Abstract
ORP2 [OSBP (oxysterol-binding protein)-related protein 2] belongs to the 12-member mammalian ORP gene/protein family. We characterize in the present study the effects of inducible ORP2 overexpression on cellular cholesterol metabolism in HeLa cells and compare the results with those obtained for CHO cells (Chinese-hamster ovary cells) that express ORP2 constitutively. In both cell systems, the prominent phenotype is enhancement of [14C]cholesterol efflux to all extracellular acceptors, which results in a reduction of cellular free cholesterol. No change was observed in the plasma membrane cholesterol content or distribution between raft and non-raft domains upon ORP2 expression. However, elevated HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase activity and LDL (low-density lipoprotein) receptor expression, as well as enhanced transport of newly synthesized cholesterol to a cyclodextrin-accessible pool, suggest that the ORP2 expression stimulates transport of cholesterol out of the endoplasmic reticulum. In contrast with ORP2/CHO cells, the inducible ORP2/HeLa cells do not show down-regulation of cholesterol esterification, suggesting that this effect represents an adaptive response to long-term cholesterol depletion in the CHO cell model. Finally, we provide evidence that ORP2 binds PtdIns(3,4,5)P3 and enhances endocytosis, phenomena that are probably interconnected. Our results suggest a function of ORP2 in both cholesterol trafficking and control of endocytic membrane transport.
Keywords: cholesterol efflux, cholesterol homoeostasis, cholesterol metabolism, endocytosis, oxysterol-binding protein (OSBP), transferrin
Abbreviations: ABCA1, ATP-binding-cassette transporter A1; ACAT, acyl-CoA:cholesterol acyltransferase; CE, cholesteryl ester; CHO cells, Chinese hamster ovary cells; LDL, low-density lipoprotein; DiI-LDL, 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanineperchlorate-labelled LDL; DRM, detergent-resistant membrane; ER, endoplasmic reticulum; FBS, fetal bovine serum; FC, free cholesterol; GST, glutathione S-transferase; HDL, high-density lipoprotein; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; HPTLC, high-performance TLC; LC–ESI, liquid chromatography–electrospray ionization; LPDS, lipoprotein-deficient serum; OSBP, oxysterol-binding protein; ORP2, OSBP-related protein 2; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PH, pleckstrin homology; PI, phosphatidylinositol; PLCδ, phospholipase Cδ; PM, plasma membrane; PS, phosphatidylserine; SCAP, sterol-regulatory-element-binding protein cleavage activating protein; SM, sphingomyelin; SR-B1, scavenger receptor B-1; SREBP, sterol-regulatory-element-binding protein; TC, total cholesterol; Tfn, transferrin
INTRODUCTION
In mammalian cells cholesterol homoeostasis is achieved through complex regulatory circuits that control, on the one hand, the biosynthesis and esterification of cholesterol and, on the other, the uptake of cholesterol from lipoprotein carriers and the efflux of cholesterol to extracellular acceptors. The major regulators of cholesterol biosynthesis and uptake, as well as fatty acid biosynthesis, are transcription factors called SREBPs (sterol-regulatory-element-binding proteins) and their sterol-sensing accessory factor, the SCAP (SREBP cleavage activating protein) [1,2]. Another route for transcriptional regulation of sterol metabolism involves the liver X receptors, nuclear receptors that bind oxidized cholesterol derivatives, oxysterols, and control the expression of several genes involved in intestinal cholesterol adsorption, cellular cholesterol efflux, plasma lipid transport, and conversion of cholesterol into bile acids (reviewed in [3]).
The subcellular distribution of cholesterol is markedly asymmetric. A majority of cell cholesterol resides in the PM (plasma membrane), where it constitutes 35–45% of the lipid molecules. Also early and recycling endosomes and the trans-part of the Golgi complex contain significant amounts of cholesterol, while the ER (endoplasmic reticulum) is cholesterol-poor (reviewed in [4]). The mechanisms responsible for this asymmetry are not well understood. The ER contains the major sensors and enzymatic activities responsible for the maintenance of cellular cholesterol homoeostasis, the SREBP–SCAP system, the ACATs (acyl-CoA:-cholesterol acyltransferases) enzymes that generate the storage form of cholesterol, CEs (cholesteryl esters), and the rate-limiting enzymes of the cholesterol biosynthetic pathway. Accordingly, the homoeostatic apparatus is responsive to changes in the ER cholesterol content (reviewed in [5,6]).
The efflux of cholesterol from cells occurs through four mechanisms, the combination of which determines the rate of cholesterol efflux from a given cell type in vivo. First, cholesterol can transfer to acceptors in the circulation via a diffusion-mediated process that is bidirectional. The net transfer here is driven by extracellular cholesterol esterification that maintains a concentration gradient between lipoprotein surfaces and cell membrane. Secondly, a similar bidirectional transport process can be facilitated by SR-B1 (scavenger receptor B-1). Thirdly, cholesterol and phospholipids are removed from cells by an active unidirectional protein-dependent mechanism involving ABCA1 (ATP-binding-cassette transporter A1) and lipid-poor apolipoprotein acceptors [7]. The recently discovered fourth mechanism involves ABCG1 and ABCG4 and spherical HDL (high-density lipoprotein) acceptors [8].
OSBP (oxysterol-binding protein) is a cytoplasmic protein that shows affinity for a number of oxysterols, 27-carbon oxygenated derivatives of cholesterol [9]. Overexpression of OSBP in CHO cells (Chinese-hamster ovary cells) increases the synthesis of cholesterol and SM (sphingomyelin) [10,11] and OSBP has been suggested to play a role in the trafficking of ceramide from the ER to the Golgi apparatus [12]. A family of human ORPs (OSBP-related proteins) was recently identified that, in addition to OSBP, consists of 11 members. Related protein families are present throughout the eukaryotic kingdom, suggesting a fundamental role of the ORPs in lipid metabolism [13]. We recently characterized one of the human OSBP-related proteins, ORP2, and presented evidence for its role in the control of cellular cholesterol efflux and esterification in stably transfected CHO cells [14]. Furthermore, analysis of Saccharomyces cerevisiae strains with disruptions of the yeast ORP (OSH) genes revealed major phenotypic effects involving sterol metabolism [15,16]. To investigate the mechanisms underlying the effects of ORP2 on cellular cholesterol metabolism, we have now established stable T-REx HeLa cell lines that can be induced with doxycycline to overexpress ORP2. Inducible expression allows the analysis of the acute effects of ORP2, in contrast with the constitutively expressing ORP2/CHO cells, the phenotype of which may be due to adaptive processes during the long selection and single-cell cloning periods in the presence of excess ORP2. We characterize in the present study the effects of inducible ORP2 overexpression on cellular cholesterol metabolism and compare the results with those obtained with the constitutive ORP2/CHO cells. Furthermore, in search for the mechanisms responsible for the observed alterations in cholesterol metabolism, we identify a phosphoinositide ligand of ORP2 and assess the effects of ORP2 overexpression on fluid-phase and receptor-mediated endocytosis.
EXPERIMENTAL
Antibodies and other reagents
Rabbit polyclonal anti-LDL receptor antibody (where LDL stands for low-density lipoprotein) was purchased from PROGEN (Heidelberg, Germany), rabbit polyclonal antibodies anti-SR-B1 and anti-ABCA1 from Novus Biologicals (Littleton, CO, U.S.A.), and rabbit polyclonal anti-GRP-94 from Santa Cruz Biotechnology (Heidelberg, Germany). Rabbit antibodies raised against the cytosolic domain of mouse syntaxin 2 were produced in the laboratory using a standard immunization method. Production of rabbit polyclonal anti-ORP2 antibody is described in [14]. Tetramethylrhodamine-conjugated dextran (10 kDa), DiI-LDL (1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanineperchlorate-labelled LDL) and FITC-conjugated Tfn (transferrin) were from Molecular Probes (Leiden, The Netherlands).
Selection of stably transfected inducible HeLa cells and cell culture
Human ORP2 cDNA [17] was cloned into a BamHI site of the pcDNA4/TO expression vector (Invitrogen, Leek, The Netherlands). T-REx HeLa cells (Invitrogen) were transfected with the expression plasmid using Lipofectamine™ 2000 (Invitrogen). Cells containing expression plasmid were selected with 1.0 mg/ml zeocin in growth medium and identified by immunofluorescence microscopy with rabbit anti-ORP2 antibody [14] after a 20 h induction with 1 μg/ml doxycycline. Single-cell cloning was carried out by limiting dilution. Cell lines 21f3, 38b10 and 40d5 were used in the present study.
T-REx HeLa cells were cultured in Eagle MEM (Sigma) supplemented with 10% (v/v) Tet System-approved FBS (fetal bovine serum; BD Biosciences, Heidelberg, Germany and ClonTech Laboratories, Heidelberg, Germany), 10 mM Hepes, 100 units/ml penicillin, 100 μg/ml streptomycin and 5 μg/ml blasticidin. The transfected cells were cultured in the same medium supplemented with 600 μg/ml zeocin. ORP2 overexpression in T-REx HeLa cells was induced with 1 μg/ml doxycycline for 42 h.
Stably transfected CHO-K1 cells were cultured in Iscove's modified Dulbecco's medium (Sigma), supplemented with 10% FBS (Life Technologies, Basel, Switzerland), 100 units/ml penicillin, 100 μg/ml streptomycin and 400 μg/ml Geneticin (G-418 sulphate; Life Technologies). The stably transfected ORP2/CHO cell lines (clone A5a9 described in [14] and clone A6d6), and CHO cells transfected with the pcDNA3.1 (Invitrogen) vector plasmid (denoted as control cells) were used.
Assay for [14C]cholesterol efflux and esterification
Cells were seeded on day 1 on 3 cm dishes and cultured in a medium containing 10% serum. On day 2, the medium was changed and 54 μCi/mmol [14C]cholesterol (0.2 μCi/dish in a volume of 2 ml; Amersham Biosciences, Little Chalfont, Bucks., U.K.), and in some experiments 2 μg/ml ACAT inhibitor PKF 058-035 (Novartis, Basel, Switzerland) were added to fresh serum containing medium. ORP2 expression was induced in T-REx HeLa cells with 1 μg/ml doxycycline in the labelling medium. After 42 h of labelling in the presence or absence of doxycycline the growth medium was discarded. The cells were washed twice with PBS, and 1 ml of serum-free medium or medium containing 20% (v/v) human serum was added. After 2 h at 37 °C, the radioactivity in the medium and in the cells was measured. In some experiments the efflux was carried out using human HDL2 (15 μg/ml of HDL; efflux time 4 h), BSA (2 mg/ml) or BSA plus human apoA-I (20 μg/ml; efflux time 4 h) as acceptors. After washing, the cells were scraped into 1 ml of ice-cold 2% (w/v) NaCl. Extraction and HPTLC (high-performance TLC) analysis of the lipids were performed as previously described in [14,18]. The amounts of 14C-labelled FC (free cholesterol) and CEs were measured by liquid-scintillation counting. The total amount of [14C]cholesterol radioactivity incorporated into the cells during the labelling period did not differ significantly between the induced and the non-induced ORP2/HeLa cells.
Quantification of cyclodextrin-accessible cell-surface cholesterol
Tet-inducible ORP2/HeLa cells were incubated for 42 h with [14C]cholesterol in a medium supplemented with 10% FBS or with 5% LPDS (lipoprotein-deficient serum), chased for 2 h, and subjected to a 5 min incubation with 5 mM methyl-β-cyclodextrin (Sigma) in Hepes-buffered serum-free medium on a shaking water bath at 37 °C. In the case of ORP2/HeLa cells, incubation in the presence or absence of doxycycline (1 μg/ml) was carried out concomitantly with the 42 h labelling. The cyclodextrin medium was collected and the cells were harvested as described above. The [14C]cholesterol content of the medium and of the cells was determined by liquid-scintillation counting.
Enrichment of PMs by the colloidal silica method
The colloidal silica method for the enrichment of PMS was first described in [19]. Chlorhydrol-coated Levasil 50 beads {Obermeier; nominal diameter 500 Å (1 Å=0.1 nm), 30% dilution prepared as in [19]} were diluted to 1% with 20 mM MES (pH 6.7), 150 mM NaCl and centrifuged at 800 g for 5 min at 4 °C to remove aggregates. Two 10 cm dishes of T-REx HeLa cells were incubated for 40 h in the absence or presence of 1 μg/ml doxycycline. The cells were washed twice with 20 mM Mes and 150 mM NaCl (pH 6.7), and overlaid with 5 ml of 1% silica solution. After incubating the cells on ice for 1 min, they were washed and overlaid with 5 ml of 1 mg/ml polyacrylic acid in the above buffer. After 1 min on ice the cells were washed and scraped in 25 mM Hepes/KOH (pH 7.4), 4 mM MgCl2 and 150 mM NaCl. The cells were then disrupted with a Dounce homogenizer and the lysate was added on 1 ml of 70% (w/v) Nycodenz (Nyegaard, Oslo, Norway), followed by centrifugation at 63000 g for 45 min at 4 °C in a SW60 Beckman rotor. Total membranes were isolated in sucrose step gradients as described in [14].
LC–ESI (liquid chromatography–electrospray ionization)–MS analysis of phospholipids
The lipids of PM fractions of the HeLa cell lines enriched using the silica method were extracted [20], spiked with internal standards, evaporated to dryness under nitrogen, and dissolved in chloroform/methanol (1:2). Several internal standards were needed to correct for the effects of polar head group and acyl chain length on the instrument response according to previously reported procedures [21,22]. The synthetic di-16:1, di-20:1 and di-22:1 PC (phosphatidylcholine) species were purchased from Avanti Polar Lipids (Alabastor, AL, U.S.A.). The di-16:1, di-20:1 and di-22:1 PE (phosphatidylethanolamine) and PS (phosphatidylserine), 34:2 and 36:2 PI (phosphatidylinositol), and 15:0, 21:0 and 25:0 SM species were synthesized and purified in our laboratory as described previously [22]. Just before the MS analysis, 1% NH4OH was added and the lipid extracts with the internal standards were infused to the electrospray source of a Quattro Micro triple quadrupole mass spectrometer (Micromass, Altrincham, Cheshire, U.K.). The PC and SM (precursor of 184), PE (neutral loss of 141), PS (neutral loss of 87) and PI (precursor of 241) species were selectively detected using head group-specific MS/MS scanning modes. The quantifications and calibrations, applying the responses of the internal standards, were performed using Visual Basic-based macros in Excel (Microsoft).
Analysis of cholesterol distribution into detergent-insoluble membranes
T-REx HeLa cells were labelled with [14C]cholesterol in the presence or absence of 1 μg/ml doxycycline for 42 h and Triton X-100 insoluble membranes were extracted as described previously [18]. Briefly, after labelling, the cells were washed with ice-cold PBS and harvested by centrifugation. The cell pellets were resuspended in TNE (25 mM Tris/HCl, pH 7.5, 150 mM NaCl and 5 mM EDTA), 10% (w/v) sucrose, 1 mM dithiothreitol, protease inhibitor mixture (25 μg/ml each of chymostatin, leupeptin, antipain and pepstatin A) and 1% Triton X-100 at 4 °C. After 10 min on ice, the cell homogenate was mixed with 60% OptiPrep™ and overlaid with steps of 35, 30, 25, 20 and 0% OptiPrep™ in TNE/10% sucrose/1% Triton X-100. The gradient was centrifuged at 164000 g for 4 h at 4 °C in a Beckman SW60 rotor. Six fractions were collected from the top and the radioactivity was measured by liquid-scintillation counting.
Analysis of the arrival of newly synthesized cholesterol at the PM
For measuring the appearance of newly synthesized [3H]cholesterol at the PM, a method described in [18] was used. Briefly, ORP2/CHO and CHO control cells prelabelled for 36 h with [14C]cholesterol were labelled for 15 min with [3H]acetate and chased for 2 h. During the last 5 min of the chase, the cells were incubated with 5 mM methyl-β-cyclodextrin (Sigma) in Hepes-buffered serum-free medium on a shaking water bath at 37 °C. The medium was collected and the cells were harvested as described above. From the cell suspension and the medium 100 μl was used to determine the [14C]cholesterol content by liquid-scintillation counting. Another 100 μl of the cell sample was used for protein analysis. Lipids were extracted from the remaining medium and cell samples, and [3H]cholesterol was analysed by HPTLC and Ag+-HPLC [18].
Assay of HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase activity
Cells were incubated in a medium supplemented with LPDS for 42 h. In the case of tet-inducible cells, 42 h induction was carried out concomitantly with the LPDS treatment. HMG-CoA reductase activity in total membranes of control and ORP2 expressing cells was assayed as described in [23]. For each assay, 100 μg of membrane protein and 0.20 μCi of [3-14C]HMG-CoA (57 mCi/mmol; Amersham Biosciences) were used. The reactions were linear between 5 and 135 min, and a reaction time of 90 min was chosen for the experiments.
Analysis of cholesterol concentrations
In order to analyse the total, free and esterified cholesterol in T-REx HeLa cell lines, cells were grown on 3 cm dishes in a medium containing 10% serum or 5% LPDS in the presence or absence of doxycycline for 42 h. Cells were homogenized in 1 ml of 2% NaCl and aliquots of 200 μl were taken for protein analysis. Lipids were extracted from the remaining 800 μl with 1:2 chloroform/methanol [24]. The lower phase containing the lipids was evaporated under nitrogen, freeze-dried for 1 h and dissolved in 120 μl of methanol. Cholesterol was measured enzymatically using either Cholesterol CHOD-PAP (kit 1489232, Roche, Mannheim, Germany) for TC (total cholesterol) or Free Cholesterol C (kit 274-47109E, Wako, Tokyo, Japan) for FC. Esterified cholesterol was calculated by subtracting the FC from the TC. The cholesterol content of PMs enriched with the silica method was determined using a more sensitive assay described in [25].
Assays for fluid-phase and receptor-mediated endocytosis
Inducible T-REx HeLa cells were grown on coverslips in the presence or absence of doxycycline for 42 h and then, in the case of DiI-LDL or FITC–Tfn uptake, incubated in serum-free medium for 2 h at 37 °C. Rhodamine–dextran (5 mg/ml in serum-containing growth medium) or DiI-LDL (10 μg/ml in serum-free medium) was internalized at 37 °C. After different times the cells were washed with PBS and fixed with 4% (w/v) paraformaldehyde for 20 min. FITC–Tfn (50 μg/ml in serum-free medium) was similarly internalized for 5, 15 or 60 min, and the cells were fixed after 30 s treatment in acetate buffer (pH 4.5) and 100 mM NaCl, to remove surface-bound Tfn conjugates. For recycling, FITC–Tfn was internalized at 16 °C for 60 min, and the cells were washed and treated with acid buffer, followed by a 5 or 15 min chase in serum-free medium containing 500 μg/ml of unlabelled human holo-Tfn. The coverslips were mounted in Mowiol containing 50 mg/ml of 1,4-diazabicyclo(2.2.2)octane and analysed using a Leica TCS SP1 laser scanning confocal microscope system. The cellular fluorescence was quantified from images recorded at identical microscope settings, with the pinhole open (Airy 5), using the Quantify program set of the Leica LCS software package.
Liposome preparation and binding assay
Liposome pull-down assays were carried out as described in [26]. Liposomes were prepared from L-α-phosphatidylcholine (Sigma, 100 nmol/assay), [14C]dipalmitoyl phosphatidylcholine (50 nCi/assay, Amersham Biosciences), butylated hydroxytoluene (1 nmol/assay) and 1 nmol of the different dipalmitoylphosphoinositides [PtdIns(3)P, PtdIns(4)P, PtdIns(5)P, PtdIns(3,5)P2, PtdIns(4,5)P2 (Matreya, Pleasant Gap, PA, U.S.A.), PtdIns(3,4)P2 and PtdIns(3,4,5)P3 (Sigma)]. The reagents were dried under nitrogen, freeze-dried for 1 h and stored in −20 °C until use. Liposomes were prepared by resuspending the lipids in 20 mM Hepes/KOH (pH 7.6) and 100 mM KCl, vortex mixing and sonicating. Aggregates and large vesicles were removed by centrifugation at 16000 g for 15 min. Purified GST (glutathione S-transferase)–ORP2 fusion protein (100 μg), GST fusion of the PH (pleckstrin homology) domain of PLCδ (phospholipase Cδ) (30 μg) or GST alone (50 μg) were immobilized on 30 μl of glutathione–Sepharose 4B beads (Amersham Biosciences) in the above buffer. The amounts of protein were designed to obtain equimolar amounts of ORP2 and PLCδ PH domain on the beads, taking into account that the GST–ORP2 preparation contained 65% intact protein and 35% proteolytically released GST. The amount of control GST equalled that of the GST moiety on the ORP2 beads and slightly exceeded that on the PLCδ PH domain beads. The beads were then washed twice with the above buffer, mixed with the liposomes and incubated for 30 min at room temperature (22 °C) on a roller. The beads were collected by centrifugation at 500 g for 5 min and washed three times with the same buffer. The radioactivity associated with the pellets and the supernatants was determined by liquid-scintillation counting.
Other methods
Protein concentrations were determined with the Bio-Rad DC Protein assay (catalogue no. 500-0116) using BSA as a standard.
For Western blotting, 15 μg of total protein was separated in SDS/12.5% (ORP2, SR-B1, syntaxin 2 or GRP-94), 8% (LDL receptor) or 5% (ABCA1) polyacrylamide gels and transferred to Hybond-C nitrocellulose membrane (Amersham Biosciences) according to the manufacturer's instructions. Unspecific binding of antibodies was blocked with 5% (w/v) fat-free cow's milk in 10 mM Tris/HCl, pH 7.4, 150 mM NaCl and 0.1% Tween-20. Primary antibodies diluted in the same buffer were incubated with the filters overnight at 4 °C, and the bound antibodies were visualized using horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad Laboratories, Glattbrugg, Switzerland) and the enhanced chemiluminescence system (ECL®, Amersham Biosciences).
GST–ORP2 and GST–PH(PLCδ) were produced in Escherichia coli BL21 from the expression vector pGEX-1λT (Pharmacia, Orsay, France) and purified using glutathione–Sepharose 4B using a standard method.
RESULTS
Characterization of inducible ORP2/HeLa cell lines
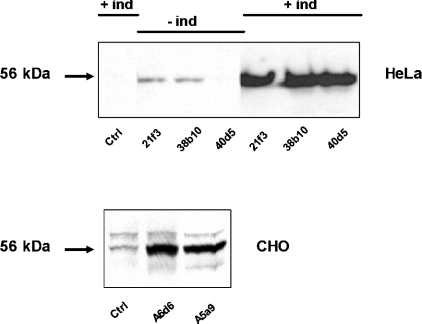
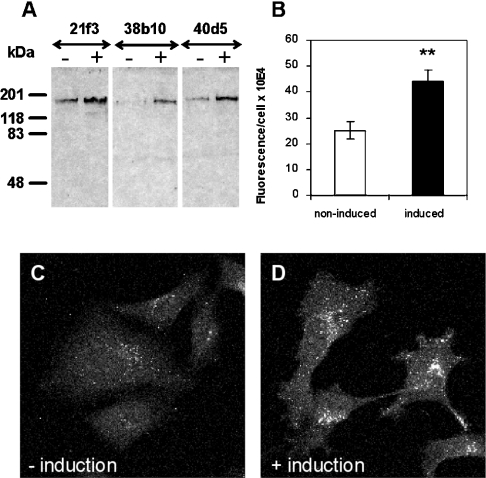
To characterize the acute effects of ORP2 overexpression we used three independent inducible T-REx ORP2/HeLa cell lines (21f3, 38b10 and 40d5). Two constitutive ORP2/CHO cell lines (A6d6 and A5a9) were included in some of the analyses for a comparison. In mock-transfected T-REx HeLa cells, no endogenous ORP2 was detectable. In the non-induced situation, two of the transfected cell lines (21f3 and 38b10) showed some leakage ORP2 expression while the third one (40d5) did not. After 42 h induction, all three lines displayed strong ORP2 expression comparable with that seen in the constitutive ORP2/CHO lines (Figure 1). In contrast with the T-REx HeLa cells, endogenous ORP2 was detectable in control CHO cells transfected with the empty vector (see also [14]).
Figure 1. Western-blot analysis of ORP2/HeLa and ORP2/CHO cells.
Lysates (15 μg of total protein) of ORP2/HeLa cells (top panel) or ORP2/CHO cells (bottom panel) were resolved by SDS/PAGE, transferred on to nitrocellulose membrane and stained with rabbit polyclonal anti-ORP2 antibody followed by visualization using HRP-conjugated anti-rabbit IgG and enhanced chemiluminescence. The cell lines are specified below the panels, and the absence or presence of a 42 h induction of the HeLa cell lines with 1 μg/ml doxycycline is indicated on the top. ‘Ctrl’ indicates cell lines transfected with the corresponding empty vector plasmid.
Effect of inducible ORP2 overexpression on cholesterol efflux
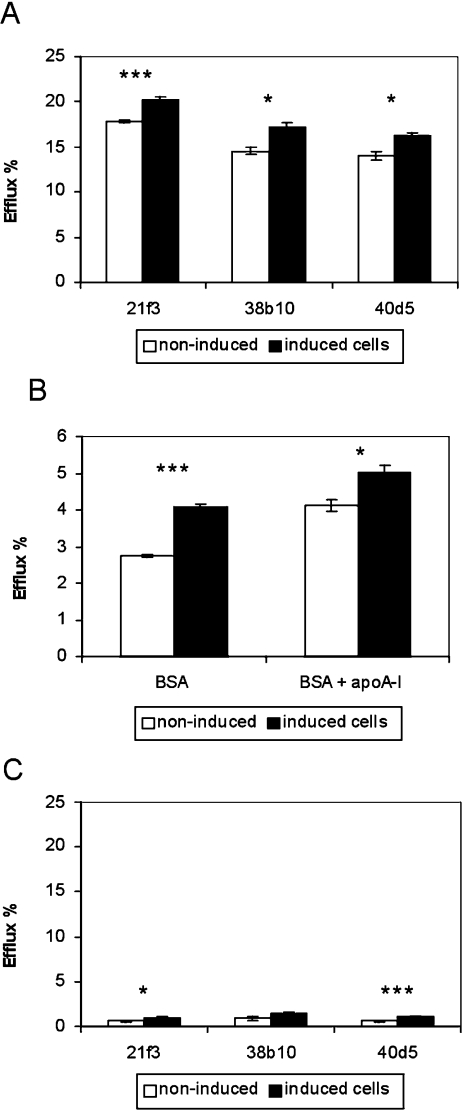
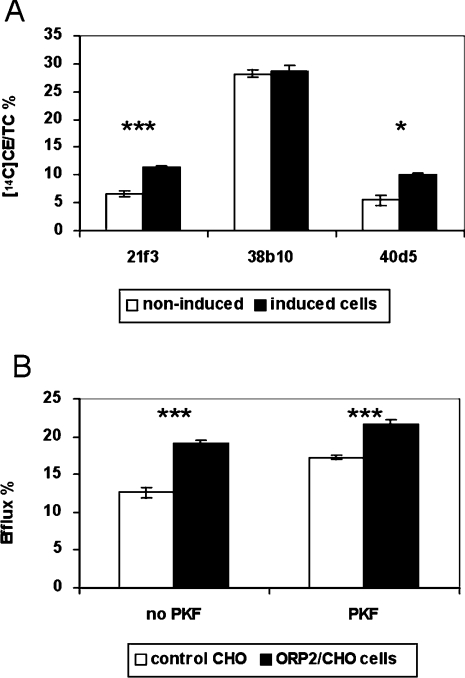
To test if the inducible ORP2/HeLa cells display increased cholesterol efflux similar to that observed in ORP2/CHO cells [14], the amount of [14C]cholesterol effluxed to 20% (v/v) human serum was measured after 42 h of [14C]cholesterol labelling and induction of ORP2 expression. After induction, all three ORP2/HeLa cell lines displayed an approx. 15% increase in cholesterol efflux as compared with non-induced cells (Figure 2A). Doxycycline treatment did not affect [14C]cholesterol efflux from cells that were stably transfected with the empty vector plasmid (results not shown). Similar increment in cholesterol efflux upon doxycycline induction of ORP2 expression was also observed when BSA (Figure 2B) or human HDL2 (results not shown) were used as acceptors. Furthermore, addition of human apoA-I (20 μg/ml) to the BSA efflux medium caused no further increment in cholesterol efflux (Figure 2B). To study the possibility that the increased amount of radioactive cholesterol in the medium could be due to shedding of PM, we also measured cholesterol efflux to the medium with no acceptors (Figure 2C). The relative amount of [14C]cholesterol released into the acceptor-free medium was negligibly low (0.4–1.4%). Even though the values for the induced, ORP2-expressing cells were higher than for the non-induced cells, the extent of putative membrane shedding was so small that it cannot explain the observed changes in cholesterol efflux to serum acceptors.
Figure 2. Effects of ORP2 overexpression on cholesterol efflux to different acceptors.
(A) The inducible ORP2/HeLa cells (identified below the panel) were labelled with [14C]cholesterol for 42 h in the absence (white bars) or presence (black bars) of 1 μg/ml doxycycline, incubated for 2 h in a medium containing 20% human serum, and the radioactivity in the efflux medium and the cells was determined. (B) Similar experiments as in (A) carried out with ORP2/HeLa line 21f3 using 0.2% BSA or 0.2% BSA with 20 μg/ml human apoA-I, in serum-free medium as acceptors (efflux time 4 h). (C) In a setting identical with that in (A), the ORP2/HeLa cell lines were labelled with [14C]cholesterol and incubated for 2 h in serum-free medium that contained no cholesterol acceptor. The radioactivity in the cells and the media was then determined by liquid-scintillation counting. The results are shown as the percentage of radioactivity found in the medium (mean±S.E.M.; n=3; ***P<0.001, *P<0.05, t test).
We also analysed by Western blotting the expression levels of two proteins known to be involved in cholesterol efflux, ABCA1 and SR-B1, in induced and non-induced ORP2/HeLa cells. Using a commercial antibody that detected a strong signal in macrophages, we did not observe ABCA1 expression in these cells. SR-B1 protein was detectable, but there was no difference in the expression level between the induced and non-induced cell specimens (results not shown).
PM lipid composition and cholesterol organization in ORP2/HeLa cells
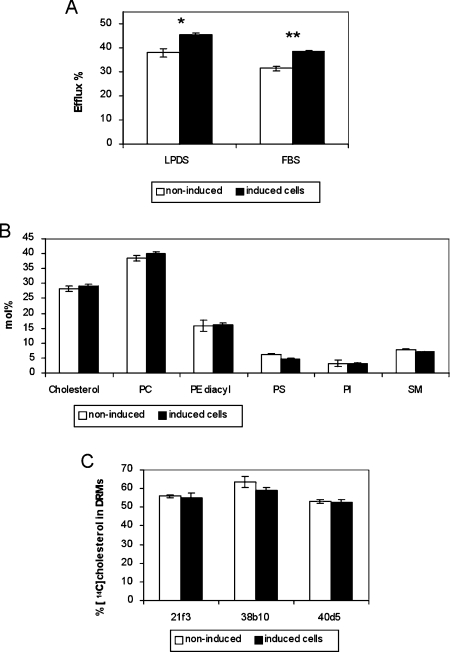
The above results suggested that the ORP2-mediated efflux enhancement may result from a change in the cellular or PM cholesterol distribution that makes it more readily available for efflux. We next probed the PM [14C]cholesterol in ORP2/HeLa 21f3 cells cultured in a medium supplemented either with 10% FBS or with 5% LPDS, under inducing or non-inducing conditions, with a brief treatment with methyl-β-cyclodextrin that does not permeabilize the PMs of the cells [18]. Under both conditions, the expression of ORP2 was associated with a significant increase in the cyclodextrin extractability of the cholesterol (Figure 3A).
Figure 3. Organization of cholesterol in the PM of ORP2/HeLa cells.
(A) The ORP2/HeLa cell line 21f3 was labelled with [14C]cholesterol for 42 h in the absence (white bars) or presence (black bars) of 1 μg/ml doxycycline, either in a medium containing 5% LPDS or one supplemented with 10% complete FBS. The cell-surface cholesterol was extracted for 5 min with 5 mM methyl-β-cyclodextrin, and radioactivity in the medium and in the cells was determined. The bars represent the portion recovered in the medium (mean±S.E.M.; n=3). (B) PMs of ORP2/HeLa cells incubated in 10% FBS containing medium in the absence of presence of induction, were enriched using the colloidal silica technique. Cholesterol was quantified by an enzymatic method and phospholipids by LC–ESI–MS, as described in the Experimental section. The results are shown as a mol% distribution, and represent the means±S.E.M. for the three cell lines. (C) The ORP2/HeLa cell lines were labelled with [14C]cholesterol for 42 h in the absence or presence of induction in a medium supplemented with 10% FBS, and distribution of the radioactivity between Triton X-100 insoluble and soluble fractions was determined as specified in the Experimental section. The bars represent the portion of [14C]cholesterol in two top fractions containing the DRM, and represent a mean±S.E.M. for three experiments. **P<0.01, *P<0.05, t test.
To determine the lipid composition of the ORP2/HeLa cell PMs in the induced and non-induced states, we enriched a PM fraction of these cells, cultured in the presence of 10% FBS, using a previously described colloidal silica method [19]. As judged from Western blotting using antibodies raised against PM (syntaxin 2) and ER (GRP-94) markers and densitometric scanning, the enrichment of PMs was 6-fold. The cholesterol content of these membranes was determined enzymatically and phospholipids were analysed by LC–ESI–MS. Cholesterol represented 27–31 mol% of the total lipids, and there was no difference between the induced and non-induced ORP2/HeLa membrane preparations (Figure 3B). Furthermore, there was no significant difference between the induced and non-induced cells in the relative amounts of the SM, PC, diacyl-PE, PS or PI classes (Figure 3B), nor in the molecular species composition within these classes (results not shown).
To investigate the possibility that the distribution of cholesterol between DRM (detergent-resistant membrane) and sensitive membrane domains might have changed in the induced ORP2/HeLa cells, we isolated Triton X-100-resistant membrane domains of the cells and analysed the proportion of the cellular [14C]cholesterol found in the DRM (Figure 3C). The analysis revealed no difference between the induced and non-induced ORP2/HeLa cells. A similar result was obtained when a milder detergent, Brij96, was used for raft isolation.
To address the question whether association of the ORP2 protein directly with the cellular PMs might provide an explanation for the increased cholesterol efflux, we Western blotted total membrane and PM fractions isolated from the inducible HeLa cells using the colloidal silica method, with ORP2 antibodies. Even though, as shown previously by Laitinen et al. [14], a minor portion of the ORP2 was membrane associated, the protein was not detected in the PM fraction (results not shown).
Intracellular transport of newly synthesized cholesterol in cells overexpressing ORP2
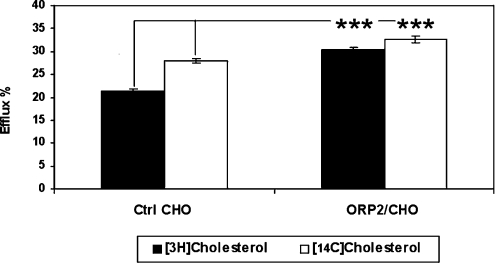
To investigate whether ORP2 affects the transport of newly synthesized cholesterol from the ER to the PM, we measured the appearance of newly synthesized [3H]cholesterol in a cell-surface pool accessible for extraction with methyl-β-cyclodextrin [18]. These experiments were carried out in the stably transfected ORP2/CHO cell line A5a9, the efflux phenotype of which is markedly more pronounced than that of the inducible HeLa cells [14]. To be able to compare the results with those obtained for prelabelled cholesterol which mainly resides in the PM, the cells were first incubated for 36 h with [14C]cholesterol. Labelling of newly synthesized cholesterol was then carried out as described in the Experimental section. Cyclodextrin was added to the medium in the last 5 min of the 2 h chase. The medium and the cells were collected, and both [3H]cholesterol and [14C]cholesterol were quantified. In control cells, 21% of the newly synthesized [3H]cholesterol was accessible to cyclodextrin after the 2 h chase, while in the ORP2 cells the proportion of newly synthesized cholesterol accessible to cyclodextrin was significantly higher, 30% (Figure 4). Also the extractability of prelabelled [14C]cholesterol was significantly higher in the ORP2 cells as compared with the controls (33% versus 28%). However, the difference was less pronounced than for [3H]cholesterol. The results indicate that the transport of newly synthesized cholesterol to the cell surface in general or to cell-surface domains preferentially accessible to cyclodextrin extraction is enhanced in the cells that overexpress ORP2.
Figure 4. Effect of ORP2 overexpression on the efflux of newly synthesized cholesterol to methyl-β-cyclodextrin.
Control (Ctrl) CHO and ORP2 expressing A5a9 (ORP2/CHO) cells were cultured in lipoprotein-deficient medium containing [14C]cholesterol as described in the Experimental section. To measure the appearance of newly synthesized [3H]cholesterol at the PM, cells were labelled with [3H]acetate for 15 min and chased for 2 h. During the last 5 min of the chase the cells were incubated with 5 mM methyl-β-cyclodextrin. The synthesized [3H]cholesterol and the pre-existing [14C]cholesterol in the cells and the media were isolated by HPTLC and HPLC and quantified by liquid-scintillation counting. The results are shown as the percentage of the total [3H] (black bars) or [14C] (white bars) cholesterol found in the efflux medium (mean±S.E.M.; n=7; ***P<0.001, t test).
Cholesterol esterification in ORP2/HeLa cells
To investigate how the homoeostatic control of cholesterol metabolism functions in the ORP2/HeLa cells apparently losing FC from the PM due to ORP2 expression, we determined the proportion of the [14C]cholesterol that was esterified after 42 h of ORP2 induction and labelling (Figure 5A). In contrast with ORP2/CHO cells that display a reduction of cholesterol esterification [14], the induced ORP2 overexpression led to enhanced cholesterol esterification in two HeLa cell lines out of the three. In the third line (38b10), the esterification was high already in the non-induced state and there was no change upon induction. The fact that the ORP2/HeLa and the ORP2/CHO cells both show enhanced cholesterol efflux but the cholesterol esterification phenotypes are different suggested that the observed increase in efflux is not a secondary phenomenon due to reduced cholesterol esterification. To clarify the issue further, we employed the ORP2/CHO A5a9 cell line that shows a marked (approx. 50%) enhancement of [14C]cholesterol efflux as compared with control CHO cells. The cells were labelled with [14C]cholesterol in the presence or absence of the ACAT inhibitor PKF 058-035. In the presence of PKF, esterification of [14C]cholesterol was abolished (99% inhibition) in both cell lines. The efflux of labelled cholesterol to human serum was measured and the results in the presence and absence of the inhibitor were compared. The ORP2/CHO cells displayed a significant increase in cholesterol efflux as compared with controls both in the absence and in the presence of PKF (Figure 5B). We therefore conclude that the increase in [14C]cholesterol efflux in ORP2 overexpressing cells is not due to decreased ACAT activity and a resulting increase in FC available for efflux, but due to other mechanisms that are involved.
Figure 5. Cholesterol esterification in cells expressing ORP2.
(A) Effect of ORP2 overexpression on cholesterol esterification. The three inducible HeLa cell lines were labelled with [14C]cholesterol for 42 h in the absence (white bars) or presence (black bars) of 1 μg/ml doxycycline. Labelled FC and CEs were separated from cell extracts using HPTLC and quantified by liquid-scintillation counting. The results are shown as a percentage of 14C-labelled CEs of total labelled cholesterol (mean±S.E.M.; n=9, ***P<0.001, *P<0.05, t test). (B) Effect of ACAT inhibition on the efflux of [14C]cholesterol from control CHO or ORP2/CHO cells. Control (white bars) and ORP2/CHO A5a9 cells (black bars) were labelled with [14C]cholesterol for 42 h in the absence (no PKF) or presence (PKF) of an ACAT inhibitor (PKF 058-035). Cells were then incubated for 2 h in a medium containing 20% human serum. The radioactivity of the cells and the media was determined by liquid-scintillation counting. The results are shown as the percentage of radioactivity found in the medium (mean±S.E.M.; n=6, ***P<0.001, t test).
HMG-CoA reductase activity in cells overexpressing ORP2
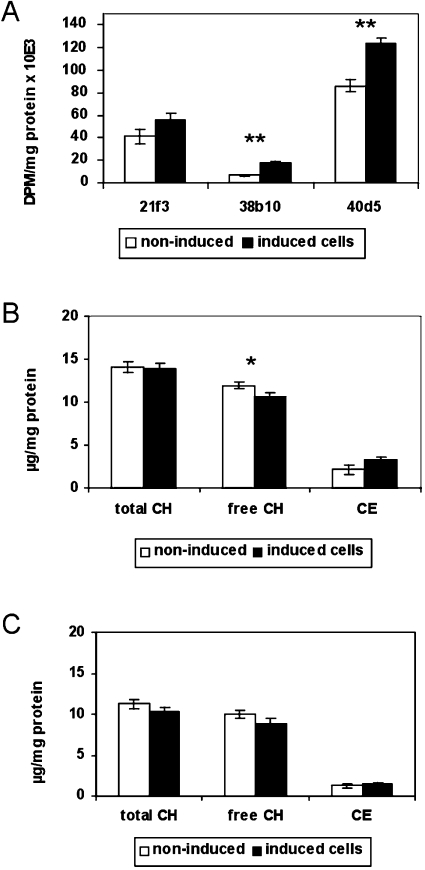
Since ACAT activity is subject to allosteric regulation by ER cholesterol (reviewed in [27]), the above results indicated that the cholesterol concentration in the ER may have changed in opposite directions in the ORP2/HeLa and ORP2/CHO cell models. We therefore determined the activity of the major ratelimiting enzyme of cholesterol biosynthesis, HMG-CoA reductase, which is also regulated by the cholesterol content of ER membranes [28]. To determine the effects of ORP2 overexpression on the regulation of cholesterol biosynthesis, the inducible ORP2/HeLa cells were cultured for 42 h in lipoprotein-deficient medium and the activity of the HMG-CoA reductase was assayed (Figure 6A). In all three inducible ORP2/HeLa cell lines, an increase in HMG-CoA reductase activity (33% for 21f3, 168% for 38b10 and 43% for 40d5) was observed upon ORP2 induction. The activity was markedly low both in the non-induced and the induced situation in the cell line 38b10, which also showed higher cholesterol esterification activity than the other two ORP2/HeLa lines, indicating that, even though this cell line behaves similarly to the other two upon doxycycline induction, it differs from them in cholesterol homoeostatic status. Like the ORP2/HeLa cell lines, both ORP2/CHO cell lines showed an increase (146% for A5a9 and 55% for A6d6) in HMG-CoA reductase activity compared with the control cells (results not shown). Thus it seems that cells overexpressing ORP2 display a homoeostatic response to an increase cholesterol biosynthesis.
Figure 6. HMG-CoA reductase activity and cholesterol content of cells expressing ORP2.
(A) Effect of ORP2 overexpression on cellular HMG-CoA reductase activity. The three ORP2/HeLa cell lines (identified below the panel) were cultured for 42 h in lipoprotein-deficient medium in the absence (white bars) or presence (black bars) of 1 μg/ml doxycycline, after which HMG-CoA reductase activity was assayed as described in the Experimental section. The results are shown as d.p.m. [14C]mevalonic acid lactone/mg of protein during a 90 min reaction (mean±S.E.M.; n=3, **P<0.01, t test). (B) Effect of ORP2 overexpression on the cellular content of total, free and esterified cholesterol. After the incubation of inducible HeLa cells in normal serum-containing medium for 42 h in the absence (white bars) or presence (black bars) of 1 μg/ml doxycycline, the amounts of total and free cholesterol (CH) were measured from cell extracts with enzymatic methods. The amount of CEs was determined by subtracting FC from TC. (C) Cholesterol amounts were similarly measured from cells cultured in 5% LPDS in the presence or absence of 1 μg/ml doxycycline. The results in (B and C) are representative of three measurements for three cell lines and are shown as μg of cholesterol/mg of protein (mean±S.E.M.; n=9, *P<0.05, t test).
The results obtained with radioisotope-labelled cholesterol suggest that the cellular cholesterol balance has changed in ORP2 expressing cells. We therefore determined the absolute cholesterol content of the inducible ORP2/HeLa cells cultured either in normal serum-containing medium or in lipoprotein-deficient medium containing 5% LPDS. TC and FC in the cells were quantified, and CEs were calculated by subtracting FC from TC. The TC content of the cells cultured in the presence of serum did not change upon induction (Figure 6B). However, the amount of FC was significantly decreased in the induced cells, the average reduction in the three cell lines being 11%. The level of CEs tended to be higher in the induced cells, in accordance with the results of the [14C]cholesterol labelling experiments. If cell line 38b10, with an aberrant cholesterol esterification activity (see above), was excluded from the analysis, the increase of cellular CE became statistically significant (average increase of 100%, P<0.001). In cells cultured in LPDS-containing medium, a slight decrease was observed in both TC (−8%) and FC (−11%) upon induction of ORP2 expression. However, there was no change in the cellular content of CE (Figure 6C). The results are consistent with the interpretation that the ORP2 expressing HeLa cells are depleted of FC, but the TC level is not affected when the cells are able to compensate for the increased cholesterol efflux by cholesterol uptake from serum lipoproteins.
Effects of ORP2 overexpression on LDL receptor expression and LDL uptake
The results suggesting a homoeostatic response to up-regulate cholesterol biosynthesis upon ORP2 induction prompted us to investigate the expression of LDL receptors and the endocytotic uptake of LDL in the ORP2/HeLa cell model. Cells were incubated in the absence or presence of doxycycline for 42 h and total protein samples were prepared for SDS/PAGE and Western blotting with LDL receptor antibodies. Up-regulation of the cellular LDL receptor that migrates at 160 kDa was detected in all three cell lines upon induction of ORP2 expression in complete serum-containing medium (Figure 7A). According to densitometric scanning of the ECL® films, the up-regulation was approx. 1.6-fold. When the cells were cultured in LPDS-containing medium, LDL receptor expression was strongly up-regulated under these conditions of lipid depletion, and no further change was observed upon induction of ORP2 expression (results not shown). We next assayed the cellular uptake of fluorescently labelled DiI-LDL in non-induced and induced ORP2/HeLa cells using a confocal microscope. The fluorescence observed in the endocytic compartments was clearly more intense in the induced cells (Figures 7B–7D), demonstrating enhancement of LDL uptake upon ORP2 overexpression. This result is in accordance with the increase in HMG-CoA reductase activity and supports the interpretation that a homoeostatic response to FC depletion occurs in the ORP2 expressing cells. Furthermore, it provides a plausible explanation for the fact that there is no significant decrease in the TC content of ORP2/HeLa induced in the presence of serum.
Figure 7. Effects of ORP2 overexpression on LDL receptor expression and the uptake of DiI-LDL.
(A) Western-blot analysis of LDL-receptor expression in the non-induced (–) and induced (+) ORP2/HeLa cell lines (identified on the top) cultured in serum-containing medium. Total protein was loaded at the rate of 15 μg/lane. Mobility of molecular-mass standards is indicated on the left. (B–D) Quantification of cellular DiI-LDL uptake. ORP2/HeLa cells (representative data for line 40d5 is shown) were incubated for 42 h in the absence (−induction) or presence (+induction) of 1 μg/ml doxycycline, followed by a 2 h incubation in serum-free medium. DiI-LDL was internalized for 15 min and the intracellular fluorescence was imaged with a confocal microscope. Quantification of intracellular fluorescence is presented in (B) (mean±S.E.M., n=30; **P<0.01, t test) and images of the non-induced and induced cells in (C) and (D) respectively.
The effects of ORP2 overexpression on the endocytosis of dextran and Tfn
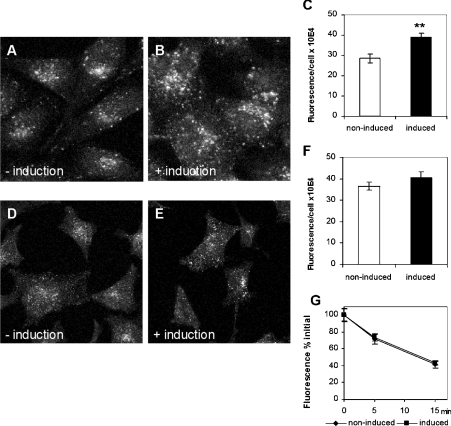
It was recently reported [16] that S. cerevisiae strains with disruptions of the yeast ORP (OSH) genes display defects in endocytosis accompanied by alterations in the subcellular distribution of ergosterol. This, together with the results demonstrating an increase in LDL uptake in induced ORP2/HeLa cells prompted us to investigate other endocytotic processes in these cells. To study fluid-phase endocytosis, non-induced and induced ORP2/HeLa cells were allowed to internalize rhodamine–dextran for 15 min. The intracellular dextran was imaged with a confocal microscope and quantified. The results revealed a significant (30–35%) increase in rhodamine–dextran uptake in the induced ORP2/HeLa as compared with non-induced cells (Figures 8A–8C). We then quantified the uptake and recycling of FITC-conjugated Tfn, a marker that is internalized via a receptor-mediated, clathrin-dependent route [29]. The uptake of the FITC–Tfn was monitored using the confocal microscope from cell specimens that had internalized the marker for 5 min. There was a marginal (10%), not statistically significant increase in the uptake of the marker in the induced cells as compared with the non-induced ones (Figures 8D–8F). To determine whether recycling of the FITC–Tfn is affected by ORP2 overexpression, we internalized the marker for 1 h at 16 °C and then chased for 5 or 15 min in the presence of excess unlabelled holo-Tfn. The proportion of the internalized fluorescence remaining in the cells after the chase was determined. The results revealed that FITC–Tfn was recycled from the non-induced and the induced cells at a similar rate (Figure 8G). The data suggest that ORP2 overexpression has, in addition to enhancement of LDL uptake, a profound effect on fluid-phase endocytosis, which may connect to the observations suggesting altered subcellular distribution of cholesterol in these cells.
Figure 8. Effects of ORP2 overexpression on endocytosis.
(A–C) Effect of ORP2 overexpression on fluid-phase endocytosis. ORP2/HeLa cells (representative data for cell line 40d5 is shown) were incubated for 42 h in the absence (−induction) or presence (+ induction) of 1 μg/ml doxycycline, followed by internalization of rhodamine–dextran for 15 min. The intracellular fluorescence was imaged (A, B) and quantified (C; mean±S.E.M., n=30; **P<0.01, t test) with a confocal microscope. (D–G) Effects of ORP2 overexpression on the uptake and recycling of FITC–Tfn. After a 42 h incubation in the absence (−induction) or presence (+induction) of doxycycline and 2 h in serum-free medium, FITC–Tfn was internalized for 5 min at 37 °C. Images of cells after 5 min internalization are shown in (D, E) and quantification of intracellular fluorescence in (F) (mean±S.E.M., n=30). (G) After 1 h internalization at 16 °C, FITC–Tfn was recycled at 37 °C into a medium containing excess unlabelled holo-Tfn for 5 or 15 min, and the remaining intracellular fluorescence quantified (mean±S.E.M., n=30).
Phosphoinositide binding by ORP2
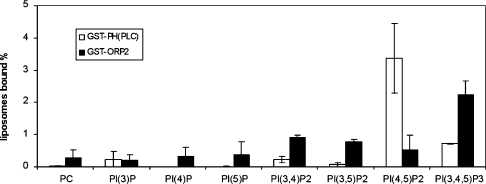
ORP2 was reported in [30] to bind PtdIns(3)P and phosphatidic acid. This finding was made using phospholipids immobilized on nitrocellulose. We therefore decided to carry out similar experiments with a liposome pull-down assay that represents a more native setting for protein–lipid interactions [26]. A GST–ORP2 fusion protein produced in E. coli was immobilized on glutathione–Sepharose and incubated with radioactive PC liposomes containing 1 mol% of the seven different phosphoinositides. A GST fusion of the PH domain of PLCδ, which binds PtdIns(4,5)P2 with high affinity and specificity (reviewed in [31]) was used as a positive control, and plain GST immobilized on Sepharose as a negative one. The radioactivity found in the beads after pull-down and washes was measured. While the PLCδ PH domain bound 3.4% of the PtdIns(4,5)P2 and a small fraction of PtdIns(3,4,5)P3, the ORP2 fusion protein was found to bind PtdIns(3,4,5)P3 with a reasonably high efficiency (2.2%), and also showed detectable but low affinity for PtdIns(3,4)P2 and PtdIns(3,5)P2 (Figure 9). No affinity for phosphatidic acid was observed (results not shown). The results suggest that ORP2 is capable of binding to phosphoinositides in membranes, which may account for its partial membrane association [14,30]. Furthermore, since phosphoinositides and protein factors that associate with these lipids play key roles in endocytic membrane trafficking (reviewed in [32,33]), the interaction of ORP2 with these lipids may account for its effect on endocytosis.
Figure 9. Binding of ORP2 to phosphoinositides.
Liposome pull-down assay was carried out as described in [26]. GST–ORP2 fusion protein (black bars) bound to glutathione–Sepharose beads was incubated with vesicles consisting of unlabelled PC, [14C]PC and 1 mol% of the indicated phosphoinositide. ‘PC’ indicates binding to liposomes that consist of PC only. GST was used as a negative control (values subtracted from the results) and PH domain of PLCδ fused to GST (white bars) as a positive control. The radioactivity associated with the beads was measured by liquid-scintillation counting. The lipid binding is expressed as the percentage of total radioactivity recovered (mean±S.E.M., n=2).
DISCUSSION
In the present study, we show that the acute increase in cellular ORP2 concentration in inducible HeLa cell lines leads to enhancement of cholesterol efflux. The increase in efflux is, as also in the case of constitutively expressing CHO cell lines [14], detectable using serum or non-apolipoprotein containing acceptors, strongly suggesting that ORP2 confers increased capacity for cholesterol removal on any acceptor. The increased efflux is not due to an increase in the PM cholesterol content, and furthermore, we found no evidence for a change in the distribution of cholesterol between raft and non-raft membrane domains upon ORP2 expression. Although a minor portion of ORP2 is membrane-associated, no significant association with isolated PMs was detected, ruling out the possibility that insertion of the protein directly into the PM would alter the accessibility of cholesterol for efflux. However, a transient peripheral interaction with the PM cannot be excluded based on this data. Analysis of the transport of newly synthesized [3H]acetate-labelled cholesterol to a cyclodextrin-accessible PM pool suggested that ORP2 could enhance the intracellular trafficking of cholesterol, which would provide a possible explanation for the efflux increment caused by the protein.
It remained unclear during our previous study whether the ORP2-induced efflux enhancement is a primary effect of ORP2 or a secondary one due to the observed inhibition of ACAT activity and resulting increase of FC available for efflux. The fact that the ORP2/HeLa and the ORP2/CHO cells both show enhanced cholesterol efflux, but the cholesterol esterification phenotypes are different suggested that the observed increase of efflux is not a secondary phenomenon due to reduced cholesterol esterification. This interpretation is supported by the present data demonstrating that the ORP2 enhancement of cholesterol efflux remains even after abolishment of ACAT activity in control and ORP2/CHO cells with a pharmacological inhibitor.
The increased cholesterol efflux from both the inducible HeLa cells and the CHO cell lines that overexpress human ORP2 constitutively was accompanied by a homoeostatic response to up-regulate the cellular HMG-CoA reductase activity. The observed elevation of LDL receptor expression and DiI-LDL uptake in the induced ORP2/HeLa cells are consistent with a homoeostatic response to cholesterol depletion or enhanced transport of cholesterol out of the ER. The ORP2/HeLa cells also showed a tendency to increase cholesterol esterification. The latter result differed from those obtained for the ORP2/CHO cells, which were previously shown to display down-regulation of ACAT activity and cholesterol esterification [14]. Both ACAT and HMG-CoA reductase are subject to allosteric regulation by ER cholesterol levels [27,28]. Therefore the data on ORP2/CHO would be consistent with a model in which loss of cholesterol from the PM leads to a decrease of ER cholesterol concentration and subsequent homoeostatic responses in cholesterol biosynthesis and esterification. However, the results obtained with ORP2/HeLa cells are not easily interpreted by the above simple paradigm of cholesterol homoeostasis. How is it possible that the HeLa cells show a tendency to increase cholesterol esterification concomitantly with enhanced efflux from the cell surface and depletion of FC? It was shown that cholesterol efflux via the diffusion-mediated mechanism is not directly linked with the regulation of ACAT activity, whereas the ACAT-accessible cholesterol pool is intimately connected with the pool effluxed via the apolipoprotein-dependent mechanism [34]. On the other hand, Du et al. [35] recently reported that cholesterol esterification by ACAT can be dissociated from cholesterol transport to the homoeostatic machinery in the ER. It is therefore possible that, even though the efflux increment caused by the acute overexpression of ORP2 during a 42 h induction of the HeLa cell lines leads to a homoeostatic response detected as increased HMG-CoA reductase activity and LDL receptor expression, it does not involve the ACAT substrate cholesterol pool. The constitutive cholesterol depletion that occurs in the ORP2/CHO cell lines is, however, likely to affect both pools of cholesterol. It also induces other adaptive processes in cellular lipid metabolism, such as changes in the fatty acid composition of phospholipids [36], an effect not detected in the inducible HeLa cell model.
The results obtained from our study on the effects of ORP2 on cellular cholesterol metabolism raise the question whether the protein might directly bind cholesterol or act as a cholesterol carrier protein. The high degree of sequence homology (47% identical amino acid residues [17]) with the sterol-binding domain of OSBP suggests that ORP2 might also bind sterols. Despite our efforts, we were not able to observe direct binding of cholesterol or oxysterols to ORP2. Furthermore, ORP2 was not observed to act as a cholesterol transfer protein in an assay that measures the transfer of radiolabelled cholesterol between vesicle populations in vitro (R. Hynynen, K. Tanhuanpää and V. M. Olkkonen, unpublished work). The mechanisms of inter-organelle cholesterol transport are poorly understood, and several alternative or parallel models have been suggested, one of which involves direct membrane contacts [4,6]. ORP proteins have recently been implicated in the formation or regulation of contact sites between ER and other cellular membrane compartments [37]. Thus it can be envisaged that ORP2 may modulate the function of such contacts, providing one plausible explanation for its effects on the movement of cholesterol within cells.
The observed increase in endocytosis of a fluid-phase marker upon ORP2 expression is an interesting finding that may connect to alterations in cholesterol metabolism. This observation provides a parallel to the recent findings [16] of endocytosis defects in S. cerevisiae strains with disruptions of the yeast ORP (OSH) genes, accompanied by alterations in the subcellular distribution of ergosterol. Abnormality of membrane dynamics in the endocytotic routes may cause a change in the cycling of cholesterol between the early and recycling endosomal compartments and the PM. For example, manipulation of Rab GTPase function on endocytic compartments has been reported to modify cholesterol trafficking from endo/lysosomal compartments [38,39]. On the other hand, alterations in the cholesterol content of membranes are known to cause changes in membrane trafficking. There is evidence that cholesterol manipulation affects both clathrin-dependent and -independent endocytic processes [40,41]. The observed marked increase of DiI-LDL uptake by the induced, ORP2-expressing HeLa cells can be partially attributed to up-regulation of LDL receptor expression. However, when the ORP2/HeLa cells were cultured at high passage numbers, they no longer displayed up-regulation of LDL receptors, but the uptake of DiI-LDL was still significantly elevated upon ORP2 induction, indicating a component independent of the receptor expression level. A slight enhancement was also detected in the uptake of FITC–Tfn by the ORP2 expressing cells. Thus ORP2 affects both fluid-phase and receptor-mediated endocytosis.
ORP2 was found to bind PtdIns(3,4,5)P3 in an in vitro assay, and also showed weak affinity for PtdIns(3,4)P2 and PtdIns(3,5)P2. Most ORP proteins have a PH domain, and in the case of OSBP, this is known to interact with several phosphoinositides [42,43]. ORP2, however, belongs to the category of short OSBP-related proteins that lack a PH domain and consist of an OSBP-related ligand-binding domain only. Two other short ORPs, S. cerevisiae Kes1p/Osh4p [44] and human ORP1S [45], a close homologue of ORP2, were shown to bind phosphoinositides. Our results are consistent with these findings, suggesting that the C-terminal ligand-binding domains of the ORPs contain phosphoinositide-binding motifs. However, we find it unlikely that these motifs would correspond to the sterol-binding site of OSBP. PtdIns(3,4,5)P3 is a species that arises transiently at the PM as a result of phosphoinositide 3-kinase activity, stimulated by a number of cell-surface receptors (reviewed in [33,46]). In addition to central functions in signalling cascades, phosphoinositides with a phosphate in the 3-position play important roles in phagosome formation and endocytosis (reviewed in [32]). PtdIns(3,4,5)P3 interacts directly with the clathrin adaptor complexes AP-2 [47] and AP-3 [48]. Furthermore, PtdIns(3,4,5)P3 binds to several GDP/GTP exchange factors (Grp1, cytohesin 1 and ARNO) and a GTPase-activating protein (centaurin-α1) of the small Arf GTPases that play central roles in membrane trafficking in both the secretory and the endocytic routes (reviewed in [46]). Interaction of ORP2 with PtdIns(3,4,5)P3 can be envisioned to impact on endocytic processes, e.g. through modulation of the binding of other protein machineries to these lipid regulators. Another interesting possibility worth future investigation is that ORP2 might affect the synthesis or turnover of membrane phosphoinositides.
Acknowledgments
T. Grundström, S. Puomilahti, P. Ranta and B. Rantala are acknowledged for skilled technical assistance. Dr T. Balla (National Institutes of Health, Bethesda, MD, U.S.A.) is acknowledged for kindly providing a PLCδ PH domain expression construct. Dr M. Suomalainen (Haartman Institute, University of Helsinki) is thanked for her kind help with the colloidal silica method. The ACAT inhibitor PKF 058-035 was a gift from Novartis. This work was supported by grants from the Academy of Finland (grants 50641, 54301 and 206298 to V.M.O.; 49987 to E.I.; 48905 to S.L.), the Sigrid Jusélius Foundation (to V.M.O., E.I. and P.S.), the Finnish Cultural Foundation (to S.L. and V.M.O.), and the International HDL Research Awards Programme (to C.E.).
References
- 1.Brown M. S., Goldstein J. L. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams C. M., Reitz J., De Brabander J. K., Feramisco J. D., Li L., Brown M. S., Goldstein J. L. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- 3.Tontonoz P., Mangelsdorf D. J. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- 4.Maxfield F. R., Wüstner D. Intracellular cholesterol transport. J. Clin. Invest. 2002;110:891–898. doi: 10.1172/JCI16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons K., Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 6.Soccio R. E., Breslow J. L. Intracellular cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 2004;24:1150–1160. doi: 10.1161/01.ATV.0000131264.66417.d5. [DOI] [PubMed] [Google Scholar]
- 7.Yancey P. G., Bortnick A. E., Kellner-Weibel G., de la Llera-Moya M., Phillips M. C., Rothblat G. H. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 8.Wang N., Lan D., Chen W., Matsuura F., Tall A. R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor F. R., Saucier S. E., Shown E. P., Parish E. J., Kandutsch A. A. Correlation between oxysterol binding to a cytosolic binding protein and potency in the repression of hydroxymethylglutaryl coenzyme A reductase. J. Biol. Chem. 1984;259:12382–12387. [PubMed] [Google Scholar]
- 10.Lagace T. A., Byers D. M., Cook H. W., Ridgway N. D. Altered regulation of cholesterol and cholesteryl ester synthesis in Chinese-hamster ovary cells overexpressing the oxysterol-binding protein is dependent on the pleckstrin homology domain. Biochem. J. 1997;326:205–213. doi: 10.1042/bj3260205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagace T. A., Byers D. M., Cook H. W., Ridgway N. D. Chinese hamster ovary cells overexpressing the oxysterol binding protein (OSBP) display enhanced synthesis of sphingomyelin in response to 25-hydroxycholesterol. J. Lipid Res. 1999;40:109–116. [PubMed] [Google Scholar]
- 12.Wyles J. P., McMaster C. R., Ridgway N. D. Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J. Biol. Chem. 2002;277:29908–29918. doi: 10.1074/jbc.M201191200. [DOI] [PubMed] [Google Scholar]
- 13.Lehto M., Olkkonen V. M. The OSBP-related proteins: a novel protein family involved in vesicle transport, cellular lipid metabolism, and cell signalling. Biochim. Biophys. Acta. 2003;1631:1–11. doi: 10.1016/s1388-1981(02)00364-5. [DOI] [PubMed] [Google Scholar]
- 14.Laitinen S., Lehto M., Lehtonen S., Hyvärinen K., Heino S., Lehtonen E., Ehnholm C., Ikonen E., Olkkonen V. M. ORP2, a homolog of oxysterol binding protein, regulates cellular cholesterol metabolism. J. Lipid Res. 2002;43:245–255. [PubMed] [Google Scholar]
- 15.Beh C. T., Cool L., Phillips J., Rine J. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics. 2001;157:1117–1140. doi: 10.1093/genetics/157.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beh C. T., Rine J. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J. Cell Sci. 2004;117:2983–2996. doi: 10.1242/jcs.01157. [DOI] [PubMed] [Google Scholar]
- 17.Lehto M., Laitinen S., Chinetti G., Johansson M., Ehnholm C., Staels B., Ikonen E., Olkkonen V. M. The OSBP-related protein family in humans. J. Lipid Res. 2001;42:1203–1213. [PubMed] [Google Scholar]
- 18.Heino S., Lusa S., Somerharju P., Ehnholm C., Olkkonen V. M., Ikonen E. Dissecting the role of the golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8375–8380. doi: 10.1073/pnas.140218797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaney L. K., Jacobson B. S. Coating cells with colloidal silica for high yield isolation of plasma membrane sheets and identification of transmembrane proteins. J. Biol. Chem. 1983;258:10062–10072. [PubMed] [Google Scholar]
- 20.Folch J. M., Lees M., Sloane-Stanley G. H. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Koivusalo M., Haimi P., Heikinheimo L., Kostiainen R., Somerharju P. Quantitative determination of phospholipid compositions by ESI-MS: effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J. Lipid Res. 2001;42:663–672. [PubMed] [Google Scholar]
- 22.Käkelä R., Somerharju P., Tyynelä J. Analysis of phospholipid molecular species in brains from patients with infantile and juvenile neuronal-ceroid lipofuscinosis using liquid chromatography-electrospray ionization mass spectrometry. J. Neurochem. 2003;84:1051–1065. doi: 10.1046/j.1471-4159.2003.01602.x. [DOI] [PubMed] [Google Scholar]
- 23.Wilce P. A., Kroon P. A. Assay of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. In: Converse C. A., Skinner E. R., editors. Lipoprotein Analysis, A Practical Approach. Oxford: Oxford University Press; 1992. pp. 203–214. [Google Scholar]
- 24.Bligh E. G., Dryer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 25.Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J. Lipid Res. 1978;19:1068–1070. [PubMed] [Google Scholar]
- 26.Schiavo G., Gu Q. M., Prestwich G. D., Söllner T. H., Rothman J. E. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang T. Y., Chang C. C., Cheng D. Acyl-coenzyme A:cholesterol acyltransferase. Annu. Rev. Biochem. 1997;66:613–638. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 28.Sever N., Yang T., Brown M. S., Goldstein J. L., DeBose-Boyd R. A. Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol. Cell. 2003;11:25–33. doi: 10.1016/s1097-2765(02)00822-5. [DOI] [PubMed] [Google Scholar]
- 29.Maxfield F. R., McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y., Liu Y., Ridgway N. D., McMaster C. R. Novel members of the human oxysterol-binding protein family bind phospholipids and regulate vesicle transport. J. Biol. Chem. 2001;276:18407–18414. doi: 10.1074/jbc.M101204200. [DOI] [PubMed] [Google Scholar]
- 31.Lemmon M. A. Phosphoinositide recognition domains. Traffic. 2003;4:201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 32.Owen D. J. Linking endocytic cargo to clathrin: structural and functional insights into coated vesicle formation. Biochem. Soc. Trans. 2004;32:1–14. doi: 10.1042/bst0320001. [DOI] [PubMed] [Google Scholar]
- 33.Wenk M. R., De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q., Tsujita M., Yokoyama S. Selective down-regulation by protein kinase C inhibitors of apolipoprotein-mediated cellular cholesterol efflux in macrophages. Biochemistry (Moscow) 1997;36:12045–12052. doi: 10.1021/bi970079t. [DOI] [PubMed] [Google Scholar]
- 35.Du X., Pham Y. H., Brown A. J. Effects of 25-hydroxycholesterol on cholesterol esterification and sterol regulatory element-binding protein processing are dissociable: implications for cholesterol movement to the regulatory pool in the endoplasmic reticulum. J. Biol. Chem. 2004;279:47010–47016. doi: 10.1074/jbc.M408690200. [DOI] [PubMed] [Google Scholar]
- 36.Käkelä R., Tanhuanpää K., Laitinen S., Somerharju P., Olkkonen V. M. Over-expression of OSBP-related protein 2 (ORP2) in CHO cells induces alterations of phospholipid species composition. Biochem. Cell Biol. 2005 doi: 10.1139/o05-056. in the press. [DOI] [PubMed] [Google Scholar]
- 37.Olkkonen V. M., Levine T. P. Oxysterol binding proteins: in more than one place at one time? Biochem. Cell Biol. 2004;82:87–98. doi: 10.1139/o03-088. [DOI] [PubMed] [Google Scholar]
- 38.Höltta-Vuori M., Tanhuanpää K., Möbius W., Somerharju P., Ikonen E. Modulation of cellular cholesterol transport and homeostasis by Rab11. Mol. Biol. Cell. 2002;13:3107–3122. doi: 10.1091/mbc.E02-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choudhury A., Dominguez M., Puri V., Sharma D. K., Narita K., Wheatley C. L., Marks D. L., Pagano R. E. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 2002;109:1541–1550. doi: 10.1172/JCI15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naslavsky N., Weigert R., Donaldson J. G. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol. Biol. Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichler H., Riezman H. Where sterols are required for endocytosis. Biochim. Biophys. Acta. 2004;1666:51–61. doi: 10.1016/j.bbamem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Levine T. P., Munro S. The pleckstrin homology domain of oxysterol-binding protein recognises a determinant specific to Golgi membranes. Curr. Biol. 1998;8:729–739. doi: 10.1016/s0960-9822(98)70296-9. [DOI] [PubMed] [Google Scholar]
- 43.Levine T. P., Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- 44.Li X., Rivas M. P., Fang M., Marchena J., Mehrotra B., Chaudhary A., Feng L., Prestwich G. D., Bankaitis V. A. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J. Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fairn G. D., McMaster C. R. Identification and assessment the role of a nominal phospholipid binding region of oxysterol binding protein related protein ORP1S in the regulation of vesicular transport. Biochem. J. 2004;387:889–896. doi: 10.1042/BJ20041915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payrastre B., Missy K., Giuriato S., Bodin S., Plantavid M., Gratacap M. Phosphoinositides: key players in cell signalling, in time and space. Cell. Signalling. 2001;13:377–387. doi: 10.1016/s0898-6568(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 47.Gaidarov I., Chen Q., Falck J. R., Reddy K. K., Keen J. H. A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 alpha subunit. Implications for the endocytic pathway. J. Biol. Chem. 1996;271:20922–20929. doi: 10.1074/jbc.271.34.20922. [DOI] [PubMed] [Google Scholar]
- 48.Hao W., Tan Z., Prasad K., Reddy K. K., Chen J., Prestwich G. D., Falck J. R., Shears S. B., Lafer E. M. Regulation of AP-3 function by inositides. Identification of phosphatidylinositol 3,4,5-trisphosphate as a potent ligand. J. Biol. Chem. 1997;272:6393–6398. doi: 10.1074/jbc.272.10.6393. [DOI] [PubMed] [Google Scholar]