BJ5183 cells were added and electroporation was performed in a 2.0 mm cuvette at 2.5 kV, 200 Ω and 25 μF in a Bio-Rad Gene Pulser electroporator. The smaller colony in an L-agar plate containing 50 μg/ml kanamycin were picked and grown in Luria–Bertani broth (10 g of Bacto tryptone, 5 g of yeast extract and 10 g of NaCl in 1 litre of water) containing kanamycin. Recombinant viral plasmids were screened by analysing their sizes and restriction sites. The viral plasmids were digested with PacI, and transfected to HEK-293 (human embryonic kidney 293) cells. Transfected cells were collected 9 days after transfection by scraping the cells off the dishes. Viral lysates were obtained by three cycles of freezing and thawing. The expression of recombinant proteins was checked with appropriate antibodies.
PLC activity assay
Full-length PLC-η was expressed with a FLAG-epitope tag in COS7 cells by using adenoviruses. FLAG–PLC-η and its lipase inactive mutant were affinity-purified with the resin, and conjugated with anti-FLAG antibody (M2 clone; Sigma). PLC activities were measured essentially as described previously [16]. Briefly, purified PLC-η and PLC-β1 were incubated in 200 μl of reaction mixture (50 mM Hepes, pH 7.0, 120 mM KCl, 10 mM NaCl, 10 μM Ca2+/EGTA, 0.2 mg/ml BSA, 90 μM [3H]PtdIns-(4,5)P2 and 120 μM phosphatidylethanolamine) at 30 °C for 15 min. [3H]Ins(1,4,5)P3 was extracted and measured by liquid scintillation counting. Various EGTA/Ca2+ buffers giving different concentrations of free Ca2+ were prepared as described in [16].
In situ hybridization
PCR products using the sense primer (5′-CGGAATTCAAGAAGTTGCCGTATCACCTTGG-3′) and antisense primer (5′-ACGCGTCGACGGTTTGGGGAGTTGCTGTCCACTG-3′) were digested with EcoRI and SalI, and inserted into EcoRI/SalI sites of pBluescript SK vector. The plasmids were digested with EcoRI (antisense) or SalI (sense). Riboprobes were synthesized using the DIG (digoxigenin)-labelling mixture and RNA polymerase: T7 for the antisense probe and T3 for the sense probe (Roche, Indianapolis, IN, U.S.A.). Hybridization of frozen sections was performed as described previously [17]. Mouse brains were collected from adult male C57BL/6 mice. Frozen tissues were sectioned into 20 μm thick samples. The probes were hybridized at 65 °C for more than 16 h. Then the slides were incubated with preadsorbed anti-DIG (1:2000) antibodies at room temperature for 1 h.
RESULTS AND DISCUSSION
Molecular cloning of PLC-η
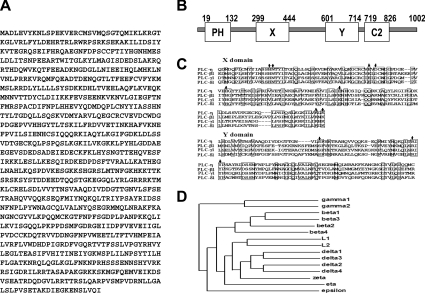
To identify novel PLC, we searched the databases for sequences with similarity to the X and Y domains of PLC. The highly homologous sequence had been deposited as a hypothetical protein KIAA1069 in the HUGE database (Kazusa DNA Research Institute), which was neither assigned any function nor submitted as a full-length coding sequence. Full-length cloning of KIAA1069 revealed a 3.0 kb cDNA with an ORF of 1002 amino acid residues (Figure 1A). Sequence analysis indicated that this gene product possesses the typical X and Y catalytic domains identified in all known PLCs (residues 299–444 and 601–714 respectively) (Figure 1B). In the N-terminal region, there is a PH domain known to be involved in membrane phospholipids interaction [13]. Next to the Y domain, a C2 domain is also found (residues 719–826). Since this gene was considered to be a subtype of PLC, we named it PLC-η. All PLCs contain amino acids essential for enzymatic activity in the X and Y domains. In the case of PLC-L1 and -L2, one or two amino acids are replaced with other amino acids. Therefore they cannot hydrolyse phosphoinositides, although they have high similarity with other PLCs. PLC-η has the same amino acids as other PLC isoenzymes in the X and Y domains as shown in Figure 1(C), suggesting that it possesses PLC activity. A phylogeny analysis of the 15 identified mammalian PLCs supports the classification of PLC-η as a distinct isoenzyme, which suggests that the PLC-η is a divergent PLC isoform from a hypothetical precursor (Figure 1D).
Figure 1. Molecular cloning of PLC-η.
(A) The predicted amino acid sequence of human PLC-η. (B) Schematic representation of the predicted domain features of human PLC-η. PH, X, Y and C2 represent their respective domains. (C) Alignment of amino acid sequences corresponding to the X and Y domains of PLCs. The amino acid sequence of PLC-η is compared with PLC-β1, PLC-γ1 and PLC-δ1. Identical amino acids are boxed. The amino acids indicated with arrows are known to be essential for PLC activity. (D) Dendrogram illustrating phylogeny of CLUSTAL W aligned mammalian PLC sequences.
PLC-η possesses PLC activity
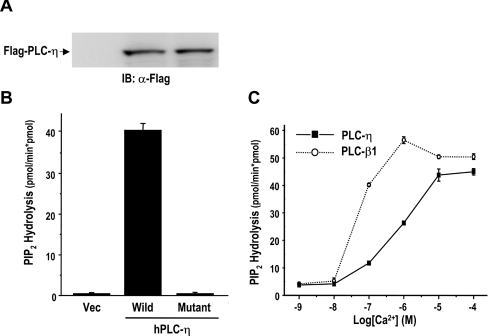
As mentioned above, all known PLCs except the L-type have a enzymatic activity that hydrolyses PtdIns(4,5)P2, a membrane phospholipid. The catalytic activity of purified PLC-η was measured with [3H]PtdIns(4,5)P2. PLC-η was hydrolysed with [3H]PtdIns(4,5)P2, thereby generating [3H]Ins(1,4,5)P3. However, substituting a glutamine residue that had been identified in L-type for His359 eliminated the activity, suggesting that this residue is essential for enzymatic activity (Figures 2A and 2B).
Figure 2. PLC-η has PtdIns(4,5)P2-hydrolysing enzyme activity.
(A) Expressions of FLAG-tagged PLC-η. Recombinant adenoviruses for Flag–PLC-η (wild), and its mutant form (mutant), in which a glutamine residue is substituted for His358, were infected into COS-7 cells. Cells were lysed, 2 days post-infection, and subjected to Western-blot analysis using anti-Flag antibody. (B) PLC-η has all amino acids necessary for enzymatic activity. Purified wild-type PLC-η (wild) and its mutant (H358Q) were assayed for the PtdIns(4,5)P2-hydrolysing activity. (C) The Ca2+-dependence of PLC-η enzyme activity. Purified PLC-η (0.5 pmol) was examined for PtdIns(4,5)P2-hydrolysing activity in the presence of various concentrations of free Ca2+.
In vitro PLC activity is influenced by the assay conditions such as detergent and Ca2+ concentration. The catalytic activity of PLC-η was measured at various concentrations of free Ca2+ (Figure 2C). Like most PLC isoenzymes, the catalytic property of PLC-η exhibited dependence on Ca2+ concentration, and the maximal activity was obtained at approx. 10 μM Ca2+. PLC-β1 used as a control showed maximal activity at 1 μM Ca2+, indicating that a higher Ca2+ concentration is necessary to activate PLC-η when compared with PLC-β1.
Analysis of PLC-η gene structure
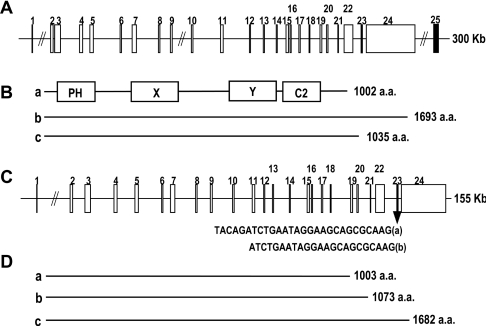
Using the NCBI BLAST search and the RT–PCR method, we identified several alternative spliced forms of PLC-η mRNA and analysed their genome structure in human and mouse chromosomes. The originally identified human PLC-η gene from KIAA1069 is translated into a protein of 1002 amino acids and consists of 23 exons for ORF (Figures 3A and 3B) (hPLC-ηa; GenBank® accession no. AY691170). Another splicing form whose 23rd exon is missed and the 22nd exon is linked to the long 24th exon encodes a protein of 1693 amino acids (splice variant hPLC-ηb; GenBank® accession no. AY691171). The third form (splice variant hPLC-c; GenBank® accession no. XM_042635) translates into a 1035 amino acid protein, which links the 22nd exon with the 25th exon without the 23rd and the 24th exons. These exons are spread over a 300 kb region of chromosome 3.
Figure 3. Organization of the PLC-η gene.
A linear presentation of the exon locus shows the relative positions and approximate sizes of 24 exons spanning 200 kb (human) or 160 kb (mouse) of chromosome 3. (A) The cDNA co-ordinates for the exons of human gene are as follows: 1, 1–43; 2, 44–190; 3, 191–435; 4, 436–564; 5, 565–735; 6, 736–829; 7, 830–1033; 8, 1034–1154; 9, 1155–1327; 10, 1328–1434; 11, 1435–1596; 12, 1597–1668; 13, 1669–1760; 14, 1761–1858; 15, 1859–2038; 16, 2039–2146; 17, 2147–2271; 18, 2272–2356; 19, 2357–2503; 20, 2504–2583; 21, 2584–2643; 22, 2644–2998; 23, 2999–3026; 24, 3027–6196; 25, 2999–3208. (B) Schematic presentation of splicing variants (see the Results and discussion section). (a) hPLC-ηa (GenBank® accession no. AY691170), (b) hPLC-ηb (GenBank® accession no. AY691171) and (c) hPLC-ηc (GenBank® accession no. XM_042635). (C) The cDNA co-ordinates for the exons of mouse gene are as follows: 1, 1–43; 2, 44–190; 3, 191–435; 4, 436–565; 5, 566–735; 6, 736–829; 7, 830–1033; 8, 1034–1154; 9, 1155–1326; 10, 1327–1434; 11, 1435–1596; 12, 1597–1668; 13, 1669–1763; 14, 1764–1861; 15, 1862–2040; 16, 2041–2149; 17, 2150–2274; 18, 2275–2359; 19, 2360–2506; 20, 2507–2586; 21, 2587–2646; 22, 2647–3001; 23, 3002–3024 or 3029; 24, 3025 or 3030–6273. (D) Schematic presentation of splicing variants (see the Results and discussion section). (a) mPLC-ηa (GenBank® accession no. AY691172), (b) mPLC-ηb (GenBank® accession no. AY691173) and (c) mPLC-ηc (GenBank® accession no. AY691174).
We also found three splicing forms of mouse PLC-η gene (Figures 3C and 3D). The 23rd exon has two splicing sites at the 5′-region to generate two mRNA fragments consisting of 28 and 23 nucleotides respectively, thereby each splicing form makes 1003 amino acids (mPLC-ηa; GenBank® accession no. AY691172) and 1073 amino acid proteins (splice variant mPLC-ηb; GenBank® accession no. AY691173). The other form containing no 23rd exon is translated into a 1682 amino acid protein (splice variant mPLC-ηc; GenBank® accession no. AY691174). The exons are spread over a 155 kb region of mouse chromosome 3. These splicing forms of human and mouse gene make proteins containing different C-terminal amino acids. However, their PLC activity does not appear to be influenced by the variation in splicing, since there is no functional domain in the C-terminal region and the functional domains for catalytic activity are intact.
Spreading of exons over a very wide chromosomal region and transcription of various splicing forms suggest that the splicing regions are unstable and thereby easily attacked by splicing enzymes. The splicing variants can be expressed in different tissues or cell types and in this way could play different roles in cellular responses besides enzymatic activity.
PLC-η is expressed in the brain
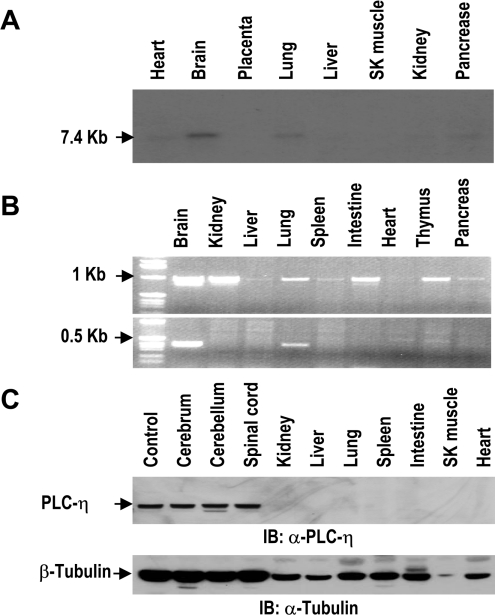
To analyse the tissue distribution of PLC-η, we performed three different experiments with human and mouse tissues. In Northern-blot analysis with human tissue mRNAs, PLC-η was detected as a 7.4 kb transcript in the brain and lung (Figure 4A). Then we determined the distribution of mouse mRNAs using PCR analysis. We designed two pairs of PCR primers for a common region of three splicing forms and the region including the 23rd exon. The PCR products of the common region were observed in all tissues we examined, except liver and heart as they were of 1 kb size. However, the 23rd exon-containing PCR products of 0.35 kb size were observed in the brain and lung, indicating that the splicing machinery for the 23rd exon-containing mRNA is restricted to the brain and lung (Figure 4B), and protein of 1003 amino acids is specifically expressed in these tissues. Expression of the splicing variant containing the 23rd exon was confirmed by Western-blot analysis (Figure 4C). PLC-η was expressed abundantly only in nerve tissues such as cerebrum, cerebellum and the spinal cord. Contrary to PCR analysis, the protein of PLC-η was not detected in the lung, suggesting that it may be present at a level that is not detectable with our antibody.
Figure 4. Tissue distributions of PLC-η.
(A) Northern-blot analysis of human tissues detected by PLC-η cDNA. SK muscle, skeletal muscle. (B) PCR analysis of PLC-η in cDNA from mouse tissues. The primer pair for common nucleotides of three splicing forms (upper panel) and the specific primer for the 23rd exon-containing cDNA (lower panel) were used in each PCR reaction. (C) Immunoblot analysis of PLC-η with mouse tissue extracts. Mouse PLC-η protein expressed in HEK-293 cells was used as a control. Immunoblot of tubulin was used as a loading control.
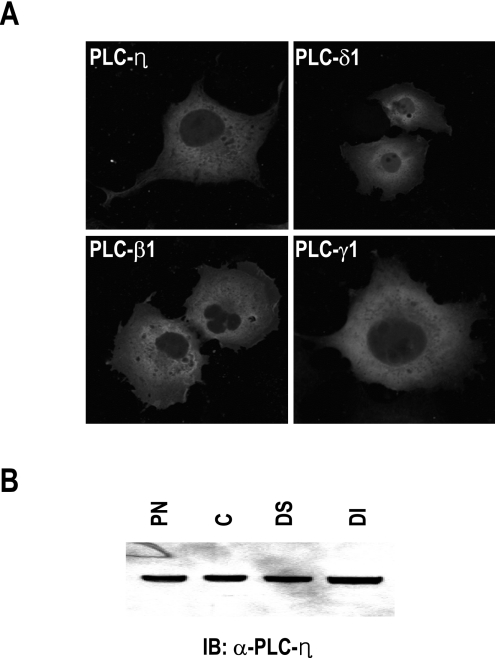
The localization of PLC-η in cells was investigated by the expression of a GFP-tagged form in HEK-293 cells and subcellular fractionation of the brain tissue. GFP signals were detected in the cytosol and membrane but not in the nucleus (Figure 5A), which is similar to the expression pattern of the other PLCs, such as PLC-β1, PLC-γ1 and PLC-δ1. To confirm the localization of PLC-η, we fractionated the brain tissue. As shown in Figure 5(B), it was detected in all cytosolic and membrane fractions, which suggests that PLC-η may be involved in various cellular events.
Figure 5. Subcellular localization of PLC-η.
(A) Expression pattern of GFP-tagged PLC isoenzymes. pEGFP-C1 plasmids carrying PLC genes were transfected into HEK-293 cells, using a liposome-mediated gene transfer method. After 24 h, cells were fixed with 4% (w/v) paraformaldehyde and observed under fluorescent microscope. (B) Subcellular fractionation of brain tissue. PN, postnuclear; C, cytosol; DS, detergent-soluble membrane; DI, detergent-insoluble membrane.
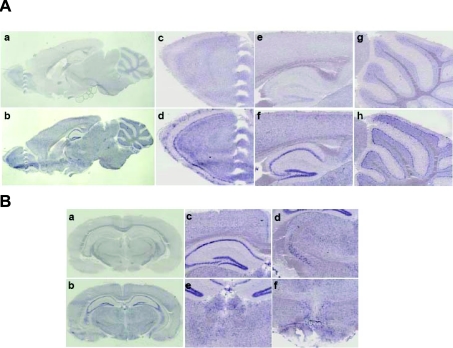
In situ hybridization using DIG-labelled riboprobes was performed to investigate the expression pattern in the brain (Figure 6). This analysis revealed that the PLC-η was expressed throughout the whole brain, especially in the inner layer of the olfactory bulb, CA region and dentate gyrus of hippocampus, Purkinje layer of cerebellum, cerebral cortex, zona incerta, habenular nuclei and hypothalamus, which are neuronal cell-enriched regions, implying that PLC-η plays a role in nerve-specific signal transduction.
Figure 6. Expression pattern of PLC-η in mouse brain.
In situ hybridization with cRNA probes detecting mouse PLC-η. (A) Parasagittal section (a, c, e and g). Sense probe as negative control; (b, d, f and h) antisense probe; (c, d) olfactory bulb; (e, f) hippocampus; and (g, h) cerebellum. (B) Coronal section (a) sense probe; (b–f) antisense probe; (c) hippocampus; (d) zona incerta; (e) habenular nuclei and (f) hypothalamus.
In conclusion, we report that PLC-η was identified in the human and mouse and hydrolyses PtdIns(4,5)P2 to produce Ins(1,4,5)P3 in a Ca2+-dependent manner. The gene of PLC-η encodes several splicing variants by exon exchange in 3′-regions. One form of PLC-η encoding 1003 amino acids is highly expressed in nerve tissue and may play fundamental roles in nerve tissue function.
Acknowledgments
This study was supported by the National R&D Program for Fusion Strategy of Advanced Technologies of Ministry of Science Technology and by the Korea Science and Engineering Foundation through the Center for Cell Signalling Research at Ewha Womans University, Korea.
References
- 1.Rhee S. G., Choi K. D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J. Biol. Chem. 1992;267:12393–12396. [PubMed] [Google Scholar]
- 2.Berridge M. J. Inositol trisphosphate and calcium signalling. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 3.Rhee S. G., Bae Y. S. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 4.Song C., Hu C.-D., Masago M., Kariya K., Yamawaki-Kataoka Y., Shibatohge M., Wu D., Satoh T., Kataoka T. Regulation of a novel human phospholipase C, PLC-ε, through membrane targeting by Ras. J. Biol. Chem. 2001;276:2752–2757. doi: 10.1074/jbc.M008324200. [DOI] [PubMed] [Google Scholar]
- 5.Saunders C. M., Larman M. G., Parrington J., Cox L. J., Royse J., Blayney L. M., Swann K., Lai F. A. PLC-ζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 6.Kanematsu T., Misumi Y., Watanabe Y., Ozaki S., Koga T., Iwanaga S., Ikehara Y., Hirata M. A new inositol 1,4,5-trisphosphate binding protein similar to phospholipase C-δ1. Biochem. J. 1996;313:319–325. doi: 10.1042/bj3130319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otsuki M., Fukami K., Kohno T., Yokota J., Takenawa T. Identification and characterization of a new phospholipase C-like protein, PLC-L2. Biochem. Biophys. Res. Commun. 1999;266:97–103. doi: 10.1006/bbrc.1999.1784. [DOI] [PubMed] [Google Scholar]
- 8.Wu D., Jiang H., Katz A., Simon M. I. Identification of critical regions on phospholipase C-β1 required for activation by G-proteins. J. Biol. Chem. 1993;268:3704–3709. [PubMed] [Google Scholar]
- 9.Zhang S., Coso O. A., Collins R., Gutkind J. S., Simond W. F. A C-terminal mutant of the G protein β subunit deficient in the activation of phospholipase C-β. J. Biol. Chem. 1996;271:20208–20212. doi: 10.1074/jbc.271.33.20208. [DOI] [PubMed] [Google Scholar]
- 10.Kim C. G., Park D., Rhee S. G. The role of carboxyl-terminal basic amino acids in Gqα-dependent activation, particulate association, and nuclear localization of phospholipase C-β1. J. Biol. Chem. 1996;271:21187–21192. doi: 10.1074/jbc.271.35.21187. [DOI] [PubMed] [Google Scholar]
- 11.Kamat A., Carpenter G. Phospholipase C-γ1: regulation of enzyme function and role in growth factor-dependent signal transduction. Cytokine Growth Factor Rev. 1997;8:109–117. doi: 10.1016/s1359-6101(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim M. J., Chang J.-S., Park S. K., Hwang J.-I., Ry S. H., Suh P.-G. Direct interaction of SOS1 Ras exchange protein with the SH3 domain of phospholipase C-γ1. Biochemistry. 2000;39:8674–8682. doi: 10.1021/bi992558t. [DOI] [PubMed] [Google Scholar]
- 13.Rebecchi M. J., Pentyala S. N. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 2000;80:1291–1335. [Google Scholar]
- 14.Liu N., Fukami K., Yu H., Takenawa T. A new phospholipase Cδ4 is induced at S-phase of the cell cycle and appears in the nucleus. J. Biol. Chem. 1996;271:355–360. doi: 10.1074/jbc.271.1.355. [DOI] [PubMed] [Google Scholar]
- 15.He T.-G., Zhou S., Costa L. T., Yu J., Kinzler K. W., Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D., Jhon D.-Y., Kriz R., Knopf J., Rhee S. G. Cloning, sequencing, expression, and Gq-independent activation of phospholipase C-β2. J. Biol. Chem. 1992;267:16048–16055. [PubMed] [Google Scholar]
- 17.Perez S. E., Rebelo S., Anderson D. J. Early specification of sensory neuron fate revealed by expression and function of neurogenins in the chick embryo. Development. 1999;126:1715–1728. doi: 10.1242/dev.126.8.1715. [DOI] [PubMed] [Google Scholar]