Abstract
Bacterial histone-like DNA-binding proteins are best known for their role in compacting the genomic DNA. Of these proteins, HU is ubiquitous and highly conserved across the eubacterial kingdom. Using the HBsu (Bacillus subtilis-encoded HU homologue) as a model, we explore here the molecular basis for the ability of some HU homologues to engage a longer approx. 35 bp DNA site as opposed to the much shorter sites reported for other homologues. Using electrophoretic mobility-shift assays, we show that the DNA site size for HBsu is approx. 10–13 bp and that a specific surface salt bridge limits the DNA site size for HBsu. Surface exposure of the highly conserved Lys3, achieved by substitution of its salt-bridging partner Asp26 with Ala, leads to enhanced DNA compaction by HBsu-D26A (where D26A stands for the mutant Asp26→Ala), consistent with the interaction of Lys3 with the ends of a 25 bp duplex. Both HBsu and HBsu-D26A bend DNA, as demonstrated by their equivalent ability to promote ligase-mediated DNA cyclization, indicating that residues involved in mediating DNA kinks are unaltered in the mutant protein. We suggest that Lys3 is important for DNA wrapping due to its position at a distance from the DNA kinks where it can exert optimal leverage on flanking DNA and that participation of Lys3 in a surface salt bridge competes for its interaction with DNA phosphates, thereby reducing the occluded site size.
Keywords: DNA bending, DNA site size, HBsu, integration host factor (IHF), histone-like HU protein, salt bridge, type II DNA-binding protein
Abbreviations: BstHU, Bacillus stearothermophilus HU; EMSA, electrophoretic mobility-shift assay; HBsu, Bacillus subtilis-encoded HU homologue; IHF, integration host factor
INTRODUCTION
The histone-like HU proteins are ubiquitous in the eubacterial kingdom. Primarily studied in Escherichia coli and Bacillus subtilis, their role in compaction of the bacterial genome is well documented [1–4]. HU proteins also act as accessory factors in a host of cellular processes, such as DNA replication, recombination and repair, but their precise functions remain only partially delineated [5–11]. Structurally, HU proteins are very similar to E. coli IHF (integration host factor), which engages approx. 35 bp of duplex DNA [12–16]. In its complex with IHF, the DNA is kinked by intercalation of two proline residues at 2 bp steps separated by 9 bp of duplex, generating an overall DNA bend of approx. 160° [16]. Flanking DNA is wrapped about the surface of the protein in a process that is only modestly dependent on salt concentration, leading to the proposal that surface salt bridges exist that must be disrupted to allow contacts to DNA phosphates [17]. In contrast, the binding site for E. coli HU has been reported to be only approx. 9 bp, although binding to prebent DNA has suggested a binding geometry similar to that exhibited by IHF [18–21]. This variability of DNA site size is further illustrated in the structure of Anabaena HU with DNA, which shows the ability of Anabaena HU to stabilize a range of bending angles while engaging a DNA target of up to approx. 19 bp [22].
In an effort to delineate molecular determinants of binding site size by this highly conserved family of proteins, we recently analysed DNA binding by the B. subtilis bacteriophage SPO1-encoded HU homologue TF1 [23,24]. TF1, like IHF, binds to preferred sites and it engages 37 bp of duplex [25]. With two proline residues intercalating at a spacing of 9 bp, the ability to bring flanking DNA segments into protein contact and to produce a binding site longer than 9 bp was suggested to require precise stabilization of the proline-mediated DNA kinks [24]. Our studies also identified Lys3 as a critical residue for wrapping of DNA longer than approx. 25 bp and suggested that optimal interactions with flanking DNA occur at a specific distance from the DNA kinks, in a position where optimal leverage may be exerted on the DNA; Lys3 is predicted to contact DNA 8–9 bp distal to the proline-mediated DNA kinks [15,23].
The ability to engage a longer DNA duplex may require electrostatic interaction with Lys3, yet Lys3 is almost completely conserved among HU homologues regardless of the occluded site size [26]. To address this question, we have compared TF1 with the B. subtilis-encoded HU homologue, HBsu. Both HBsu and Bacillus stearothermophilus HU (BstHU), for which structural information is available, have an aspartic residue at position 26, and the structure of BstHU shows Asp26 forming a salt bridge with Lys3 (∼3.7 Å separation between charged groups [12,14]; 1 Å=10−10 m). In contrast, TF1 has an alanine residue at position 26. We show in the present study that substitution of Asp26 with Ala increases the ability of HBsu to engage a longer DNA site and to form a more tightly wrapped complex. These observations show that surface salt bridges can modulate DNA site size and DNA wrapping by HU proteins and suggest that the absence of Asp26 may contribute an enhanced capacity for wrapping of duplex DNA by HU homologues.
EXPERIMENTAL
Molecular modelling
The α-carbon traces for TF1 (1WTU) were fitted to IHF (1IHF) using a least-squares fit. The intercalating arms (A53–A76 and B54–B75) of TF1 were aligned with the arms of 1IHF and major steric stresses in the backbone geometry and side chains were relieved. The TF1 chains were then combined with the DNA from the IHF–DNA structure after the removal of two nucleotides (+15G and −60C). All H-bonds between the protein chains and the DNA were manually catalogued in Insight II and the complex was minimized using PEACH 3.8 (Program for Energetic Analysis of bioCHemical molecules). All hydrogens were minimized in vacuum, followed by minimization in vacuum of the intercalating arms. After adding water to the system, 60 sodium ions were added to neutralize the system charge and the water and sodium ions were minimized for 100 steps. Finally, the whole system was minimized by the steepest descents (SD) method. Illustrations were generated with RasMol.
Protein preparation and cross-linking
The gene encoding B. subtilis HU (HBsu) was amplified from B. subtilis genomic DNA (A.T.C.C. #6051-U) and cloned into NdeI-digested pET5a, generating plasmid pHBsu. Plasmid pHBsu-D26A (where D26A stands for the mutant Asp26→Ala) was generated by PCR amplification of plasmid pHBsu using a forward primer designed to introduce the appropriate substitution at position 26 of HBsu (5′-AAAGCAGTTGCCTCTGTTTTTG-3′; codon specifying A26 underlined) and a reverse primer positioned to abut the forward primer (5′-TGTAGCGTCTTTTTTAGACAATTC-3′). The PCR, performed with a mixture of Taq and Pfu polymerases, generated a full-length plasmid containing the mutated HBsu gene. The original template DNA was removed by DpnI digestion. Both constructs were confirmed by sequencing. Primer sequences are available on request.
Plasmids pHBsu and pHBsu-D26A were used to transform E. coli BL21(DE3)pLysS. Overexpression was induced with 1 mM isopropyl β-D-thiogalactoside. Cell lysates were fractionated by ammonium sulphate precipitation as described in [27]. The precipitate formed with 75% ammonium sulphate was dissolved in buffer A (20 mM potassium phosphate, pH 7.0, 50 mM KCl, 5%, v/v, glycerol, 1 mM EDTA, 0.2 M PMSF and 3.5 mM 2-mercaptoethanol), dialysed extensively against buffer A and applied to a CM-Sepharose column equilibrated in buffer A. The proteins were eluted with a linear gradient from 50 mM to 1 M KCl in buffer A. Peak fractions were purified by chromatography on phenyl-Sepharose using buffer A, as described in [27]. Protein concentrations were determined by Coomassie Blue staining of SDS/polyacrylamide gels using the HU homologue TF1 as a standard.
Proteins were cross-linked in a total volume of 10 μl of 10 mM Bicine (pH 8.5) and 50 mM NaCl with 0.1% glutaraldehyde at room temperature (24 °C) for 30 min. Reactions were terminated by the addition of an equal volume of Laemmli sample buffer and the cross-linked products were analysed on SDS/17% (w/v) polyacrylamide gels.
EMSAs (electrophoretic mobility-shift assays) using agarose gels
Supercoiled or EcoRI-linearized pUC18 (100 ng) was mixed with HBsu in 10 μl of binding buffer (20 mM Tris/HCl, pH 8.0, 100 mM KCl, 0.1 mM EDTA, 0.1 mM dithiothreitol, 0.05% Brij58, 10 μg/ml BSA and 5% glycerol). The entire reaction was loaded on a 0.5% agarose gel in TBE (45 mM Tris/borate, pH 8.3 and 1 mM EDTA). Nicked circular DNA was prepared by incubation of pET5a with N.BstNBI (New England Biolabs, Beverly, MA, U.S.A.) and the presence of nicks confirmed by susceptibility of the DNA to digestion with exonuclease III. Binding reactions were performed as described for pUC18 and analysed on 0.5% agarose gels in TAE (40 mM Tris/acetate, pH 8.0 and 1 mM EDTA). DNA was visualized by ethidium bromide staining after electrophoresis.
EMSA and quantification of protein–DNA complexes
Oligonucleotides used to generate DNA constructs were purchased and purified by denaturing PAGE. The top strand was 32P-labelled at the 5′-end with T4 polynucleotide kinase. Equimolar amounts of complementary oligonucleotides were mixed, heated to 90 °C and slowly cooled to 4 °C to form duplex DNA. Fourway junction DNA was prepared as described in [28], using the following oligonucleotides: (1) 5′-CCCTATAACCCCTGCATTGAATTCCAGTCTGATAA-3′, (2) 5′-GTAGTCGTGATAGGTGCAGGGGTTATAGGG-3′, (3) 5′-AACAGTAGCTCTTATTCGAGCTCGCGCCCTATCACGACTA-3′ and (4) 5′-TTTATCAGACTGGAATTCAAGCGCGAGCTCGAATAAGAGCTACTGT-3′.
EMSAs were performed using 10% polyacrylamide gels (acrylamide/bisacrylamide, 39:1) in TBE [27]. Gels were prerun for 30 min at 20 mA at room temperature before loading the samples with the power on, except for experiments with four-way junction DNA for which gels were run at 4 °C. DNA and protein were mixed in binding buffer and each sample contained 25 fmol of DNA in a total reaction volume of 10 μl, unless specified otherwise. After electrophoresis, gels were dried and protein–DNA complexes were visualized and quantified by phosphoimaging, using a software supplied by the manufacturer (ImageQuant 1.1). Data were fitted to the Hill equation Y=Ymax[HU]h/(Kdh+[HU]h), where [HU] is the protein concentration, h the Hill coefficient, Y the fractional saturation and Kd represents an apparent ‘aggregate’ affinity for the DNA. Fits were performed using the program KaleidaGraph. All experiments were performed at least in triplicate.
Supercoiling assays
Negatively supercoiled pGEM5 was relaxed by incubation with Vaccinia topoisomerase I in 50 mM Tris (pH 8.0), 50 mM NaCl and 0.1 mM EDTA. Increasing concentrations of HBsu were added and the reaction was allowed to continue for 60 min at 37 °C. Reactions were terminated by the addition of proteinase K to a final concentration of 0.17 mg/ml and incubation at 37 °C for 60 min. DNA topoisomers were resolved by electrophoresis on 1% agarose gels in TBE at approx. 3 V/cm for 16 h and visualized by ethidium bromide staining. To distinguish positive and negative supercoiling, gels were soaked in 3 μg/ml chloroquine after electrophoresis in the first dimension, turned 90° and the DNA resolved by electrophoresis in the second dimension at approx. 5 V/cm for 5 h.
Cyclization assays
A 105 bp DNA was prepared by digestion of plasmid pET5a with BspHI. DNA was purified on a 7% polyacrylamide gel and 32P-labelled with T4 polynucleotide kinase. Reactions were initiated by addition of 12 units of T4 DNA ligase to a final volume of 100 μl of reaction buffer (20 mM Tris/HCl, pH 8, 50 mM NaCl, 10 mM MgCl2, 0.1 mM Na2-EDTA, 1 mM dithiothreitol and 0.05% Brij58) containing 700 fmol of DNA and 280 fmol of HU. Aliquots of 10 μl were removed at the times indicated and the reaction mixture was quenched by the addition of 5 μl of stop buffer (75 mM EDTA, 15% glycerol and 6 μg/μl proteinase K). Samples were heated at 55 °C for 15 min and resolved on pre-run 6% (w/v) native polyacrylamide gels (acrylamide/bisacrylamide, 39:1) at 4 °C. Quantification was performed on a Molecular Dynamics Storm PhosphorImager using a software supplied by the manufacturer.
RESULTS
Structural considerations
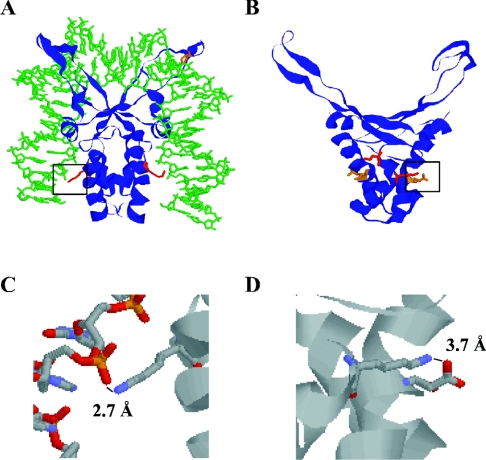
Structurally, TF1 and BstHU are very similar, with only 2–3 Å pairwise root mean square deviations between α-carbons of the protein cores (Figure 1) [12,14,15]. In general, HU proteins are characterized by a large number of positively charged surface residues and a comparable number of negatively charged residues (each monomer of TF1 contains 15 Arg+Lys and 12 Asp+Glu, and HBsu has 16 and 14 basic and acidic residues respectively). An extensive network of surface salt bridges may therefore exist, as inferred from the modest salt-dependence of DNA binding by HU homologues [17,24]. Examination of the structures reveals that the conserved Lys3 is disposed differently in the two proteins. A possible path for DNA along the surface of TF1 is illustrated in Figure 1(A), showing an energy minimized model based on the solution structure of free TF1 and the 35 bp DNA duplex from the IHF–DNA co-crystal structure (the Protein Data Bank file of TF1-DNA model is available at http://www.BiochemJ.org/bj/390/bj3900049add.htm). In TF1, Lys3 is completely surface exposed and is in a position to form a salt bridge with DNA phosphates (Figure 1C). The closest anionic residue is Glu29 of the opposite chain whose C-terminal oxygen is 10–12 Å from the N-terminal nitrogen of Lys3 [15]. The 0.4 M NaCl present during structure elucidation would have competed for intramolecular surface salt bridges between Lys3 and neighbouring anionic residues; indeed, allowable rotations of side chains can bring the charged groups of Lys3 and Glu5 within salt-bridging distance. In contrast, Lys3 of BstHU appears to be engaged in a stable salt bridge with Asp26, with a charge separation of 3.7 Å in 200 mM KCl (Figures 1B and 1D) [14]. Thus this ionic bond would have to be disrupted upon complex formation to liberate Lys3 for interaction with DNA phosphates. The presence of surface salt bridges would compete with contacts to DNA phosphates and lower the observed binding constant, perhaps to the extent of reducing the occluded site size.
Figure 1. Comparison of TF1 and BstHU structures.
(A) Energy minimized model of TF1 (1WTU) in complex with DNA derived from the IHF-DNA structure (1IHF). Lys3, shown to contact DNA 8–9 bp distal to the DNA kinks [23] is indicated in red, and the DNA-intercalating Pro63 is shown in orange. (B) Lys3 (red) forms a salt bridge with Asp26 (orange) in B. stearothermophilus HU (1HUE). (C) Expanded view of TF1 salt bridge between the ϵ-amino group of Lys3 and phosphate oxygen of the DNA backbone. (D) Expanded view of the surface salt bridge between Lys3 and Asp26 of BstHU. In (C, D), nitrogen is coloured blue, oxygen is red, carbon is grey, and phosphorous is orange. Expanded views are identified in (A, B) by a black frame.
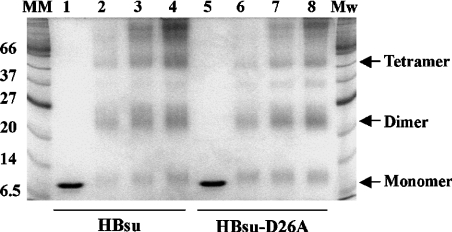
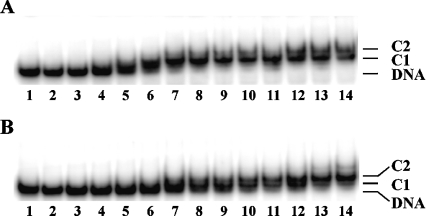
To address the hypothesis that a salt bridge with Asp26 serves to limit accessibility of Lys3, thereby attenuating interaction with longer DNA sites that must be brought into protein contact through interaction with DNA sites distal to the proline-mediated kinks, we substituted Asp26 with Ala in HBsu. As expected, both wild-type HBsu and HBsu-D26A exist predominantly as dimers in solution, consistent with unaltered dimer interfaces, and both form higher-order oligomeric assemblies at higher protein concentrations (Figure 2). We note also that significant levels of monomeric protein remain for both proteins when cross-linking is performed at pH 5.2, whereas dimers predominate at pH>7.0 (Figure 2 and results not shown). This is consistent with reports showing the significant presence of unfolded or partly folded monomeric states at low pH and the inability of the unfolded monomers to dimerize [29,30]. A species corresponding to a trimer is also present for both proteins; as these proteins self-associate, as evidenced by the higher-order oligomeric structures seen at higher protein concentrations, association of free monomer (in which the hydrophobic face otherwise buried in the dimer interface is exposed), either with itself or with properly assembled dimer, would be expected to result in non-specific aggregation.
Figure 2. Purified HBsu and HBsu-D26A.
Coomassie Blue-stained SDS/polyacrylamide gel showing purified HBsu (lane 1) and HBsu-D26A (lane 5). Lanes 2–4 contain 1, 2 and 3 μg HBsu cross-linked with 0.1% glutaraldehyde for 30 min, lanes 6–8 contain 1, 2 and 3 μg HBsu-D26A cross-linked in the presence of glutaraldehyde. Molecular-mass standards are identified on the left (in kDa). Oligomeric assemblies are indicated on the right.
Interaction with plasmid DNA
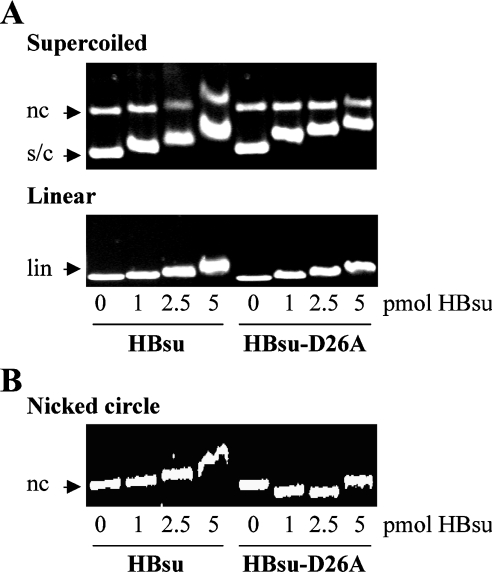
Both wild-type HBsu and HBsu-D26A appear to bind preferentially to supercoiled DNA compared with linear DNA (Figure 3A). For HBsu, this preference is also evidenced by the detection of a shift in mobility of the nicked circular DNA only at higher protein concentrations. Preferred binding to supercoiled DNA would be an expected property of Hbsu, which also binds preferentially to curved DNA [31]. These experiments also show that increasing concentrations of HBsu or HBsu-D26A result in a retardation of the entire population of DNA as a relatively sharp band, suggesting preferred occupancy of unbound DNA over DNA already bound by protein. Such behaviour would be associated with negative co-operativity, as opposed to the broad binomial distribution of bound DNA species characteristic of non-co-operative binding, or the bimodal distribution between bound and unbound DNA seen in the case of positive co-operativity [32].
Figure 3. Compaction of DNA by HBsu-D26A.
(A) Agarose gel electrophoresis of HBsu and HBsu-D26A binding to supercoiled (s/c; top panel; nc refers to nicked circle) or linear (lin; bottom panel) pUC18 DNA. (B) HBsu and HBsu-D26A binding to nicked pET5a DNA. HBsu variants are identified at the bottom, and protein concentrations, identical for both panels, are indicated below the bottom panel.
We also note that no shift in the mobility of nicked circular DNA is evident after incubation with HBsu-D26A. To determine if this is due to enhanced DNA compaction by HBsu-D26A, which would be expected to increase mobility of the DNA, we explicitly examined interaction of both proteins with nicked circular DNA using the longer 4134 bp plasmid pET5a (Figure 3B). The significantly increased mobility of nicked, circular DNA seen in the presence of HBsu-D26A suggests that the Ala-for-Asp substitution confers an enhanced ability to wrap and compact the DNA. An implication of this observation is also that the greater retardation of supercoiled DNA compared with linear or relaxed DNA in the presence of HBsu-D26A may reflect not only enhanced affinity, but also protein binding to already compacted DNA.
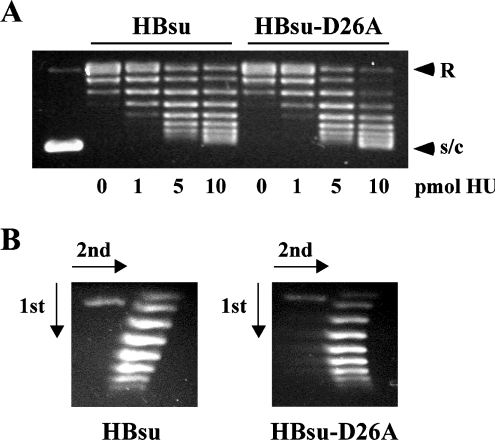
The increased capacity of HBsu-D26A for DNA wrapping was also explored by analysis of the efficiency with which either protein supercoils relaxed plasmid DNA. HBsu and HBsu-D26A were incubated with relaxed pGEM5, prepared by incubation of supercoiled plasmid with Vaccinia topoisomerase I (Figure 4A). Consistent with enhanced DNA wrapping, HBsu-D26A supercoils DNA more efficiently than wild-type HBsu. Two-dimensional agarose gel electrophoresis confirms that both proteins introduce negative supercoils (Figure 4B).
Figure 4. HBsu-D26A supercoils DNA more efficiently.
(A) HBsu and HBsu-D26A supercoil relaxed plasmid DNA in the presence of topoisomerase I. Protein concentrations are indicated below the panels. Relaxed (R) and supercoiled (s/c) DNA are identified on the right. The first lane shows supercoiled DNA before relaxation by topoisomerase. Reactions with 0 pmol of HU represent relaxed plasmid DNA to which increasing concentrations of HU are added. (B) Two-dimensional electrophoresis of DNA topoisomers generated in the presence of 10 pmol of HU. Negatively supercoiled DNA loses superhelicity in the presence of chloroquine, forming the left branch of the arc. Directions of the first and second dimensions are indicated by arrows.
Interaction with short duplexes
DNA-bending proteins increase the rate of ligase-mediated cyclization of short DNA fragments. As shown in Figure 5, HBsu and HBsu-D26A cyclize 105 bp DNA with equivalent efficiency, suggesting that the bending angle is unaltered in the mutant protein. This observation is consistent with the role of DNA-intercalating proline residues and surrounding basic residues in stabilizing the DNA kinks, residues that are unaltered in the mutant protein [16,22,24].
Figure 5. Cyclization of 105 bp DNA.
Linear DNA was incubated with HBsu (lanes 2–8) or HBsu-D26A (lanes 9–15) and T4 DNA ligase for 0.5, 1, 2.5, 5, 10 or 20 min. Reaction in lane 1 contained no ligase. Reactions in lanes 8 and 15 were ligated for 20 min, followed by addition of exonuclease III to confirm the formation of a circular ligation product. The 105 bp DNA was previously shown not to cyclize effectively under these conditions [28].
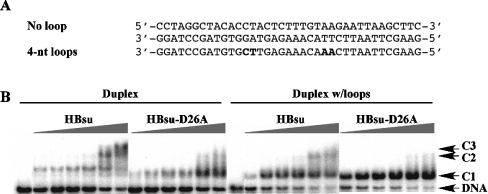
The effect of the D→A substitution on DNA site size was evaluated further using EMSA. The length of the 37 bp DNA probe used corresponds to the DNA engaged by the sequence-specific HU homologues IHF and TF1. Binding to perfect duplex DNA was compared with DNA in which two 4 nt loops were placed symmetrically about the centre with a separation of 9 bp (Figure 6A). Such looped DNA was previously shown to serve as preferred substrates for Anabaena HU and TF1 [27,33]. Incubation of 37 bp perfect duplex DNA with wild-type HBsu results in the formation of three distinct complexes (Figure 6B), indicating a site size longer than the 9 bp site reported for E. coli HU (which would have resulted in four complexes [18]), but significantly shorter than the approx. 19 bp site seen with Anabaena and Helicobacter pylori HU (which would have led to two complexes [22,34]). Combined with the observation that HBsu forms two complexes with 26 bp DNA (results not shown), a site size of 10–13 bp is indicated. In contrast, HBsu-D26A forms a second unstable complex with 37 bp DNA only at higher protein concentrations, suggesting a site size longer than the 19 bp site seen for Anabaena and H. pylori HU, and consistent with the interaction of Lys3 with the ends of an approx. 25 bp duplex. Notably, complex 1 for HBsu-D26A has greater mobility compared with complex 1 formed with wild-type HBsu, indicating a more tightly wrapped complex.
Figure 6. HBsu-D26A engages a longer DNA site.
(A) The sequence of 37 bp duplex and duplex with loops. Four nt loops are generated as tandem mismatches (mismatched bases in boldface). (B) Electrophoretic analysis of HBsu and HBsu-D26A binding to 37 bp duplex (left panels) and 37 bp duplex with loops (right panels). Complexes (C1, C2 and C3) and free DNA are identified on the right. Protein concentrations (identical for each panel) are 2.5, 5.0, 7.5, 10, 25 and 50 nM. Reactions contained 200 mM KCl.
Fractional complex formation for HBsu or HBsu-D26A with 37 bp DNA was quantified, with the best fits obtained using nonlinear fits to the Hill equation. Although both HBsu and HBsu-D26A bind 37 bp duplex DNA with a calculated apparent Kd of 16±1 nM at 200 mM KCl, the Hill co-efficients for HBsu and HBsu-D26A are 1.3±0.3 and 1.1±0.2 respectively. For HBsu-D26A, the Hill coefficient indicates non-co-operative binding, consistent with the predominant formation of one complex (identified as C1 in Figure 6B). For HBsu, the Hill co-efficient of 1.3 suggests positive co-operativity, an interpretation confirmed by nonlinearity of Scatchard plots (results not shown; [35]). However, HBsu is expected to bind DNA non-specifically, implying the existence of numerous overlapping sites on the DNA. Because of overlap binding, available sites become less accessible with increasing saturation of the DNA. Overlap binding therefore results in negative co-operativity as it becomes increasingly difficult to bind additional ligands as saturation is approached. The calculated Hill co-efficient must therefore be interpreted with caution. We also note that the apparent Kd obtained from fits to the Hill equation reports an ‘aggregate’ affinity for the DNA, represented as the product of macroscopic binding constants for binding of each additional ligand to the DNA. As the net number of HBsu and HBsu-D26A molecules that may be accommodated on the 37 bp duplex is different, the equivalent Kd obtained from Hill plots, which corresponds to the protein concentration at which half-maximal saturation is observed, therefore suggests higher affinity binding by HBsu-D26A (for which no more than two HU dimers may be accommodated) compared with HBsu (for which three protein molecules may bind, leading to a calculated Kd that is derived from the product of three macroscopic binding constants). As expected, both HBsu and HBsu-D26A exhibit modest salt-dependence of binding, consistent with the presence of numerous surface salt bridges that must be disrupted to allow complex formation. Quantification of fractional saturation measured at 50 mM KCl yields Hill coefficients of 1.1±0.2 for both proteins and a calculated Kd of 3.0±0.2 nM (results not shown), again with the above mentioned considerations for the interpretation of parameters obtained from the fits.
The ability of both HBsu and HBsu-D26A to bind with higher affinity for DNA in which flexure points are introduced at the sites of kinking is reflected in their preferred binding to 37 bp DNA with loops (Figure 6B). Whereas two complexes may be seen with HBsu, HBsu-D26A forms a single complex with the more flexible DNA. For HBsu-D26A, fits of the data to a rectangular hyperbola (which assumes binding to a single site) yield an apparent Kd=1.0±0.0 nM at 200 mM KCl, whereas fits of fractional saturation of HBsu binding to the looped DNA to the Hill equation yield Kd=3.3±0.1 nM, both binding constants reflecting higher affinity binding compared with perfect duplex DNA. As seen also for binding to perfect duplex DNA, HBsu-D26A binds with higher affinity for the looped DNA construct than does wild-type HBsu (for which two distinct complexes form). We also note that on the basis of calculated site size in duplex DNA, HBsu may exhibit a competition between preferred binding to DNA loops and non-specific binding to perfect duplex regions, implying that the calculated Kd is a composite of binding constants of different magnitude. Both proteins also exhibit preferred binding to 37 bp DNA with a central nick or a 1–2 nt gap (results not shown). These data further document the ability of more flexible DNA to promote DNA wrapping by HBsu by stabilizing the protein-induced DNA kinks [24]. As seen for perfect duplex DNA, both proteins exhibit modestly higher affinity binding at 50 mM KCl (results not shown), with a Kd for HBsu calculated from Hill plots of 2.1±0.1 nM, and an apparent Kd for HBsu-D26A (calculated from fits to rectangular hyperbola) of 0.6±0.0 nM.
Consistent with their ability to bend DNA, and with properties of other HU homologues, HBsu and HBsu-D26A bind preferentially to four-way junction DNA (Figure 7). Both proteins form two predominant complexes, presumably corresponding to one HU dimer binding to a pair of junction arms on either side of the crossover [19,21,28]. Although the shift in mobility of complex is modest when compared with free DNA for both proteins, a particularly modest mobility shift is seen for the HBsu-D26A–DNA complex, again consistent with a more tightly wrapped complex.
Figure 7. HBsu and HBsu-D26A bind preferentially to four-way junction DNA.
(A) HBsu and (B) HBsu-D26 binding to 1 nM four-way junction DNA. Protein concentrations, identical for both panels, are 0.06, 0.12, 0.25, 0.5, 0.75, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0 and 10 nM. Reactions were analysed on 6% native gels. The essentially quantitative conversion of available DNA to complex at 1 nM protein (lanes 7) indicates that reactions are performed under stoichiometric conditions (Kd<[DNA]).
DISCUSSION
HBsu-D26A has enhanced capacity for DNA wrapping
Recent structural and biophysical studies have indicated that protein assemblies that organize long DNA fragments wrap the double-stranded DNA across the protein surface. Proteins that wrap DNA tend to have not only numerous cationic surface residues, but also a large number of anionic side chains with which the cationic residues may form ion pairs. Such ion pairs would compete with formation of electrostatic contacts to the DNA backbone, modulating both affinity and the salt-dependence of binding [17,24,36]. In the HU homologue TF1, substitution of Lys3 with Ala results in a shorter DNA site size, an observation suggesting that Lys3 is required for interaction with the ends of an approx. 25 bp duplex and for wrapping of longer DNA duplexes across the protein surface [23]. TF1, whose structure suggests that Lys3 does not participate in a surface salt bridge, acts as a transcription factor by binding to preferred 37 bp sites within certain promoters, and has not been explicitly implicated in the organization of genomic DNA through non-specific binding to shorter DNA sites [37]. Since Lys3 is almost universally conserved in HU proteins, this observation would predict that the interaction of Lys3 with DNA phosphates must be generally attenuated by its participation in a surface salt bridge, particularly in HU homologues characterized by a short DNA-binding site. Indeed, the majority of HU proteins have an acidic residue at position 26 [26].
We have shown in the present study that substitution of Asp26 with Ala in HBsu results in a longer DNA site size, a higher affinity and a greater capacity for DNA wrapping by the mutant protein (Figures 3, 5 and 6). This substitution is designed to disrupt the surface salt bridge between Lys3 and Asp26 identified in the structure of the homologous BstHU. HBsu has 16 basic residues and 14 acidic side chains per monomer, with a net positive surface potential across the region known to contact the central 9 bp between the proline-mediated DNA kinks, but no obvious charged path along the sides of the protein that would engage DNA distal to the kinks [12,14]. The presence of Asp26 is therefore unlikely to reduce DNA site size due to electrostatic repulsion. The existence of a salt bridge between Lys3 and Asp26 in BstHU, the very modest salt-dependence of binding seen for HBsu, combined with the reported effects on disruption of surface salt bridges for other HU homologues, suggest that the observed effects on DNA site size on substitution of Asp26 are due to disruption of this salt bridge. Based on the formation of three complexes by HBsu with 37 bp DNA and two with 26 bp DNA, we reason that the optimal site size in duplex DNA must be longer than the 9 bp site reported for E. coli HU (or four complexes would have been seen on 37 bp DNA), yet shorter than 14 bp (as occlusion of 28 bp would leave only 9 bp available on 37 bp DNA). This is consistent with the approx. 10 bp site previously reported for the related B. globigii HU. By comparison, HBsu-D26A must have a site size significantly longer than 19 bp, as the formation of a second complex with 37 bp DNA is disfavoured (Figure 5). This interpretation is consistent with the interaction of Lys3 with the ends of an approx. 25 bp duplex.
The distinct sites sizes of HBsu and HBsu-D26A result in the formation of protein–DNA complexes of different stoichiometry at saturation. As the observed dissociation constant Kd obtained from fits to the Hill equation reflects the product of macroscopic binding constants for binding of each successive ligand to the DNA, the equivalent Kd measured for HBsu and HBsu-D26A binding to 37 bp duplex suggests higher affinity binding to a single site for HBsu-D26A. As mentioned above, indications of positive co-operativity for HBsu are complicated by the fact that non-specific overlap binding to the DNA will lead to negative co-operativity as saturation is approached. Additionally, although the apparent Kd for HBsu-D26A binding to DNA with loops largely represents the affinity for binding to a single site, interpretation of the parameters for HBsu is confounded by the likely existence of a competition between binding to non-specific and preferred DNA sites. We conclude from these semi-quantitative considerations that (1) both HBsu and HBsu-D26A bind with higher affinity for DNA with loops compared with perfect duplex DNA, (2) HBsu-D26A binds with higher affinity for both perfect duplex DNA and to DNA with loops compared with wild-type HBsu, and (3) both proteins exhibit only modest salt-dependence of binding. The modest salt-dependence of binding is consistent with the reported salt-dependence of other HU homologues that has been interpreted in terms of a disruption of surface salt bridges that must accompany DNA binding to liberate lysine side chains for interaction with DNA phosphates [17,24].
Although tighter wrapping of duplex DNA is seen for HBsu-D26A, as evidenced by faster-migrating DNA complexes with both plasmid DNA and short DNA duplexes, cyclization assays suggest that the bending angle is essentially unaltered in the mutant protein. This observation is expected based on the reported roles of the DNA-intercalating proline residues and surrounding cationic residues in stabilizing the DNA kinks, residues that are unaltered in HBsu-D26A [16,22,24]. Our data also show that both proteins exhibit preferred binding to DNA in which the sites of DNA kinking have been rendered more flexible through the introduction of nicks, gaps or mismatches. Such flexure points also promote contact between flanking DNA and the protein surface, for example as evidenced by the greater mobility of HBsu–DNA complex with loop compared with perfect duplex DNA (Figure 6). Such preferred binding to more flexible DNA sites is also seen for E. coli HU, for which this binding mode has been correlated with a role in DNA repair [5–7]. However, preferred binding to such repair intermediates is not a universal property of HU proteins; for example, both Deinococcus radiodurans HU and H. pylori HU fail to bind preferentially to DNA with nicks, gaps or mismatches [28,34]. We suggest that in duplex DNA, HBsu was unable to stabilize the proline-induced DNA kinks, leading to low-affinity binding to short DNA sites, whereas flexure points such as nicks or gaps allow such stabilization, promoting interaction between flanking DNA and the body of the protein, leading to increased affinity and greater DNA compaction. This mode of DNA interaction is consistent with a role for HBsu in recognition of DNA repair intermediates [8]. In contrast, HBsu-D26A wraps longer DNA, even in perfect duplex DNA. Accordingly, our data show that the absence of a salt bridge involving Lys3 promotes wrapping of perfect duplex DNA, whereas the participation of Lys3 in a surface salt bridge may be important for substrate specificity.
HBsu appears to bind plasmid DNA with apparent negative co-operativity
In its interaction with plasmid DNA, HBsu appears to bind preferentially to unbound DNA as opposed to DNA already complexed with protein, leading to a shift in mobility of the entire population of DNA species as a relatively sharp band (Figure 3). Such behaviour would be expected if HBsu bound with negative co-operativity. For a non-sequence-specific DNA-binding protein, negative co-operativity is expected at higher protein concentrations due to overlap binding, as it becomes increasingly difficult to saturate the DNA; however, such a behaviour would be particularly evident on shorter DNA, not on plasmid DNA. We therefore interpret the binding pattern primarily in terms of the DNA topology induced on protein binding [32]. HBsu and HBsu-D26A coil DNA in a toroidal superhelix, leading to negative supercoiling (Figure 4). This would lead to compensatory positive supercoils in protein-free DNA in the absence of DNA-nicking activities, as well as positive superturns resulting from the change in DNA twist upon intercalation of proline residues. The apparent negative co-operativity suggests the generation of a substrate for which the protein has reduced affinity; accordingly, protein binding to covalently closed DNA would result in the generation of positive superhelicity (in relaxed DNA) or in DNA relaxation (in already negatively supercoiled DNA), in both circumstances generating a substrate for which the protein has reduced affinity. Cruciform structures may also form in highly supercoiled DNA due to plectonemic writhing, generating a DNA conformation for which HBsu has very high affinity, thus leading to preferred binding (Figure 7). Preferred binding to a DNA nick would also lead to apparent negative co-operativity, as unbound plasmid DNA is preferentially bound over perfect duplex regions. Secondly, rotation about a protein-bound nick would likely be attenuated, leading to stabilization of toroidal superturns by bound protein, with compensatory positive superhelicity in unbound DNA. In linear DNA, HBsu, which binds preferentially to DNA overhangs, would be expected to bind preferentially to DNA ends generated by restriction enzyme digestion as well as to DNA sequences characterized by intrinsic flexure. The aggregation of HBsu observed in solution (Figure 2) also suggests that even linear DNA, although not covalently closed, may be topologically constrained through protein–protein interactions. Whereas only precise quantification of DNA complex formation can definitively document negative co-operativity, the delineated DNA-binding properties of HBsu are consistent with its manifestation; we also point out that net positive co-operativity is unlikely as it would have led to identifiable populations of bound and unbound DNA species.
Single-molecule experiments with E. coli HU have shown condensation of DNA at lower protein/DNA ratios (e.g. 1 dimer/92 bp) whereas high protein/DNA ratios (up to 1 dimer/1.8 bp) result in an approx. 8% increase in length and an increased stiffness of the resulting protein–DNA filament [39]. Consistent with these reports, we observe condensation of nicked DNA up to 1 HBsu-D26A dimer/62 bp after which increased retardation is seen. With a cellular concentration of HBsu estimated at approx. one dimer/170 bp of genomic DNA [11], its primary role in conferring DNA flexibility and condensation is consistent with these observations.
Multimedia adjunct
Acknowledgments
We thank S. Oard and M. Oldham for assistance with molecular modelling and R. Ellis and K. McCain for their participation at early stages of this project. This work was supported by grants from Louisiana Board of Regents Support Fund [LEQSF(2000-03)-RD-A-07] and the National Science Foundation (MCB-0414875) to A.G.
References
- 1.Drlica K., Rouvière-Yaniv J. Histone-like protein of bacteria. Microbiol. Rev. 1987;51:301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gualerzi C. O., Pon C. L. Berlin: Springer-Verlag; 1986. Bacterial Chromatin. [Google Scholar]
- 3.Kellenberger E., Arnold-Schultz-Gahmen B. Chromatins of low-protein-content: special features of their compaction and condensation. FEMS Microbiol. Lett. 1992;100:361–370. doi: 10.1111/j.1574-6968.1992.tb14064.x. [DOI] [PubMed] [Google Scholar]
- 4.Micka B., Marahiel M. A. The DNA-binding protein HBsu is essential for normal growth and development in Bacillus subtilis. Biochimie. 1992;74:641–650. doi: 10.1016/0300-9084(92)90136-3. [DOI] [PubMed] [Google Scholar]
- 5.Huisman O., Faelen M., Girard D., Jaffe A., Toussaint A., Rouvière-Yaniv J. Multiple defects in Escherichia coli mutants lacking HU protein. J. Bacteriol. 1989;171:3704–3712. doi: 10.1128/jb.171.7.3704-3712.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dri A. M., Moreau P. L., Rouvière-Yaniv J. Role of the histone-like proteins Osmz and HU in homologous recombination. Gene. 1992;120:11–16. doi: 10.1016/0378-1119(92)90003-8. [DOI] [PubMed] [Google Scholar]
- 7.Boubrik F., Rouvière-Yaniv J. Increased sensitivity to γ irradiation in bacteria lacking protein HU. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3958–3962. doi: 10.1073/pnas.92.9.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez S., Rojo F., Alonso J. C. The Bacillus subtilis chromatin-associated protein Hbsu is involved in DNA repair and recombination. Mol. Microbiol. 1997;23:1169–1179. doi: 10.1046/j.1365-2958.1997.3061670.x. [DOI] [PubMed] [Google Scholar]
- 9.Köhler P., Marahiel M. A. Association of the histone-like protein HBsu with the nucleoid of Bacillus subtilis. J. Bacteriol. 1997;179:2060–2064. doi: 10.1128/jb.179.6.2060-2064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura K., Yahagi S., Yamazaki T., Yamane K. Bacillus subtilis histone-like protein, HBsu, is an integral component of a SRP-like particle that can bind the Alu domain of small cytoplasmic RNA. J. Biol. Chem. 1999;274:13569–13576. doi: 10.1074/jbc.274.19.13569. [DOI] [PubMed] [Google Scholar]
- 11.Ross M. A., Setlow P. The Bacillus subtilis HBsu protein modifies the effects of alpha/beta-type, small acid-soluble spore proteins on DNA. J. Bacteriol. 2000;182:1942–1948. doi: 10.1128/jb.182.7.1942-1948.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka I., Appelt K., Dijk J., White S. W., Wilson S. 3-Å resolution structure of a protein with histone-like properties in prokaryotes. Nature (London) 1984;310:376–381. doi: 10.1038/310376a0. [DOI] [PubMed] [Google Scholar]
- 13.White S. W., Appelt K., Wilson K. S., Tanaka I. A protein structural motif that bends DNA. Proteins Struct. Funct. Genet. 1989;5:281–288. doi: 10.1002/prot.340050405. [DOI] [PubMed] [Google Scholar]
- 14.Vis H., Mariani M., Vorgias C. E., Wilson K. S., Kaptein R., Boelens R. Solution structure of the HU protein from Bacillus stearothermophilus. J. Mol. Biol. 1995;254:692–703. doi: 10.1006/jmbi.1995.0648. [DOI] [PubMed] [Google Scholar]
- 15.Jia X., Grove A., Ivancic M., Hsu V. L., Geiduschek E. P., Kearns D. R. Structure of the Bacillus subtilis phage SPO1-encoded type II DNA-binding protein TF1 in solution. J. Mol. Biol. 1996;263:259–268. doi: 10.1006/jmbi.1996.0573. [DOI] [PubMed] [Google Scholar]
- 16.Rice P. A., Yang S. W., Mizuuchi K., Nash H. A. Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn. Cell (Cambridge, Mass.) 1996;87:1295–1306. doi: 10.1016/s0092-8674(00)81824-3. [DOI] [PubMed] [Google Scholar]
- 17.Holbrook J. A., Tsodikov O. V., Saecker R. M., Record M. T., Jr Specific and non-specific interactions of integration host factor with DNA: thermodynamic evidence for disruption of multiple IHF surface salt-bridges coupled to DNA binding. J. Mol. Biol. 2001;310:379–401. doi: 10.1006/jmbi.2001.4768. [DOI] [PubMed] [Google Scholar]
- 18.Bonnefoy E., Rouvière-Yaniv J. HU and IHF, two homologous histone-like proteins of Escherichia coli, form different protein-DNA complexes with short DNA fragments. EMBO J. 1991;10:687–696. doi: 10.1002/j.1460-2075.1991.tb07998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnefoy E., Takahashi M., Rouvière-Yaniv J. DNA-binding parameters of the HU protein of Escherichia coli to cruciform DNA. J. Mol. Biol. 1994;242:116–129. doi: 10.1006/jmbi.1994.1563. [DOI] [PubMed] [Google Scholar]
- 20.Lavoie B. D., Shaw G. S., Millner A., Chaconas G. Anatomy of a flexer-DNA complex inside a higher-order transposition intermediate. Cell (Cambridge, Mass.) 1996;85:761–771. doi: 10.1016/s0092-8674(00)81241-6. [DOI] [PubMed] [Google Scholar]
- 21.Kamashev D., Balandina A., Rouvière-Yaniv J. The binding motif recognized by HU on both nicked and cruciform DNA. EMBO J. 1999;18:5434–5444. doi: 10.1093/emboj/18.19.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swinger K. K., Lemberg K. M., Zhang Y., Rice P. A. Flexible DNA bending in HU-DNA cocrystal structures. EMBO J. 2003;22:3749–3760. doi: 10.1093/emboj/cdg351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grove A., Saavedra T. C. The role of surface-exposed lysines in wrapping DNA about the bacterial histone-like protein HU. Biochemistry. 2002;41:7597–7603. doi: 10.1021/bi016095e. [DOI] [PubMed] [Google Scholar]
- 24.Grove A. Surface salt bridges modulate DNA wrapping by the type II DNA-binding protein TF1. Biochemistry. 2003;42:8739–8747. doi: 10.1021/bi034551o. [DOI] [PubMed] [Google Scholar]
- 25.Greene J. R., Geiduschek E. P. Site-specific DNA binding by the bacteriophage SP01-encoded type II DNA-binding protein. EMBO J. 1985;4:1345–1349. doi: 10.1002/j.1460-2075.1985.tb03783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grove A., Lim L. High-affinity DNA binding of HU protein from the hyperthermophile Thermotoga maritima. J. Mol. Biol. 2001;311:491–502. doi: 10.1006/jmbi.2001.4763. [DOI] [PubMed] [Google Scholar]
- 27.Grove A., Galeone A., Mayol L., Geiduschek E. P. On the connection between inherent DNA flexure and preferred binding of hydroxymethyluracil-containing DNA by the type II DNA-binding protein TF1. J. Mol. Biol. 1996;206:196–206. doi: 10.1006/jmbi.1996.0392. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S., Grove A. Histone-like protein HU from Deinococcus radiodurans binds preferentially to four-way DNA junctions. J. Mol. Biol. 2004;337:561–571. doi: 10.1016/j.jmb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Welfle H., Misselwitz R., Welfle K., Groch N., Heinemann U. Salt-dependent and protein-concentration-dependent changes in the solution structure of the DNA-binding histone-like protein, HBsu, from Bacillus subtilis. Eur. J. Biochem. 1992;204:1049–1055. doi: 10.1111/j.1432-1033.1992.tb16727.x. [DOI] [PubMed] [Google Scholar]
- 30.Vis H., Heinemann U., Dobson C. M., Robinson C. V. Detection of a monomeric intermediate associated with dimerization of protein Hu by mass spectrometry. J. Am. Chem. Soc. 1998;120:6427–6428. [Google Scholar]
- 31.Köhler P., Marahiel M. A. Mutational analysis of the nucleoid-associated protein HBsu of Bacillus subtilis. Mol. Gen. Genet. 1998;260:487–491. doi: 10.1007/s004380050921. [DOI] [PubMed] [Google Scholar]
- 32.Ellen T. P., van Holde K. E. Linker histone interaction shows divalent character with both supercoiled and linear DNA. Biochemistry. 2004;43:7867–7872. doi: 10.1021/bi0497704. [DOI] [PubMed] [Google Scholar]
- 33.Grove A., Galeone A., Mayol L., Geiduschek E. P. Localized DNA flexibility contributes to target site selection by DNA-bending proteins. J. Mol. Biol. 1996;206:120–125. doi: 10.1006/jmbi.1996.0386. [DOI] [PubMed] [Google Scholar]
- 34.Chen C., Ghosh S., Grove A. Substrate specificity of Helicobacter pylori histone-like HU protein is determined by insufficient stabilization of DNA flexure points. Biochem. J. 2004;383:343–351. doi: 10.1042/BJ20040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hensley P., Yang Y. R., Schachman H. K. On the detection of homotropic effects in enzymes of low co-operativity. Application to modified aspartate transcarbamoylase. J. Mol. Biol. 1981;152:131–152. doi: 10.1016/0022-2836(81)90098-x. [DOI] [PubMed] [Google Scholar]
- 36.Saecker R. M., Record M. T., Jr Protein surface salt bridges and paths for DNA wrapping. Curr. Opin. Struct. Biol. 2002;12:311–319. doi: 10.1016/s0959-440x(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 37.Greene J. R., Geiduschek E. P. Site-specific DNA binding by the bacteriophage SP01-encoded type II DNA-binding protein. EMBO J. 1985;4:1345–1349. doi: 10.1002/j.1460-2075.1985.tb03783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe F., Stankowski S., Schwarz G. Interaction of the HB protein of Bacillus globigii with nucleic acids. Analysis of the binding to DNA and polynucleotides. Eur. J. Biochem. 1984;140:215–219. doi: 10.1111/j.1432-1033.1984.tb08089.x. [DOI] [PubMed] [Google Scholar]
- 39.van Noort J., Verbrugge S., Goosen N., Dekker C., Dame R. T. Dual architectural roles of HU: formation of flexible hinges and rigid filaments. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6969–6974. doi: 10.1073/pnas.0308230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.