Abstract
The hepatocyte growth factor (HGF) is a multifunctional cytokine that is produced as latent scHGF (single chain HGF). Various proteases reportedly cleave scHGF to generate the active two-chain form (HGF), including u-PA (urokinase-type plasminogen activator), t-PA (tissue-type plasminogen activator), kallikrein, Factor XIa, Factor XIIa, HGF activator and matriptase. Considerable evidence indicates that, in vivo, u-PA activates scHGF in the liver; however, the in vivo results have not been uniformly supported by in vitro experiments. We now report that cleavage of scHGF by high-molecular-mass u-PA (abbreviated u-PA throughout) is sensitive to ionic strength. scHGF cleavage by u-PA was accelerated as the ionic strength was decreased. This result was equivalent irrespective of whether the predominant anion was chloride or acetate. Lmw-u-PA (low-molecular-mass u-PA) was ineffective at cleaving scHGF, regardless of ionic strength. Although scHGF shares homology with plasminogen, EACA (ϵ-amino-caproic acid) did not regulate u-PA-mediated scHGF cleavage. Soluble HGF receptor (MET) and soluble u-PAR (u-PA receptor) inhibited the scHGF cleavage. These results support a model in which the ability of u-PA to activate scHGF in vivo may be highly dependent on local conditions within the extracellular space.
Keywords: ϵ-amino-caproic acid (EACA), hepatocyte growth factor (HGF), HGF receptor (MET), ionic strength, urokinase-type plasminogen activator (u-PA), u-PA receptor (u-PAR)
Abbreviations: EACA, ϵ-amino-caproic acid; EpoR, erythropoietin receptor; HGF, hepatocyte growth factor; scHGF, single chain HGF; NaOAc, sodium acetate; u-PA, urokinase-type plasminogen activator; lmw-u-PA, low-molecular-mass u-PA; u-PAR, u-PA receptor; Su-PAR, soluble u-PA receptor
INTRODUCTION
The hepatocyte growth factor (HGF; also known as scatter factor [1]) is a pleiotrophic cytokine that was initially isolated based upon its ability to stimulate hepatic regeneration [2]. In addition to stimulating hepatocellular proliferation, HGF exerts a variety of other effects, regulating motility, tubule formation and cytotoxicity in multiple cell types such as endothelium, macrophages and fibroblasts [3,4]. Although generally considered paracrine in action, HGF autocrine loops have also been demonstrated in selected normal cell types and in cancer [5,6].
HGF is produced as a latent, scHGF (single chain HGF) protein of approx. 100 kDa. Because the protein exerts diverse activities in a wide range of cell types, an important biological consideration is the mechanism by which it becomes activated. Various proteases cleave latent scHGF to its active two-chain form (HGF), including the u-PA (urokinase-type plasminogen activator), t-PA (tissue-type plasminogen activator), HGF activator, Factor XIIa, kallikrein, Factor XIa and matriptase [7–11]. There is also one protease known to inactivate an HGF splice variant consisting of the N-terminus and kringle 1 (NK1). When NK1, a mitogen for hepatocytes, is cleaved by Factor Xa it loses activity [12].
Structurally, scHGF exhibits substantial similarity to plasminogen [13]. Thus the ability of the plasminogen activators, u-PA and t-PA, to activate scHGF was anticipated. Indeed, many groups have demonstrated that u-PA-mediated cleavage of scHGF is a biologically relevant mechanism in vivo [14–17]. Nevertheless, reports are mixed regarding the ability of u-PA to generate HGF in vitro [7,16,18].
The various studies that examined scHGF activation by u-PA in vitro utilized significantly different assay conditions. We hypothesized that these differences may explain the variability in results. To better understand u-PA-mediated cleavage of scHGF, we considered it important to reconcile the in vitro and in vivo results. Thus experiments were undertaken to determine whether scHGF activation by u-PA is regulated by solution conditions.
In the present study, we report that scHGF activation by two-chain high-molecular-mass u-PA (referred to throughout as u-PA) is strictly regulated by ionic strength. Although scHGF is genetically similar to plasminogen, the effects of ionic strength on scHGF cleavage are not specific for chloride anions, as previously demonstrated for plasminogen [19]. Our studies also demonstrate that soluble MET (HGF receptor) and Su-PAR (soluble u-PA receptor) inhibit scHGF cleavage, a potentially important result given by recent evidence demonstrating that naturally occurring forms of Su-PAR are present in the plasma and extracellular spaces [20,21].
EXPERIMENTAL
Materials
Recombinant forms of soluble murine MET (527-ME), human u-PAR (u-PA receptor; 807-UK/CF) and human EpoR (erythropoietin receptor) (963-ER-050) were purchased from R & D Systems (Minneapolis, MN, U.S.A.). MET and u-PAR were ≥95% pure as indicated by the manufacturer, whereas the purity of EpoR was listed as ≥90%. scHGF was a gift from D. Kirchhofer at Genentech (South San Francisco, CA, U.S.A.). The preparation we refer to as scHGF actually included 60% scHGF with the remainder in two-chain form. The two chain u-PA was provided by Dr J. Henkin and Dr A. Mazar of Abbott Laboratories (Abbott Park, IL, U.S.A.). Lmw-u-PA (low-molecular-mass u-PA) was obtained from Molecular Innovations (Southfield, MI, U.S.A.) and American Diagnostica (Greenwich, CT, U.S.A.). The concentration of active lmw-u-PA and u-PA was determined from the velocity of hydrolysis (v) of H-L-glutamyl-glycyl-L-arginyl-p-nitroanilide (Bachem, Torrance, CA, U.S.A.) using the equation [Ea]=v([S]+Km)/[S]kcat., where Ea is the concentration of active enzyme and [S] is the concentration of the chromogenic substrate. The published kcat and Km values are 20.76±1.8 s−1 and 200±40 μM for u-PA and 16.45±0.78 s−1 and 270±30 μM for lmw-u-PA [22]. Based on these values, we calculated our preparations to be approx. 55 and 33% active respectively. Concentrations of active enzyme are reported in the present study. Na125I, which was used to radiolabel scHGF, was purchased from Amersham Biosciences (Piscataway, NJ, U.S.A.). Iodo-BEADS were purchased from Pierce Biotechnology (Rockford, IL, U.S.A.). All other reagents were purchased from Sigma (St. Louis, MO, U.S.A.).
Radioiodination of scHGF
scHGF was radioiodinated as previously described [12]. An iodo-BEAD was prepared according to the manufacturer's instructions and used to label approx. 5 μg of protein. The reaction solution contained 400 μl of PBS and 1 mCi of Na125I. Radioiodination was allowed to continue for 10 min, after which time, the bead was removed and the reaction was quenched with 10 μl of saturated N-acetyl-tyrosine. Radiolabelled scHGF was resolved from free radioiodine by chromatography on Sephadex G-25. Collection tubes were pretreated with Sigmacoat and then with 1% (w/v) BSA for 4 h at 37 °C. Specific radioactivities were 1–1.5 μCi/μg protein. 125I-scHGF was stored at 4 °C and used in experiments within 10 days.
Cleavage of scHGF by u-PA and lmw-u-PA
125I-scHGF (2.5 nM) was equilibrated at 4 °C in solutions that contained 0.02% (w/v) protease-free BSA in 20 mM Tris/acetate (pH 7.4) and increasing concentrations of NaCl, NaOAc (sodium acetate) or KCl. Su-PAR, soluble MET, EpoR, EACA (ϵ-amino-caproic acid), amiloride or aprotinin were added as indicated. u-PA or lmw-u-PA (2–40 nM) was added and incubation was allowed to proceed for various times at 37 °C. The reactions were terminated by adding SDS-containing sample buffer that included 100 mM dithiothreitol, and heating to 65 °C for 15 min. SDS/PAGE was performed using 10 or 12% gels. Radioactivity in specific bands was quantified using PhosphorImager analysis (Storm 860; Molecular Dynamics, Palo Alto, CA, U.S.A.).
RESULTS
Ionic strength regulates u-PA-mediated scHGF cleavage
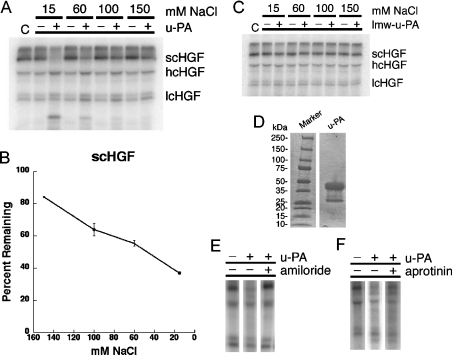
There are substantial results supporting the hypothesis that u-PA-mediated scHGF cleavage is biologically significant in vivo [14–17]; however, in vitro studies have yielded variable results [7,16,18]. To explore this discrepancy, we studied the cleavage of 2.5 nM 125I-scHGF by 15 nM u-PA in vitro under variable solution conditions. Figure 1 shows that our preparation of u-PA was primarily high molecular mass. The 125I-scHGF preparation consisted of slightly more than 60% scHGF, with the remainder already converted into hcHGF (heavy-chain HGF) and lcHGF (light-chain HGF) bands. In the presence of 150 mM NaCl, very little scHGF was cleaved by u-PA; however, as the NaCl concentration was decreased, scHGF cleavage significantly increased. In the presence of 15 mM NaCl, over 60% of the initial scHGF was cleaved; however, the HGF was not quantitatively recovered in the light-chain and heavy-chain bands, most probably due to secondary cleavage events as previously reported [7]. Cleavage was solely the result of u-PA activity as amiloride inhibited the reaction, but aprotinin did not. Under non-reducing conditions, HGF migrated as a single band, irrespective of NaCl concentration (results not shown), indicating that both activating cleavage and secondary cleavage events occur at sites that are bridged by disulphide bonds and thus peptides are not lost.
Figure 1. The effect of NaCl concentration on u-PA efficiency.
Radiolabelled scHGF was incubated with either no enzyme, u-PA (15 nM, A, B, E, F) or lmw-u-PA (10 nM, C) for 2 h at 37 °C in Tris/acetate buffers (pH 7.4) containing 15–150 mM NaCl. (A–C) Reaction products were run on SDS/PAGE gels, dried on filter paper, exposed for radioactivity and then quantified using PhosphorImager analyses (Storm 860, Molecular Dynamics). (A) A representative gel using u-PA. C represents the original scHGF preparation that has not undergone incubation. hcHGF and lcHGF signify the heavy and light chains of HGF respectively. (B) Remaining scHGF as a percentage of the starting scHGF in the preparation, combining data from an average of 3–7 experiments performed on different days. Error bars indicate calculated S.E.M. Analysis indicates the data are linear (R=0.99) with a slope of 0.34. (C) A representative gel using lmw-u-PA. (D–F) Verification for the purity of the u-PA preparation. Coomassie Blue-staining of 15 μg of protein shows that the majority of the protein is of high molecular mass (D). Radiolabelling experiments in the presence of specific protease inhibitors indicate that cleavage is completely inhibited by 1 μM of the u-PA inhibitor amiloride (E) whereas 1 nM of the potent plasmin inhibitor aprotinin allows activity to proceed (F).
The effects of ionic strength on scHGF activation by u-PA were also examined in experiments in which the u-PA concentration (1–20 nM) or the time of incubation (up to 8 h) was varied. In every study, the finding that reaction rate varies inversely with ionic strength was confirmed (results not shown). Activation of scHGF by lmw-u-PA was also examined. This form of the enzyme was active, based upon its ability to cleave chromogenic substrate; however, 10 nM lmw-u-PA failed to cleave scHGF at all ionic strengths examined (Figure 1).
The effects of ionic strength are not specific to either sodium or chloride
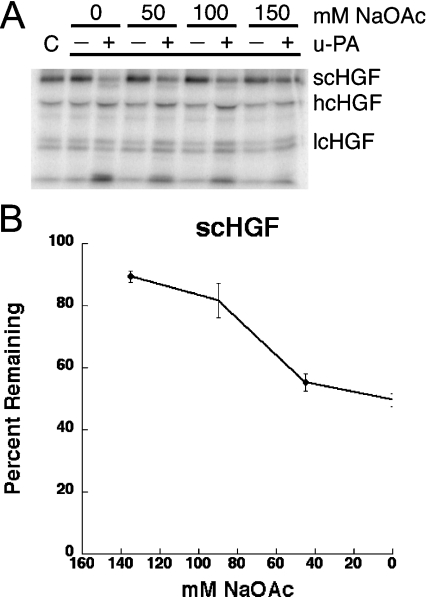
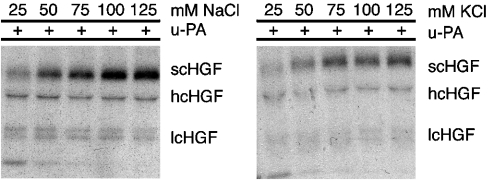
Chloride anion specifically modifies the conformation of Glu-plasminogen and as a result, Glu-plasminogen activation is inhibited [19,23]. This activity is not mimicked by other anions. To determine whether a chloride anion specifically inhibits scHGF cleavage, the reaction was assessed in the presence of increasing concentrations of NaOAc (Figure 2). A slight difference in scHGF cleavage was observed in solutions that contained 150 mM NaOAc, compared with 150 mM NaCl; however, once again, cleavage was substantially increased by decreasing the NaOAc concentration. Thus the effects of salt concentration on scHGF cleavage are not specific to chloride anions. In separate experiments, the effects of cation concentration on scHGF cleavage were examined. Figure 3 demonstrates that equivalent results were obtained using either KCl or NaCl, indicating that the sodium ion is also not specifically responsible for the observed effect of ionic strength.
Figure 2. The effect of NaOAc is similar to NaCl.
Radiolabelled scHGF (in 150 mM NaCl) with either no enzyme or 15 nM u-PA was added to Tris/acetate buffer (pH 7.4) containing 0–150 mM NaOAc and incubated at 37 °C for 2 h. Reaction products were run on SDS/PAGE gels, dried on filter paper and then viewed using PhosphorImager analyses (Storm 860, Molecular Dynamics). (A) A representative gel. C represents scHGF that has not undergone incubation. (B) Remaining scHGF as a percentage of the starting scHGF in the preparation. A combination of data from an average of 5–7 experiments performed on different days is shown. Error bars indicate calculated S.E.M. Analysis indicates that the data is linear (R=0.97) with a slope of 0.32. When the total ionic strength is considered and plotted (including the NaCl from the iodinated protein), we obtained R=0.96 with a calculated slope of 0.32 (results not shown).
Figure 3. The effect of KCl is similar to NaCl.
Radiolabelled scHGF with 15 nM u-PA was added to Tris/acetate buffer (pH 7.4) containing 25–125 mM NaCl or KCl and incubated at 37 °C for 5 h. Reaction products were run on SDS/PAGE gels, dried on filter paper and then exposed to X-ray film (Kodak XOMAT).
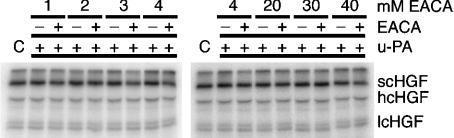
The inhibitory activity of chloride, in u-PA-mediated plasminogen activation, is counteracted by the lysine analogue, EACA, which binds directly to kringle domains in the structure of plasminogen [19,23]. Although scHGF has not been reported to bind lysine, molecular modelling suggests the potential for a lysine binding in kringle [13]. Figure 4 shows that EACA did not affect 125I-scHGF cleavage by u-PA in the presence of 150 mM NaCl.
Figure 4. EACA fails to overcome NaCl inhibition.
Radiolabelled scHGF and u-PA were incubated at 37 °C for 2 h in Tris/acetate buffer (pH 7.4) containing 150 mM NaCl and the indicated concentration of EACA. Reaction products were run on SDS/PAGE gels, dried on filter paper and then viewed using PhosphorImager analyses (Storm 860, Molecular Dynamics). C represents scHGF that has not undergone incubation.
The role of the MET (HGF receptor) and u-PAR in scHGF cleavage
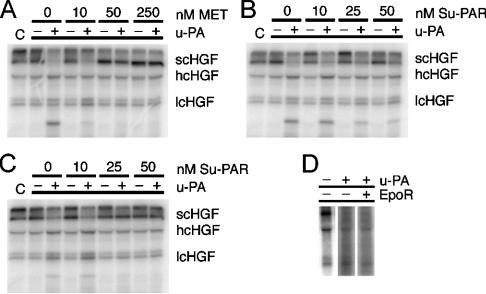
The HGF receptor, MET, efficiently binds both scHGF and HGF; however, only HGF elicits a biological response [24]. Although u-PA may also bind to MET [16,25], the effects of this interaction upon scHGF cleavage are unknown. We studied scHGF cleavage by u-PA in the presence of soluble MET (10–250 nM). As shown in Figure 5, under ionic strength conditions that optimize u-PA activity against scHGF (15 mM NaCl), 10 nM MET allowed scHGF cleavage to proceed while apparently inhibiting secondary cleavage events. Higher concentrations of MET (50–250 nM) substantially inhibited scHGF activation.
Figure 5. u-PA activity in the presence of MET or Su-PAR.
Radiolabelled scHGF, either with or without u-PA, was incubated at 37 °C for 2 h in Tris/acetate buffers (pH 7.4) containing 15 (A, B) or 100 (C) mM NaCl plus the indicated concentration of soluble MET or Su-PAR. Reaction products were run on SDS/PAGE gels, dried on filter paper and then viewed using PhosphorImager analyses (Storm 860, Molecular Dynamics). C represents scHGF that has not undergone incubation. The inability of an unrelated protein to prevent cleavage (50 nM EpoR, D) indicates that the protein interactions are specific, as opposed to a minor contaminant.
Previous in vivo studies suggest that u-PAR also may be involved in the u-PA-mediated activation of scHGF [14,15,26]. We studied scHGF cleavage by u-PA in the presence of 10, 25 or 50 nM Su-PAR. Assuming equilibrium binding conditions and a KD of 1.0 nM for u-PA-binding to Su-PAR [27], these concentrations of Su-PAR would be expected to yield 31, 74 and 96% of the u-PA in complex. The remainder of the u-PA would be free in solution. As shown in Figure 5, rather than enhancing scHGF cleavage, Su-PAR inhibited cleavage under low- and high-ionic strength conditions.
DISCUSSION
HGF is a multifaceted protein that can induce a variety of responses including mitosis, migration, tubule formation and cytotoxicity. Although latent HGF can bind to its receptor with high affinity, signalling only occurs when the protein has been activated by cleavage [24]. Thus scHGF activation is likely to be under tight regulation. Although multiple proteases cleave scHGF, compelling evidence exists that u-PA is the primary protease responsible for producing mature HGF in vivo, in the liver [14,15,17]. Despite the fact that u-PA is a well-studied protein, the conditions regulating scHGF activation by u-PA are not clearly defined. Thus in vitro studies were undertaken to identify conditions that optimize the u-PA-mediated activation of scHGF.
Successful in vitro generation of biologically active HGF has previously been demonstrated [7,16]. In both cases, the buffers used were relatively low in NaCl. Because increasing concentrations of chloride hinder the u-PA-mediated cleavage of plasminogen [23] and, because scHGF is structurally similar to plasminogen [13], experiments were designed to test the hypothesis that the chloride ions interfere with the generation of HGF. No specific chloride effect was observed; however, a marked effect of ionic strength on scHGF cleavage was demonstrated. Both NaOAc and KCl proved to be equally effective substitutes for NaCl in inhibiting scHGF cleavage (Figures 2 and 3). These results indicate that decreased ionic strength, rather than loss of a specific cation or anion, is responsible for optimizing scHGF cleavage by u-PA. Furthermore, we were able to demonstrate that the effects of ionic strength on scHGF activation by u-PA lead to real differences in biological activity. Generated HGF was used to stimulate serum-free, cultured, hepatocytes [15]. When equivalent quantities of scHGF were treated with u-PA in low-versus high-ionic strength buffers, a significant increase in thymidine incorporation was observed using the scHGF incubated with low NaCl (results not shown). These results provide a viable explanation for why u-PA functions as an in vivo activator of scHGF in the earliest stages of liver regeneration. In hepatic tissue, membrane depolarization occurs in conjunction with cellular swelling and is a very early consequence of the regenerative stimulus, probably in response to insulin [28–30]. The overall ionic strength of the pericellular spaces is probably lowered, subsequent to ions being actively pumped into the cells [30]. Hence, rapid ionic flux in response to injury may create a matrix milieu that favours scHGF activation by u-PA.
By affecting the conformation of plasminogen, EACA is able to override the chloride sensitivity of plasminogen during u-PA-mediated cleavage [19,23]. However, despite great structural similarity between scHGF and plasminogen, EACA was ineffective in overcoming the effects of ionic strength on the u-PA-mediated cleavage of scHGF. EACA binds to the lysine-binding pocket regions found within kringles 1, 2, 4 and 5 of plasminogen [31,32]. Although modelling studies indicate that there is a lysine-binding pocket in HGF kringle 2, comparisons between recognized amino acids from the plasminogen EACA binding regions and those from the homologous regions of scHGF indicate that EACA may not bind to the HGF kringles [13,33]. Our results indicate that EACA has no effect on scHGF cleavage.
The present studies indicate that MET regulates scHGF cleavage by u-PA; however, the effects are mixed. Whereas, high concentrations of MET inhibited scHGF cleavage, low MET concentrations only prevented secondary cleavage events. Because these secondary cleavage events may compromise HGF activity, the overall effect of MET on scHGF activation may be situation-specific. As scHGF that is bound to its receptor is unable to signal, u-PA may be a direct modulator of MET signalling via the proteolytic activation of receptor-bound scHGF. Notably, MET and u-PA can be co-immunoprecipitated from liver that is induced to regenerate in response to PHx (partial hepatectomy). Furthermore, cross-linking analyses performed using carcinoma cell lines also verify their association [16]. Hence, it is reasonable to hypothesize that u-PA can modulate MET signalling by regulating cleavage of bound scHGF.
Our studies with soluble MET should be interpreted with caution. Sequence similarity between human, rat and mouse MET is high [34]. Furthermore, the binding constants for human HGF to both human and rat cells are similar [35,36]. Nevertheless, there are no results formally demonstrating binding of human scHGF/HGF to mouse MET and, it is known that murine HGF is less adequate than human HGF in stimulating human MET [37]. As transgenic mice made using murine HGF are more likely to develop spontaneous tumours than mice made with human HGF [38–40], species-specificity may have affected the concentration of soluble MET required to form complexes with HGF.
Under selective conditions, u-PAR may be co-immunoprecipitated with MET from hepatic membranes [25]. Although this interaction may be transient, its timing in the process of liver regeneration coincides with an increase in HGF, and is followed by an elevation in phosphorylated MET (at 5 min) [14,25]. This finding has led to the hypothesis that u-PAR is a positive effecter of HGF generation. In the present study, we have shown that in at least some instances, Su-PAR may in fact decrease the ability of u-PA to cleave scHGF. As Su-PAR has been identified in the plasma, it may be a physiologically significant potential modifier of scHGF activation.
In summary, our studies have demonstrated that the ability of u-PA to cleave scHGF may depend upon changing conditions within the reaction milieu. Low-ionic strength promotes cleavage and scHGF activation. Differences in reaction conditions may explain why varying results have been reported previously, regarding the ability of u-PA to activate scHGF.
Acknowledgments
This work was supported by National Institutes of Health grants CA-94900, CA35373-20 and CA103958-01.
References
- 1.Gherardi E., Stoker M. Hepatocytes and scatter factor. Nature (London) 1990;346:228. doi: 10.1038/346228b0. [DOI] [PubMed] [Google Scholar]
- 2.Zarnegar R., Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989;49:3314–3320. [PubMed] [Google Scholar]
- 3.Higashio K., Shima N., Goto M., Itagaki Y., Nagao M., Yasuda H., Morinaga T. Identity of a tumor cytotoxic factor from human fibroblasts and hepatocyte growth factor. Biochem. Biophys. Res. Commun. 1990;170:397–404. doi: 10.1016/0006-291x(90)91287-3. [DOI] [PubMed] [Google Scholar]
- 4.Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G. F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 5.Galimi F., Cottone E., Vigna E., Arena N., Boccaccio C., Giordano S., Naldini L., Comoglio P. M. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J. Immunol. 2001;166:1241–1247. doi: 10.4049/jimmunol.166.2.1241. [DOI] [PubMed] [Google Scholar]
- 6.Cortner J., Vande Woude G. F., Rong S. The Met-HGF/SF autocrine signaling mechanism is involved in sarcomagenesis. EXS. 1995;74:89–121. doi: 10.1007/978-3-0348-9070-0_6. [DOI] [PubMed] [Google Scholar]
- 7.Mars W. M., Zarnegar R., Michalopoulos G. K. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am. J. Pathol. 1993;143:949–958. [PMC free article] [PubMed] [Google Scholar]
- 8.Naldini L., Tamagnone L., Vigna E., Sachs M., Hartmann G., Birchmeier W., Daikuhara Y., Tsubouchi H., Blasi F., Comoglio P. M. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992;11:4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimomura T., Miyazawa K., Komiyama Y., Hiraoka H., Naka D., Morimoto Y., Kitamura N. Activation of hepatocyte growth factor by two homologous proteases, blood-coagulation factor XIIa and hepatocyte growth factor activator. Eur. J. Biochem. 1995;229:257–261. doi: 10.1111/j.1432-1033.1995.tb20463.x. [DOI] [PubMed] [Google Scholar]
- 10.Peek M., Moran P., Mendoza N., Wickramasinghe D., Kirchhofer D. Unusual proteolytic activation of pro-hepatocyte growth factor by plasma kallikrein and coagulation factor XIa. J. Biol. Chem. 2002;277:47804–47809. doi: 10.1074/jbc.M209778200. [DOI] [PubMed] [Google Scholar]
- 11.Lee S. L., Dickson R. B., Lin C. Y. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J. Biol. Chem. 2000;275:36720–36725. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- 12.Pediaditakis P., Monga S. P., Mars W. M., Michalopoulos G. K. Differential mitogenic effects of single chain hepatocyte growth factor (HGF)/scatter factor and HGF/NK1 following cleavage by factor Xa. J. Biol. Chem. 2002;277:14109–14115. doi: 10.1074/jbc.M112196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donate L. E., Gherardi E., Srinivasan N., Sowdhamini R., Aparicio S., Blundell T. L. Molecular evolution and domain structure of plasminogen-related growth factors (HGF/SF and HGF1/MSP) Protein Sci. 1994;3:2378–2394. doi: 10.1002/pro.5560031222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mars W. M., Liu M. L., Kitson R. P., Goldfarb R. H., Gabauer M. K., Michalopoulos G. K. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21:1695–1701. [PubMed] [Google Scholar]
- 15.Mars W. M., Kim T. H., Stolz D. B., Liu M. L., Michalopoulos G. K. Presence of urokinase in serum-free primary rat hepatocyte cultures and its role in activating hepatocyte growth factor. Cancer Res. 1996;56:2837–2843. [PubMed] [Google Scholar]
- 16.Naldini L., Vigna E., Bardelli A., Follenzi A., Galimi F., Comoglio P. M. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J. Biol. Chem. 1995;270:603–611. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu M., Hara A., Okuno M., Matsuno H., Okada K., Ueshima S., Matsuo O., Niwa M., Akita K., Yamada Y., et al. Mechanism of retarded liver regeneration in plasminogen activator-deficient mice: impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. Hepatology. 2001;33:569–576. doi: 10.1053/jhep.2001.22650. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno K., Takehara T., Nakamura T. Proteolytic activation of a single-chain precursor of hepatocyte growth factor by extracellular serine-protease. Biochem. Biophys. Res. Commun. 1992;189:1631–1638. doi: 10.1016/0006-291x(92)90264-l. [DOI] [PubMed] [Google Scholar]
- 19.Urano T., Chibber B. A., Castellino F. J. The reciprocal effects of ϵ-aminohexanoic acid and chloride ion on the activation of human [Glu1]plasminogen by human urokinase. Proc. Natl. Acad. Sci. U.S.A. 1987;84:4031–4034. doi: 10.1073/pnas.84.12.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavakis T., Kanse S. M., Yutzy B., Lijnen H. R., Preissner K. T. Vitronectin concentrates proteolytic activity on the cell surface and extracellular matrix by trapping soluble urokinase receptor-urokinase complexes. Blood. 1998;91:2305–2312. [PubMed] [Google Scholar]
- 21.de Bock C. E., Wang Y. Clinical significance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med. Res. Rev. 2004;24:13–39. doi: 10.1002/med.10054. [DOI] [PubMed] [Google Scholar]
- 22.Wohl R. C., Summaria L., Robbins K. C. Kinetics of activation of human plasminogen by different activator species at pH 7.4 and 37 °C. J. Biol. Chem. 1980;255:2005–2013. [PubMed] [Google Scholar]
- 23.Urano T., Sator de Serrano V., Chibber B. A., Castellino F. J. The control of the urokinase-catalyzed activation of human glutamic acid 1-plasminogen by positive and negative effectors. J. Biol. Chem. 1987;262:15959–15964. [PubMed] [Google Scholar]
- 24.Lokker N. A., Mark M. R., Luis E. A., Bennett G. L., Robbins K. A., Baker J. B., Godowski P. J. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 1992;11:2503–2510. doi: 10.1002/j.1460-2075.1992.tb05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolz D. B., Mars W. M., Petersen B. E., Kim T. H., Michalopoulos G. K. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999;59:3954–3960. [PubMed] [Google Scholar]
- 26.Powell E. M., Mars W. M., Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 27.Blasi F., Carmeliet P. uPAR: a versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 28.Graf J., Haussinger D. Ion transport in hepatocytes: mechanisms and correlations to cell volume, hormone actions and metabolism. J. Hepatol. 1996;24(suppl. 1):53–77. [PubMed] [Google Scholar]
- 29.Minuk G. Y., Kren B. T., Xu R., Zhang X., Burczynski F., Mulrooney N. P., Fan G., Gong Y., Steer C. J. The effect of changes in hepatocyte membrane potential on immediate-early proto-oncogene expression following partial hepatectomy in rats. Hepatology. 1997;25:1123–1127. doi: 10.1002/hep.510250513. [DOI] [PubMed] [Google Scholar]
- 30.Vom Dahl S., Hallbrucker C., Lang F., Gerok W., Haussinger D. Regulation of liver cell volume and proteolysis by glucagon and insulin. Biochem. J. 1991;278:771–777. doi: 10.1042/bj2780771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Z., Chen Y. H., Wang P., Zhang J., Gurewich V., Zhang P., Liu J. N. The blockage of the high-affinity lysine binding sites of plasminogen by EACA significantly inhibits prourokinase-induced plasminogen activation. Biochim. Biophys. Acta. 2002;1596:182–192. doi: 10.1016/s0167-4838(02)00233-9. [DOI] [PubMed] [Google Scholar]
- 32.Zajicek J., Chang Y., Castellino F. J. The effects of ligand binding on the backbone dynamics of the kringle 1 domain of human plasminogen. J. Mol. Biol. 2000;301:333–347. doi: 10.1006/jmbi.2000.3972. [DOI] [PubMed] [Google Scholar]
- 33.Hoover G. J., Menhart N., Martin A., Warder S., Castellino F. J. Amino acids of the recombinant kringle 1 domain of human plasminogen that stabilize its interaction with ω-amino acids. Biochemistry. 1993;32:10936–10943. doi: 10.1021/bi00092a002. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Tolbert E. M., Sun A. M., Dworkin L. D. Primary structure of rat HGF receptor and induced expression in glomerular mesangial cells. Am. J. Physiol. 1996;271:F679–F688. doi: 10.1152/ajprenal.1996.271.3.F679. [DOI] [PubMed] [Google Scholar]
- 35.Lokker N. A., Godowski P. J. Generation and characterization of a competitive antagonist of human hepatocyte growth factor, HGF/NK1. J. Biol. Chem. 1993;268:17145–17150. [PubMed] [Google Scholar]
- 36.Arakaki N., Hirono S., Ishii T., Kimoto M., Kawakami S., Nakayama H., Tsubouchi H., Hishida T., Daikuhara Y. Identification and partial characterization of two classes of receptors for human hepatocyte growth factor on adult rat hepatocytes in primary culture. J. Biol. Chem. 1992;267:7101–7107. [PubMed] [Google Scholar]
- 37.Rong S., Bodescot M., Blair D., Dunn J., Nakamura T., Mizuno K., Park M., Chan A., Aaronson S., Vande Woude G. F. Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor. Mol. Cell. Biol. 1992;12:5152–5158. doi: 10.1128/mcb.12.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiota G., Wang T. C., Nakamura T., Schmidt E. V. Hepatocyte growth factor in transgenic mice: effects on hepatocyte growth, liver regeneration and gene expression. Hepatology. 1994;19:962–972. [PubMed] [Google Scholar]
- 39.Sakata H., Takayama H., Sharp R., Rubin J. S., Merlino G., LaRochelle W. J. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 1996;7:1513–1523. [PubMed] [Google Scholar]
- 40.Bell A., Chen Q., DeFrances M. C., Michalopoulos G. K., Zarnegar R. The five amino acid-deleted isoform of hepatocyte growth factor promotes carcinogenesis in transgenic mice. Oncogene. 1999;18:887–995. doi: 10.1038/sj.onc.1202379. [DOI] [PubMed] [Google Scholar]