Abstract
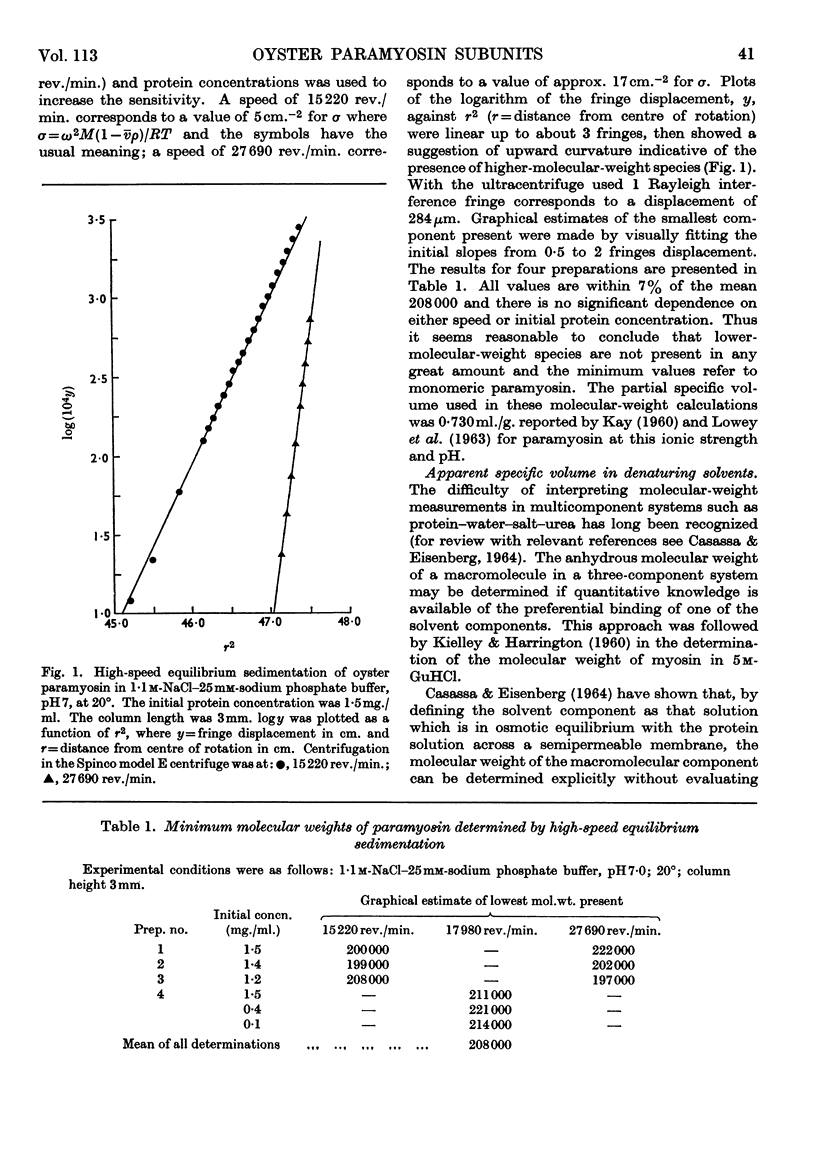
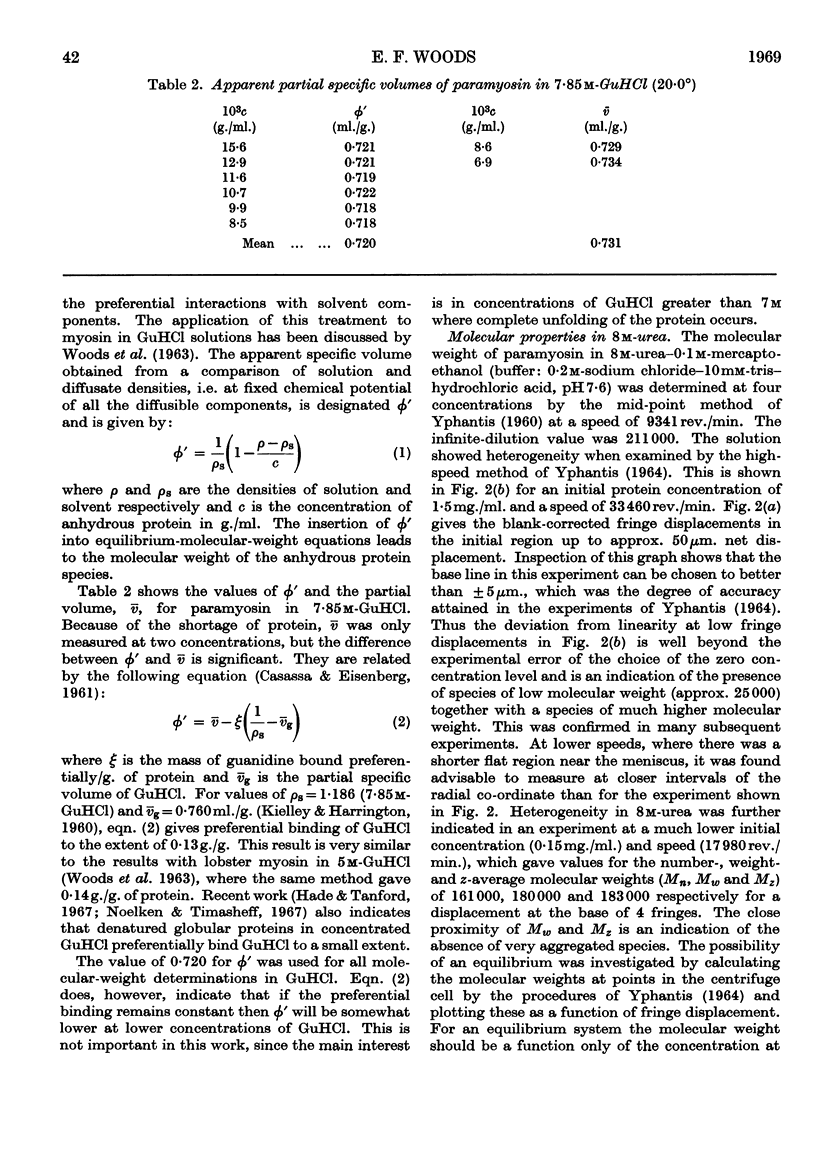
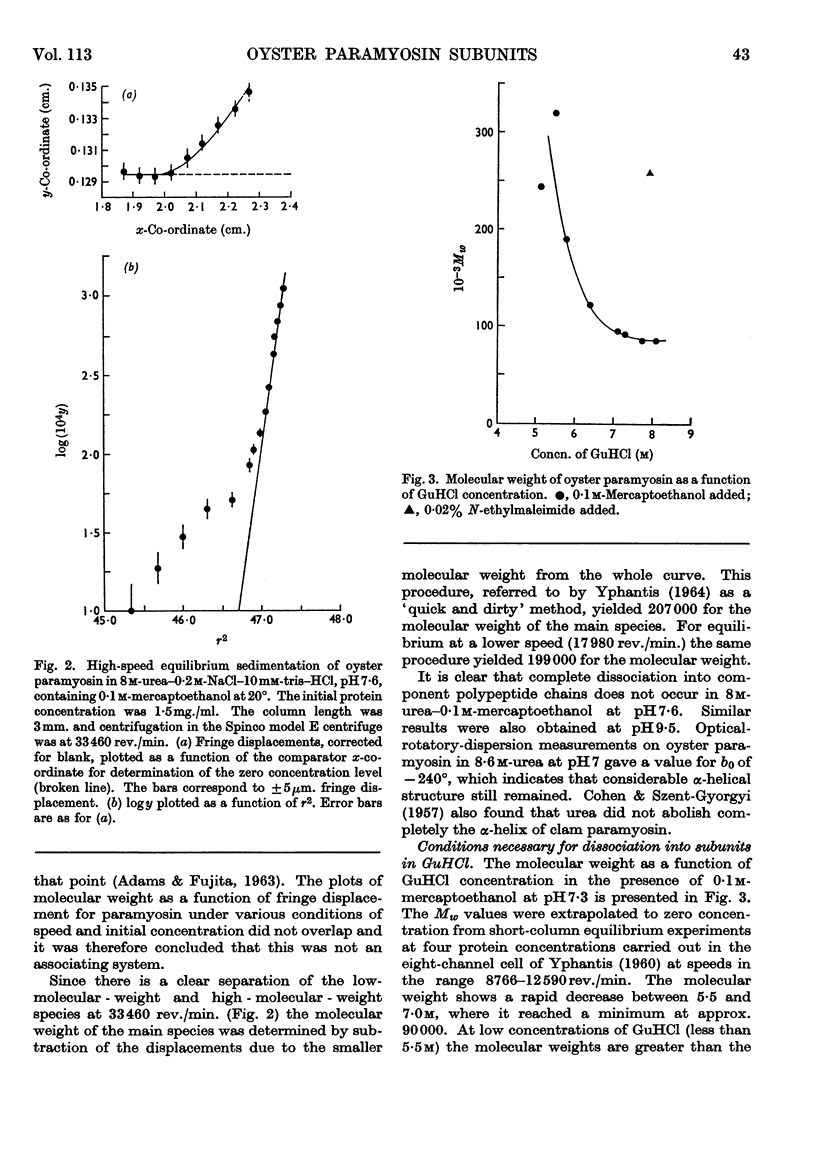
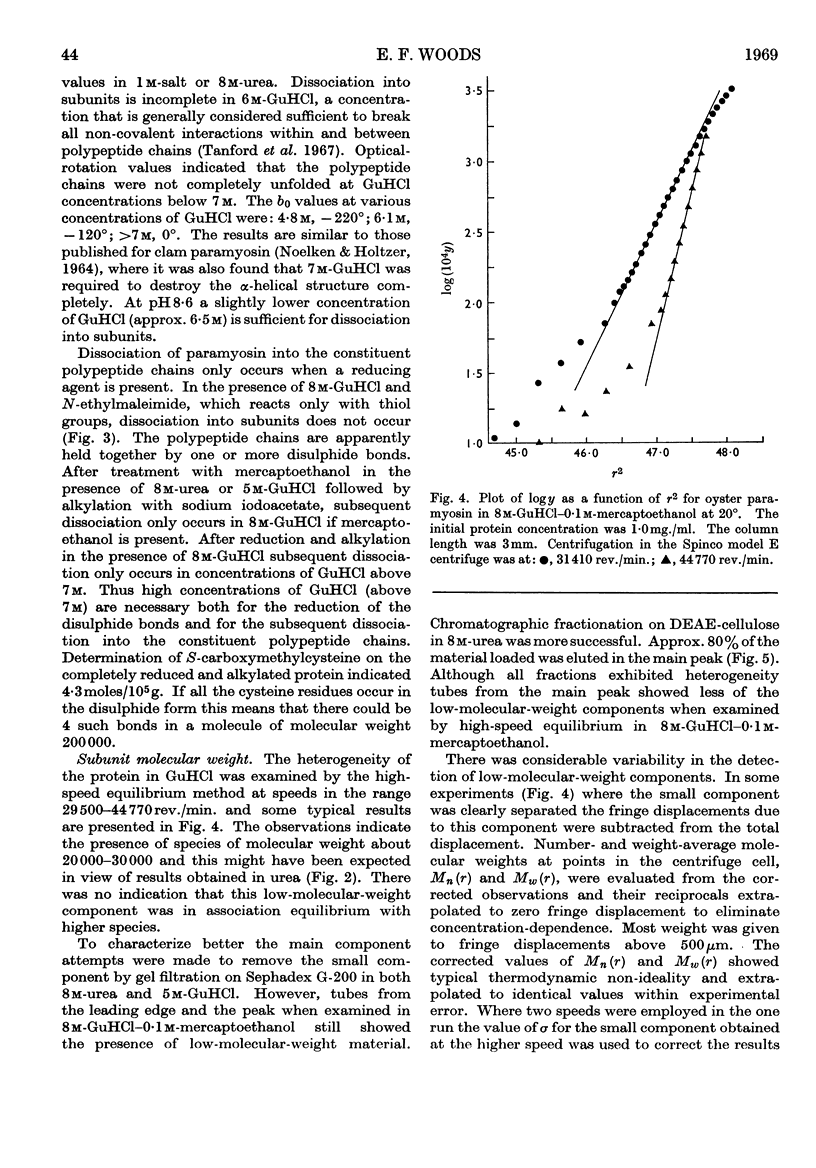
Paramyosin from the oyster Crassostrea commercialis was studied by equilibrium sedimentation. In non-denaturing solvents the minimum molecular weight is 208000. Dissociation into subunits requires complete disruption of the α-helix. This occurs at pH7 in guanidine hydrochloride solutions of concentration greater than 7m in the presence of a disulphide-bond-reducing agent. Solutions of the protein in concentrated guanidine hydrochloride are polydisperse and contain species of low molecular weight (approx. 25000) comprising approx. 5% to 10% of the protein. The molecular weight of the main component is estimated to be 97000 and the paramyosin molecule contains two of these subunits. From the present observations no decision can be made as to whether or not the small component (or components) represents part of the paramyosin molecule. Preferential binding of guanidine hydrochloride to the extent of 0·13g./g. of protein was shown in solutions of paramyosin in 7·85m-guanidine hydrochloride.
Full text
PDF

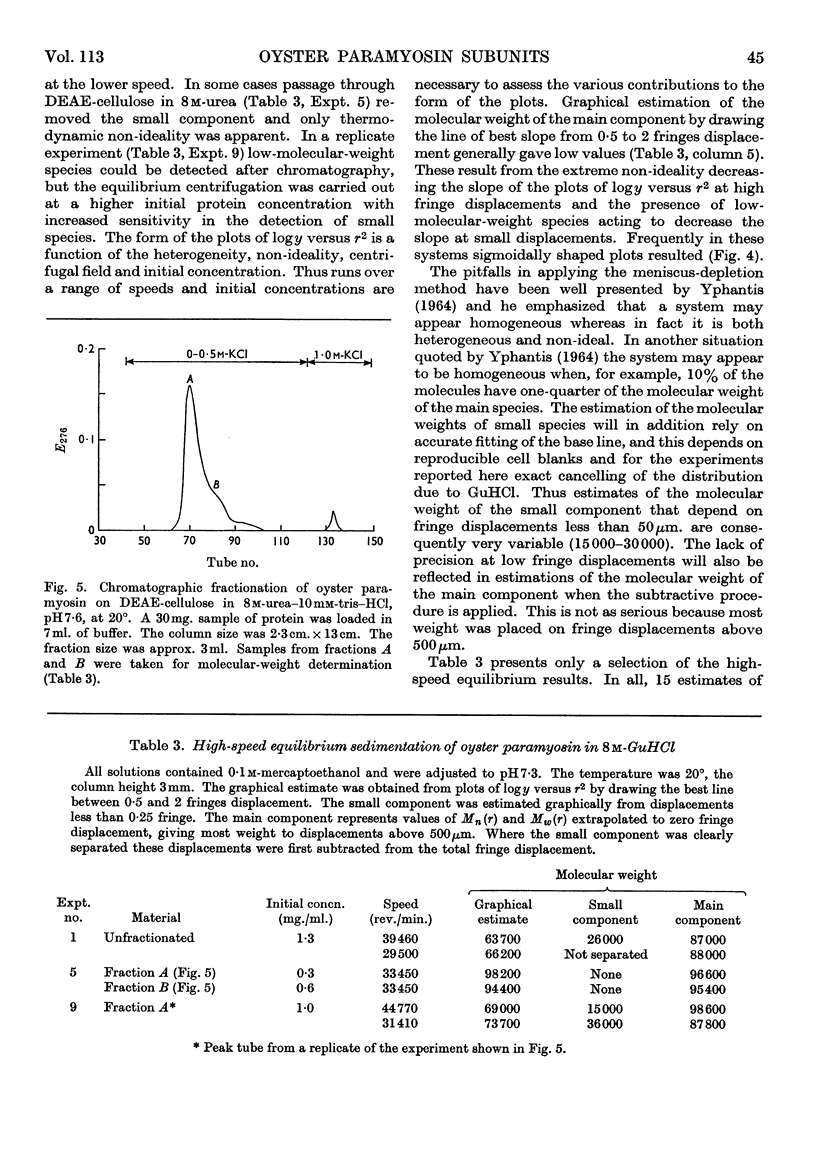
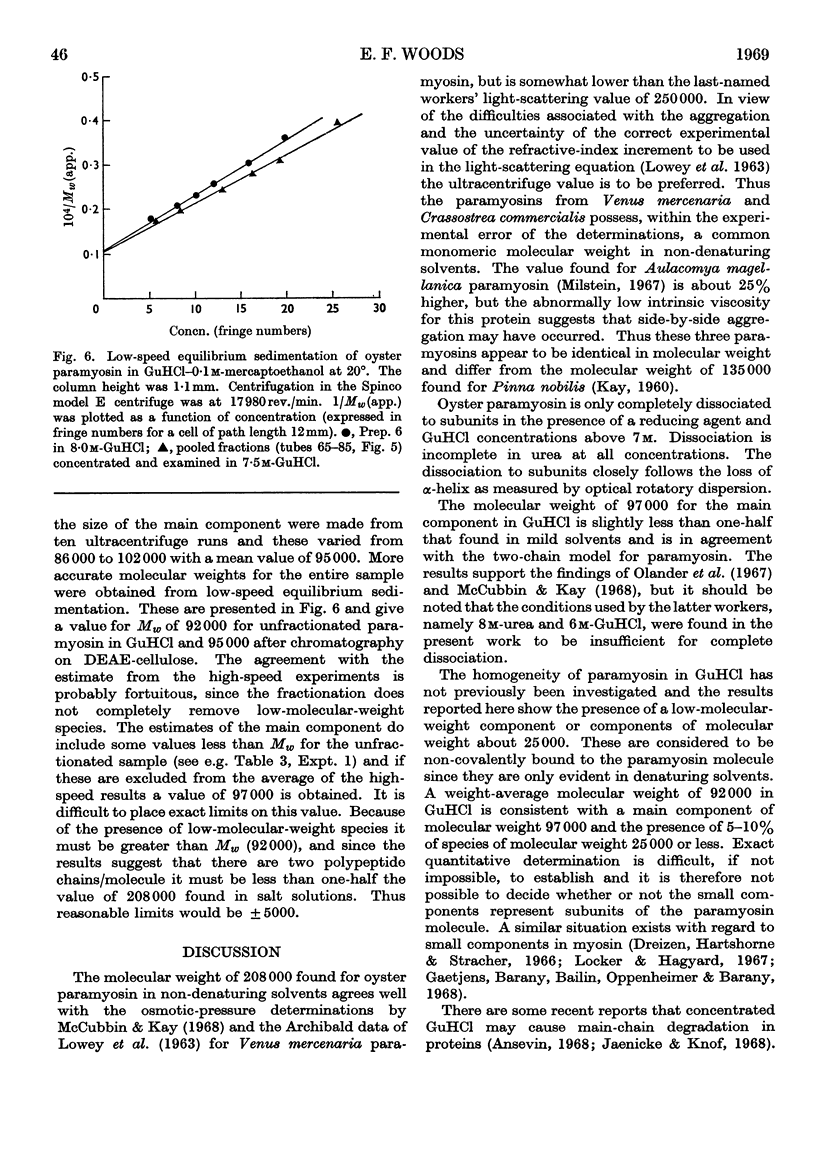

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY K. Invertebrate tropomyosin. Biochim Biophys Acta. 1957 Jun;24(3):612–619. doi: 10.1016/0006-3002(57)90255-x. [DOI] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASASSA E. F., EISENBERG H. THERMODYNAMIC ANALYSIS OF MULTICOMPONENT SOLUTIONS. Adv Protein Chem. 1964;19:287–395. doi: 10.1016/s0065-3233(08)60191-6. [DOI] [PubMed] [Google Scholar]
- Dreizen P., Hartshorne D. J., Stracher A. The subunit structure of myosin. I. Polydispersity in 5 M guanidine. J Biol Chem. 1966 Jan 25;241(2):443–448. [PubMed] [Google Scholar]
- Hade E. P., Tanford C. Isopiestic compositions as a measure of preferential interactions of macromolecules in two-component solvents. Application to proteins in concentrated aqueous cesium chloride and guanidine hydrochloride. J Am Chem Soc. 1967 Sep 13;89(19):5034–5040. doi: 10.1021/ja00995a036. [DOI] [PubMed] [Google Scholar]
- Harrap B. S., Woods E. F. Soluble derivatives of feather keratin. 2. Molecular weight and conformation. Biochem J. 1964 Jul;92(1):19–26. doi: 10.1042/bj0920019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R., Knof S. Molecular weight and quaternary structure of lactic dehydrogenase. 3. Comparative determination by sedimentation analysis, light scattering and osmosis. Eur J Biochem. 1968 Apr 3;4(2):157–163. doi: 10.1111/j.1432-1033.1968.tb00187.x. [DOI] [PubMed] [Google Scholar]
- KAY C. M. The partial specific volume of muscle proteins. Biochim Biophys Acta. 1960 Mar 11;38:420–427. doi: 10.1016/0006-3002(60)91277-4. [DOI] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- LOWEY S., KUCERA J., HOLTZER A. ON THE STRUCTURE OF THE PARAMYOSIN MOLECULE. J Mol Biol. 1963 Sep;7:234–244. doi: 10.1016/s0022-2836(63)80003-0. [DOI] [PubMed] [Google Scholar]
- Locker R. H., Hagyard C. J. Small subunits in myosin. Arch Biochem Biophys. 1967 May;120(2):454–461. doi: 10.1016/0003-9861(67)90264-0. [DOI] [PubMed] [Google Scholar]
- McCubbin W. D., Kay C. M. The subunit structure of fibrous muscle proteins as determined by osmometry. Biochim Biophys Acta. 1968 Jan 22;154(1):239–241. doi: 10.1016/0005-2795(68)90281-x. [DOI] [PubMed] [Google Scholar]
- Milstein C. P. Isolation of Aulacomya paramyosin. Biochem J. 1967 Jun;103(3):634–640. doi: 10.1042/bj1030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelken M. E., Timasheff S. N. Preferential solvation of bovine serum albumin in aqueous guanidine hydrochloride. J Biol Chem. 1967 Nov 10;242(21):5080–5085. [PubMed] [Google Scholar]
- Olander J., Emerson M. F., Holtzer A. On the dissociation and reassociation of the polypeptide chains of tropomyosin and paramyosin. J Am Chem Soc. 1967 Jun 7;89(12):3058–3059. doi: 10.1021/ja00988a051. [DOI] [PubMed] [Google Scholar]
- Tanford C., Kawahara K., Lapanje S., Hooker T. M., Jr, Zarlengo M. H., Salahuddin A., Aune K. C., Takagi T. Proteins as random coils. 3. Optical rotatory dispersion in 6 M guanidine hydrochloride. J Am Chem Soc. 1967 Sep 13;89(19):5023–5029. doi: 10.1021/ja00995a034. [DOI] [PubMed] [Google Scholar]
- URNES P., DOTY P. Optical rotation and the conformation of polypeptides and proteins. Adv Protein Chem. 1961;16:401–544. doi: 10.1016/s0065-3233(08)60033-9. [DOI] [PubMed] [Google Scholar]
- WOODS E. F., HIMMELFARB S., HARRINGTON W. F. Studies on the structure of myosin in solution. J Biol Chem. 1963 Jul;238:2374–2385. [PubMed] [Google Scholar]
- Woods E. F. Molecular weight and subunit structure of tropomyosin B. J Biol Chem. 1967 Jun 25;242(12):2859–2871. [PubMed] [Google Scholar]
- Woods E. F. Peptide chains of tropomyosin. Nature. 1965 Jul 3;207(992):82–83. doi: 10.1038/207082b0. [DOI] [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


