Abstract
Ischemic stroke is a devastating neurological disease. Brain ischemia impairs systemic immune responses and heightens susceptibility to infections, though the underlying mechanisms remain incompletely understood. Natural killer (NK) cells exhibited decreased frequency and compromised function after acute stage of stroke, resulting in NK cell-associated immune deficiency and increased risk of infection. MicroRNAs (miRNAs) are post-transcriptional molecular modulators. Our previous study revealed a significant upregulation of miR-451a in circulating NK cells from patients with ischemic stroke, but its effects and precise mechanism on immune defense remain elusive. In this study, we observed a substantial elevation of miR-451a level in brain and splenic NK cells in murine model of ischemic stroke miR-451a mimics suppressed NK cell activation and cytotoxicity within the ischemic brain and periphery, including a downregulation of activation marker CD69, and reduced production of effector molecules IFN-γ and perforin. Conversely, miR-451a inhibitor preserved NK cell activation and cytotoxicity, rescuing local inflammation, and reducing bacterial burden in the lung. Pharmacological inhibition of Akt-mTOR pathway with AZD8055 effectively blocked the impacts of miR-451a on NK cell functions. Collectively, these findings suggest miR-451a negatively regulates NK cell cytotoxicity in both the brain and periphery, which could be re-addressed by modulating the Akt-mTOR signaling pathway.
Keywords: Brain ischemia, immune suppression, miR-451a, natural killer cells, poststroke infection
Introduction
Stroke is a devastating and debilitating disease and a leading cause of death worldwide.1,2 Infectious complications, predominantly pneumonia and urinary tract infection, are associated with about 20% death and related to considerable morbidity in stroke survivors.3–5 Prophylactic antibiotics are the traditional approach to manage infections. However, the large international clinical trials, including PASS and STROKE-INF, demonstrated that prophylactic antibiotics did not reduce the incidence of post-stroke pneumonia or improve the function outcome in patients with cerebral ischemia.6,7 Emerging evidence indicates that the immune system play a role in ischemic brain injury. And in turn, the injured brain impairs systemic immune responses, increasing the susceptibility to infections.8–10 Nonetheless, the latest clinical trails CHANCE-3 and CONVINCE, did not provide evidence that anti-inflammatory therapy with colchicine could reduce recurrent stroke or other vascular events in patients with stroke.11,12 These findings highlight the critical need for a deeper understanding of these inflammatory events, including the pathophysiology of ischemic stroke and the innovative intervention for post-stroke infections.
Natural killer (NK) cells are innate lymphoid cells important for host defense against infectious pathogens and cancer.13,14 NK cells orchestrate the initial steps and intensity of both innate and adaptive immune responses because of their capacity to produce various cytokines.15–17 Notably, during the acute stage of brain ischemia, NK cells are among the earliest cell types to reach the brain, guided by neuron-derived signals that subsequently promote local inflammation, worsen neuronal death and exacerbate brain infarction.18–20 Conversely, ours findings, along with other evidence have shown that brain injury-induced activation of neurogenic pathways, including sympathetic innervation, hypothalamic-pituitary-adrenal (HPA) axis, and parasympathetic innervation collectively suppress peripheral NK cells.9,10,21 However, the molecular mechanisms underlying the alteration of NK cells after ischemic stroke remain poorly understood and identifying new targets for immunomodulatory drugs in stroke remain a critical need.
MicroRNAs (miRNAs), small non-coding RNA molecules 18–25 nucleotides in length, repress gene translation by hybridizing to the 3′-untranslated region of one or more mRNAs in a sequence-specific manner.22,23 Many miRNAs play crucial roles in cell proliferation, differentiation, death, cellular metabolism and immune responses under both physiological and pathological conditions.24,25 In ischemic stroke, the aberrant miRNA expressions have been reported, and have emerged as novel diagnostic, prognostic and therapeutic biomarkers in patients with stroke.26–28 In a previous study, we performed unbiased miRNA sequencing analysis of peripheral blood NK cells from stroke patients and identified miR-451a as dysregulated in NK cells after ischemic stroke. 29 To determine the effect and mechanism of miR-451a on NK cells function and stroke outcomes, isolated NK cells were transfected with miR-451a mimics or inhibitor in vitro, then transferred into NOD/Prkdcscid/IL-2rgnull (NPG) mice (lacking T, B, NK and NKT cells) before middle cerebral artery occlusion (MCAO) model. We explored the effect of miR-451a on NK cell activation and cytotoxicity features in the brain and periphery after brain ischemia, as well as the signaling pathway involved in the post-transcriptional modulation of miR-451a on post-stroke NK cell suppression.
Materials and methods
Mice
All animal experiments were approved by the Animal Care and Use Committees of Beijing Tiantan Hospital. All animal experiments were designed and performed in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines and the Guide for the Care and Use of Laboratory Animals (The National Academies Press, 8th Edition, 2011). Male mice aged 8–10 weeks were used in this study. C57BL/6 mice were purchased from Charles River (Wilmington, DE, USA). NOD/Prkdcscid/IL-2rgnull (NPG) mice were obtained from Beijing Vitalstar Biotechnology (Beijing, China). All mice were maintained under pathogen-free conditions and housed no more than five animals per cage under a standardized light-dark cycle with free access to food and water. For all experiments, age-matched male littermates were used between experimental groups. And All animal surgeries were performed under anesthesia.
MCAO procedure
Focal cerebral ischemia was induced by 60-minute intraluminal occlusion of the middle cerebral artery (MCA) using a filament, as described previously.19,30,31 Anesthesia was induced with 3.5% isoflurane and maintained with 1–1.5% isoflurane in 70% N2O and 30% O2. The body temperature was maintained at 37.0 ± 0.5°C using an electric warming blanket during the procedures. A standardized silicone rubber-coated monofilament (RWD Life Science, Shenzhen, China) was inserted into the left MCA to occlude circulation for 60 minutes, and reperfusion was reestablished by gently withdrawing the occluding filament to the common carotid artery. Sham mice underwent the same procedure, but the filament was not advanced far enough to occlude the MCA. Regional cerebral blood flow (CBF) was monitored for 5 minutes before and after MCAO and during the first 10 minutes of reperfusion using a Laser Speckle Imaging System (RWD Life Science, Shenzhen, China). Only MCAO mice with a residual CBF < 30% throughout the ischemic period and CBF recovery > 80% within 10 minutes of reperfusion were included in the study.
Drug administration
For in vivo assays, AZD8055 (Selleck, Houston, TX, USA) was dissolved in 30% (W/V) Captisol to a working concentration of 1 mg/ml and pH was adjusted to 3.0.32–35 The mice received a single intragastric administration of AZD8055 at a dosage of 20 mg/kg body weight immediately after brain ischemia and reperfusion. While the vehicle group were treated with an equal volume of solvent.
Neurological function assessment
Neurological function assessment was performed on days 1 and 3 after MCAO by two investigators blinded to the treatment groups. The modified Neurological Severity Score (mNSS), corner turn test and rota-rod test were conducted to evaluate neuro-deficits in MCAO mice as previously described.9,30,36 The mNSS rates neurological function and comprises a composite of motor, sensory, reflex and balance tests. Mice were given one point for the inability to perform each test, with higher scores indicating more severe impairment. The corner-turning test was conducted to evaluate sensorimotor and postural asymmetries. Each mouse was allowed to enter the corner between two boards joined at a 30° angle and then had to turn right or left. The procedure was repeated 10 times per mouse with an interval of at least 30 seconds between trials. The percentage of ipsilateral turns was calculated. The rota-rod test was conducted to evaluate motor coordination and balance. Mice were placed on a rotarod apparatus, with the speed increasing from 0 to 40 rpm. Three trials were conducted with a break of at least 5 minutes between trails. The lantency to fall from the rotating rod was recorded and the results were calculated as the average of three trials.
Neuroimaging
Magnetic resonance imaging (MRI) was performed using a 7T small-animal MRI (Bruker, Billerica, MA, USA). The axial T2-weighted images (T2) were acquired to assess the infarct size of the ischemic stroke model using the following parameters: TR = 2880 ms, TE = 41 ms, FOV = 24 mm × 30 mm, matrix size = 192 × 320, slice thickness = 0.5 mm. The MRI data were analyzed with RaDiAnt DICOM Viewer (Medixant, Poznan, Poland).
Target gene prediction
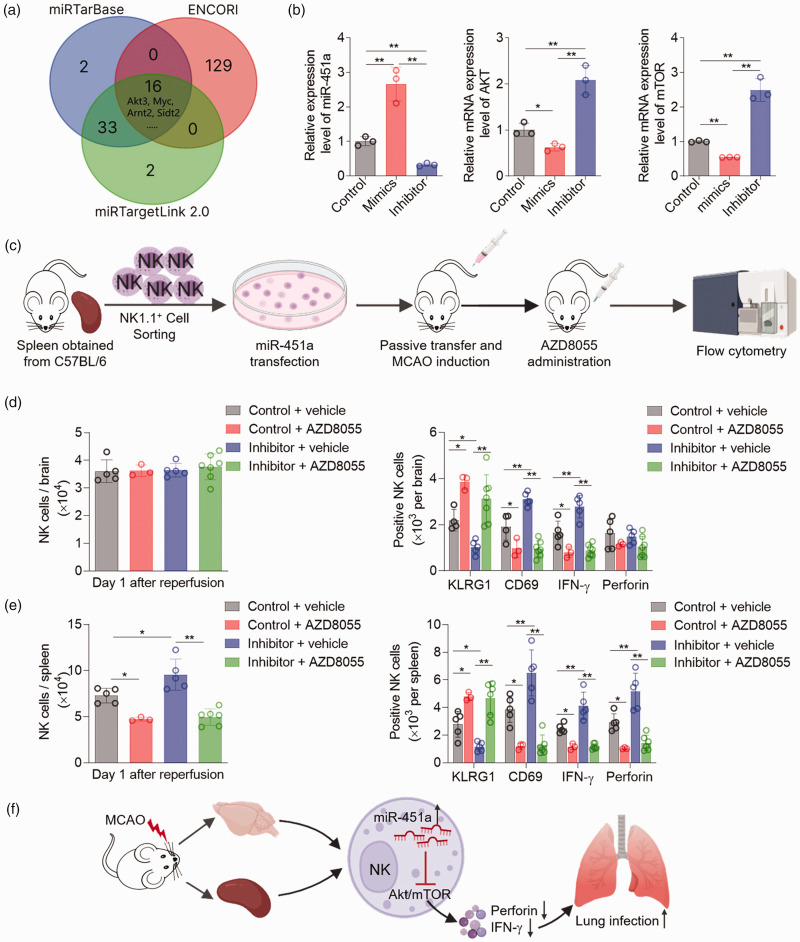
Target genes of miR-451a were predicted using miRTarbase (https://mirtarbase.cuhk.edu.cn), ENCORI (https://rnasysu.com/encori), and miRTargetLink 2.0 (https://ccb-compute.cs.uni-saarland.de/mirtargetlink2). Genes predicted by all three tools were considered target genes of miR-451a. And the Venn diagram was created using an online tool (https://bioinformatics.psb.ugent.be/webtools/Venn).
Cell isolation, miRNA transfection and passive transfer of NK cells
Cell suspensions from the spleen of naive mice were enriched for NK cells using a magnetic-bead sorting system after staining with microbeads (Miltenyi Biotech, San Diego, CA, USA), as previously described.9,19,37 The efficiency and purity of isolated NK cells were confirmed with flow cytometry (>98%). NK cells were cultured in RPMI medium (Gibco, Grand Island, NY, USA) with 10% FBS (Gibco, Grand Island, NY, USA), 1% penicillin/streptomycin (Solarbio, Beijing, China), 100 ng/ml IL-2 and 50 ng/ml IL-15 (Peprotech, Cranbury, NJ, USA).
Chemically synthesized miRNA reagents were purchased from GenePharma (Shanghai, China) to over-express or inhibit miR-451a in NK cells. In brief, purified NK cells were transfected with 100 pmol of miR-451a mimics or inhibitor mixed with 5 μl of Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Negative control miRNA (NC) was transfected in parallel. The interference sequences were miR-451a mimics (sense: 5′-AAACCGUUACCAUUACUGAGUU-3′, antisense: 5′-CUCAGUAAUGGUAACGGUUUUU-3′), miR-451a inhibitor (5′-AACUCAGUAAUGGUAACGGUUU-3′), negative control (sense: 5′-UUCUCCGAACGUGUCACGUTT-3, antisense: 5′-ACGUGACACGUUCGGAGAATT-3′). After transfection, NK cells were cultured for an additional 24 hours before use. Next, 3 × 106 NK cells were injected via the tail vein into NPG recipient mice prior to MCAO.
Quantitative real-time PCR
Total RNA of sorted NK cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the standard protocol and the concentration was measured using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Reverse transcription was performed using the Reverse Transcriptase M-MLV reagent (TaKaRa, Shiga Prefecture, Japan). Quantitative real-time PCR was conducted using SYBR Premix Ex Taq (Roche, Basle, Switzerland) on the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol. The results were analyzed based on the 2–ΔΔCt method.
Flow cytometry
Quantitative analysis of NK cell phenotypes prepared from the spleen, lung or brain were performed using fluorochrome-conjugated antibodies as previously described.9,19,30,37 All antibodies were purchased from BioLegend (San Diego, CA, USA) or BD Bioscience (San Jose, CA, USA) unless stated otherwise. Antibodies were labeled with one of the following fluorescent tags: phycoerythrin (PE), fluorescein isothiocyanate (FITC), allophycocyanin (APC), APC-Cy7, PerCP-Cy5.5 or Brilliant Violet 421. The following antibodies were used in this study: anti-mouse CD3 (17A2), NK1.1 (PK136), NKG2A (20d5), KLRG1 (2F1), NKG2D (A10), Ly49H (3D10), CD69 (H1.2F3), IFN-γ (XMG1.2) and perforin (S16009A). Flow cytometric measurements were performed on a FACS Aria III (BD Bioscience, San Jose, CA, USA). The data were analyzed using FlowJo 7.6 software (Informer Technologies, Walnut, CA, USA).
Enzyme-linked immunosorbent assay (ELISA)
The brain and lung homogenates were prepared from MCAO mice. The protein levels of TNF-α, IFN-γ, IL-6 and IL-1β were measured using the mouse detection ELISA kit (MULTISCIENCES, Hangzhou, China) according to the manufacturer’s instructions. The absorbance was detected by Spark® Multimode Microplate Reader (TECAN, Switzerland).
Microbiologic analysis
The lung tissues of NPG mice were collected using aseptic techniques on day 3 after brain ischemia and reperfusion. After collection, all tissues were minced and homogenized. All samples were diluted in sterile phosphate-buffered saline (PBS) from 1:5 to 1:100 and plated on Luria-Bertani agar plate (Solarbio, Beijing, China). After incubation at 37°C for 24 hours, the growth colonies on the agar plate were quantified. The lung infection was defined as colony-forming units (CFU) per sterilely obtained lung specimen.
Hematoxylin and eosin (H&E) staining
H&E staining was performed to evaluate the lung infiltration on day 3 after MCAO.38,39 Briefly, lung tissues were carefully collected using aseptic techniques and fixed with 4% paraformaldehyde (Solarbio, Beijing, China). Next, the isolated tissues were embedded in paraffin, and 5-mm-thick coronal sections were prepared with a microtome (Leica Biosystems, Nussloch, Germany). Then the H&E Staining Kit (Beyotime Biotechnology, Shanghai, China) was used for staining lung sections. The degree of infiltration was defined as the count of inflammatory cells per mm2 in H&E-stained slices, quantified using ImageJ (National Institutes of Health, Bethesda, MD).
Statistics
The experimental design was based on previous publications with similar mechanistic studies.9,19,37 No statistical methods were used to pre-determine sample sizes, but the sample sizes were similar to those reported in previous publications.9,31,38,40 Animals were randomly assigned to treatment conditions. All results were analyzed by investigators blinded to the treatment. Data were first examined using the Shapiro-Wilk normality test to assess distribution. Two-tailed unpaired Student’s t-test was used to determine the significance of differences between two groups. One-way ANOVA followed by Tukey post hoc test was used for three or more groups. Two-way ANOVA accompanied by Turkey post hoc test was performed for multiple comparisons. Data are expressed as mean ± SD. P values < 0.05 were considered significant. All data were analyzed using GraphPad Prism 8.0 software (GraphPad, San Diego, CA, USA).
Results
The miR-451a mimics suppresses NK cell activity and local inflammation in the brain of MCAO mice
As previously reported by our group, miRNA sequencing analysis identified that miR-451a was significantly upregulated in peripheral blood NK cells from ischemic patients. 29 To further study the effect and mechanism of miR-451a on NK cells and stroke outcomes, we verified the increased expression of miR-451a in NK cells from both the ischemic brain and spleen of wild-type mice on day 3 after MCAO (Figure 1(a)). To determine the specific post-transcriptional impact of miR-451a on NK cell features after ischemic stroke, NK cells isolated from pooled splenocytes of wild-type mice were transfected with miR-451a control, mimics or inhibitor. Following transfection, these NK cells were intravenously injected (3 × 106 cells per mouse) into NOD/Prkdcscid/IL-2rgnull (NPG) mice prior to MCAO induction (Figure 1(b)). NK cell counts, inhibitory receptors (KLRG1 and NKG2A), activating receptors (NKG2D and Ly49H), activation markers (CD69) and functional parameters (NK-cell–derived IFN-γ and perforin) were quantified in the miR-451a mimics, inhibitor or negative control groups on days 1 and 3 post-MCAO. The miR-451a mimics did not affect NK cell numbers in the ischemic brain on day 1 after reperfusion. However, miR-451a mimics resulted in a decline in NK cell response, as indicated by increased expression of KLRG1, decreased CD69, and reduced production of IFN-γ and perforin. Perforin production in NK cells transfected with miR-451a significantly decreased in the ischemic brain starting on day 3. Conversely, the miR-451a inhibitor rescued, to a large extent, the activation and cytotoxicity of NK cells in the ischemic brain (Figure 1(c) to (d)).
Figure 1.
miR-451a down-regulates the phenotype and function of CNS-infiltrating NK cells after brain ischemia and reperfusion. (a) Validation of miR-451a expression in NK cells from MCAO and sham models in wild-type mice. n = 3 per group, error bars represent mean ± SD. *P < 0.05. (b) The schematic graph of the experimental design. NK cells sorted from pooled splenocytes of wild-type mice were transfected with miR-451a mimics, negative control or inhibitor. After being transfected for 24 h, NK cells were transferred intravenously (3 × 106 per mouse) into NOD/Prkdcscid/IL-2rgnull (NPG) mice prior to MCAO. On day 1 or day 3 after MCAO and reperfusion, mice were subjected to neurological assessment, MRI scan, flow cytometry and pathology staining. (c–d) Flow cytometry plots and bar graphs show the effect of miR-451a on the phenotype and function of NK cells in the ischemic brain at indicated time points, including NK cell counts (CD3−NK1.1+), inhibitory receptors on NK cell (KLRG1 and NKG2A), activating receptors on NK cell (NKG2D and Ly49H), activation markers of NK cell (CD69), and cytotoxic function of NK cell (IFN-γ and perforin). Day 1 after reperfusion, n = 5–7 mice per group. Day 3 after reperfusion, n = 11–12 mice per group. The data were representative of three independent experiments and calculated as mean ± SD; *P < 0.05, **P < 0.01 by two-way ANOVA and (e) The expression of IFN-γ, TNF-α, IL-1β and IL-6 in the ipsilateral brain at day 3 after MCAO in NPG mice receiving transfected NK cells was analyzed by ELISA. n = 7 mice per group. Data were presented as mean ± SD; *P < 0.05, **P < 0.01 by one-way ANOVA.
To further evaluate the local brain inflammation by miR-451a-modified NK cells, we quantified inflammatory molecules in the ipsilateral brain on day 3 after reperfusion. As shown in Figure 1(e), in parallel with the suppressed NK functions, the miR-451a mimics intervention reduced the production of inflammatory factors, including TNF-α, IFN-γ and IL-6 in the brain. Conversely, the miR-451a inhibitor rescued the NK cell response, accompanied by the preserved release of TNF-α, IFN-γ and IL-6. However, the levels of IL-1β were not significantly altered. Our results demonstrate that miR-451a suppressed NK cell response and attenuated local inflammation in the ischemic brain.
The miR-451a is involved in the alteration of peripheral NK cell phenotype in MCAO mice
In the spleen of MCAO mice, miR-451a mimics dramatically reduced NK cell numbers, impaired NK cell activation, and suppressed IFN-γ and perforin production on days 1 and 3 after reperfusion. Moreover, the miR-451a inhibitor, which down-regulates miR-451a, prevented the decline of NK cells, limited the expression of KLRG1, is preserved the expression of CD69, IFN-γ and perforin after MCAO (Figure 2(a) and (b)). Together, these results indicate that miR-451a inhibits the cytotoxic and effector response of NK cells in the periphery after brain ischemia.
Figure 2.
MiR-451a is involved in the alteration of peripheral NK cells after MCAO. (a) After treatment with miR-451a mimics, negative control or inhibitor, NK cells were transferred intravenously into NPG mice before MCAO. Splenocytes were isolated at the indicated time points after surgery. Flow cytometry plots show the NK cell counts (CD3−NK1.1+), the expression of KLRG1, NKG2A, NKG2D, Ly49H, CD69, IFN-γ and perforin and (b) Summarized results show the suppression of miR-451a on peripheral NK cells at the acute stage of ischemic stroke. Day 1 after stroke, n = 5–8 mice per group; Day 3 after stroke, n = 10–12 mice per group. Data were collected from three independent experiments and expressed as mean ± SD; *P < 0.05, **P < 0.01 by two-way ANOVA.
Targeting NK cells with miR-451a is not sufficient to affect neurological deficits and infarct volume in MCAO mice
Compelling evidence has supported that brain-infiltrating NK cells exacerbate neurological deficits and infarct size during acute ischemic stroke.19,41 We further compared the neurological deficits and ischemic lesions on days 1 and 3 after stroke in NPG mice (lacking NK cells) and NPG mice receiving miR-451a-transfected NK cells. Compared to the NPG mice, the NPG mice receiving miR-451a control-transfected NK cells or miR-451a inhibitor-transfected NK cells significantly aggravated neuro-deficits (Figure 3(a)) and increased infarct volume at the acute and subacute phase of ischemic stroke (Figure 3(b)). Nevertheless, the NPG mice receiving miR-451a mimics-transfected NK cells, which exhibit deficient NK cell responses, had no significant effect on stroke outcomes compared with NPG mice (Figure 3(a) and (b)). Among the three groups transferred with miR-451a-transfected NK cells, the miR-451a mimics group displayed modest improvement in motor function and infarct lesion, although no statistical significance was observed. These data suggest that the post-transcriptional regulation of NK cells activity by miR-451a, may not be adequate to markedly impact ischemic lesion in MCAO mice, particularly during the acute stage.
Figure 3.
Targeting NK cells with miR-451a is not sufficient to affect stroke outcomes. Splenic NK cells isolated from wild-type mice were transfected with miR-451a mimics, negative control or inhibitor, and 3 × 106 NK cells were transfused intravenously into NPG mice, followed by the MCAO procedure. Mice were subjected to neurological assessment and MRI evaluation at the indicated time points. (a) Cumulative data illustrate the neurological assessment of NPG mice (without NK cells) and NPG mice receiving NK cells transfected with the indicated treatment, the neurological assessment includes mNSS scores, corner turning test and rota-rod test. NPG mice receiving NK cells transfected with miR-451a inhibitor versus NPG mice: *P < 0.05 and **P < 0.01 by two-way ANOVA; NPG mice receiving NK cells transfected with miR-451a control versus NPG mice: #P < 0.05 and ##P < 0.01 by two-way ANOVA and (b) The representative MRI images and quantification of infarct lesion of the indicated groups at day 3 after brain ischemia and reperfusion. *P < 0.05 by one-way ANOVA. n = 6–7 mice per group. The data were representative of three independent experiments and were expressed as mean ± SD.
Inhibition of miR-451a improves host immune defense against post-stroke infection
Emerging evidence suggests that stroke-induced defects in lymphocyte activation contribute to the impaired antibacterial response after stroke.9,42 We further investigated whether miR-451a-targeted NK-cell function could influence the bacterial burden in the lung in MCAO mice. H&E staining and bacteriological assay were used to examine inflammatory cell infiltration and bacterial infection of the lung on day 3 after brain ischemia (Figure 4(a) and (c)). Compared to the control group (negative control miRNA), the bacterial burden was heavier in miR-451a mimics group on day 3 after MCAO, as indicated by inflammatory infiltrates counts and CFU. In contrast, miR-451a-inhibited NK cells attenuated the spontaneous lung infectious burden (Figure 4(b) and (d)). These results indicate that NK cell deficiency reduces the capacity for bacterial clearance after MCAO, which is at least partially regulated by miR-451a.
Figure 4.
miR-451a exacerbates lung bacterial burden in MCAO mice, which is related to compromised function of NK cells in the lung. Isolated splenic wild-type NK cells were treated with miR-451a mimics, negative control or inhibitor and cultured for 24 hours, then transferred via intravenous injection to NPG transgenic mice, prior to MCAO. On day 3 after ischemia and reperfusion, lung tissues were collected for histological staining, bacteriological analysis, flowcytometry and ELISA analysis. (a) H&E-stained lung sections of NPG mice revealed the infiltration of inflammatory cells and the thickening of alveolar walls on day 3 after MCAO. Scale bras: 20 μm; insert: 10 μm. (b) Quantification analysis show the infiltrated cell counts from lung sections on day 3 after ischemia. n = 5–7 mice per group. (c–d) Representative image of bacterial culture and quantification of colony-forming units (CFU) in the lung of NPG mice on day 3 after surgery. n = 3–4 mice per group. (e) Bar graph show the effect of miR-451a on cell number and functional marker expression (CD69, IFN-γ and perforin) of lung NK cells on day 3 after MCAO. n = 5 mice per group and (f) ELISA measurement of IFN-γ, TNF-α, IL-1β and IL-6 protein levels in the lung on day 3 after MCAO. n = 7 mice per group. The results represented three independent experiments. Error bars represent mean ± SD. *P < 0.05; **P < 0.01 by one-way ANOVA.
Meanwhile, we evaluated the NK cells function and inflammatory molecules in the lung on day 3 after MCAO. As shown in Figure 4(e) and (f), the miR-451a mimics group exhibited a decrease in NK cell numbers and function, evidenced by reduced expression of the activation marker CD69, and functional molecules perforin. The dysfunction of NK cells induced by miR-451a mimics led to lower levels of inflammatory cytokines (TNF-α, IFN-γ, and IL-6) in the lung. Conversely, inhibiting miR-451a significantly prevented the decline in NK cell counts, maintained the expression of CD69, IFN-γ, and perforin, and restored cytokine levels in the lung (Figure 4(e) and (f)). Ultimately, the inhibition of miR-451a preserved NK cell cytotoxicity and enhanced the release of pro-inflammatory cytokines, thereby contributing to the improved lung antibacterial effects.
miR-451a mediated NK cell suppression through the akt-mTOR pathway
We identified possible targets of miR-451a by screening online databases (Figure 5(a)). Sixteen gene candidates appeared three times in these databases, including Akt3, Myc, Arnt2, Sidt2, etc. Among these, Akt3 has been extensively studied and is considered neuroprotective in stroke.43,44 Importantly, accumulating data suggest that the Akt-mTOR pathway is essential for modulating the development, differentiation, and activation of immune cells including NK cells.45,46 Therefore, we quantified the relative mRNA expression of Akt and mTOR in NK cells transfected with miR-451a mimics, inhibitor, and negative control. Treatment with miR-451a mimics led to decreased Akt and mTOR expression in vitro, whereas miR-451a inhibitor significantly increased Akt and mTOR expression compared to the negative control (Figure 5(b)). Isolated NK cells from pooled splenocytes of wild-type mice were treated with miR-451a inhibitor or control, then transfused to NPG recipients prior to MCAO surgery. AZD8055, a dual mTOR kinase blocker, was administered intragastrically at a dose of 20 mg/kg immediately after reperfusion (Figure 5(c)). We found that, although without affecting NK cell counts in the ischemic brain, miR-451a inhibitor-induced NK cell activation was blocked by AZD8055 (Figure 5(d)). In the spleen, miR-451a inhibitor-induced NK cell accumulation and activation were both blocked by AZD8055 (Figure 5(e)). Together, these data indicate that miR-451a contributes to NK cell deficiency after ischemic stroke, partially via the Akt-mTOR pathway (Figure 5(f)).
Figure 5.
miR-451a modulates the phenotype and function of NK cells via the Akt-mTOR pathway. (a) Three databases were searched for target prediction of miR-451a, and 16 candidates appeared three times in these databases. (b) Splenic NK cells isolated from wild-type mice were treated with miR-451a mimics, negative control or inhibitor. After culturing for 24 hours, the expression of miR-451a, AKT, and mTOR were quantified by quantitative real-time PCR. n = 3 per group. (c) The schematic diagram delineates the acute mTOR inhibition with AZD8055 in vivo. After treatment with miR-451a inhibitor or negative control, NK cells were passively transferred by intravenous injection into NPG recipients, followed by MCAO surgery. Meanwhile, the MCAO mice were treated with AZD8055 or vehicle by oral gavage at a dose of 20 mg/kg immediately after reperfusion. Brain cells and splenocytes were measured using flow cytometry on day 1 after ischemia and reperfusion. (d–e) Cumulative data show the NK cell number and the expression of KLRG1, CD69, IFN-γ and perforin in the brain and spleen of MCAO mice receiving AZD8055 or vehicle. n = 3–5 mice per group and (f) The graphical abstract depicts that miR-451a contributed to stroke-mediated NK cell deficiency through the Akt-mTOR pathway. Data were collected from three independent experiments and were expressed as mean ± SD. *P < 0.05; **P < 0.01 by one-way ANOVA.
Discussion
This study provides further evidence that ischemic stroke induces changes in miRNA expression in NK cells. As documented here, we found dramatically upregulated miR-451a in NK cells after ischemic stroke in mice. Through in vitro miR-451a transfection and in vivo transfer, we demonstrated that miR-451a mimics suppressed NK cell responses involving cell counts and cytotoxicity, and reduced the local inflammatory cytokines in the brain and periphery. Conversely, inhibition of miR-451a restores NK cell responses and rescues inflammatory molecules release, subsequently lowering bacterial burden in the lung post-MCAO. Furthermore, we show that miR-451a contributes to ischemic stroke-induced NK cell suppression, partially through the Akt-mTOR pathway.
Accumulating evidence has demonstrated that miRNAs play key roles in nearly all aspects of cerebral pathophysiology after ischemic stroke, including excitotoxicity, oxidative stress, mitochondrial disturbances, inflammation, blood-brain barrier (BBB) disruption, neuronal apoptosis, and aspects of post-stroke recovery.47–50 For example, miR-223, miR-125b, and miR-107 contribute to the glutamate accumulation and the degree of excitotoxicity after ischemic stroke.51–53 miR-124, miR-210, miR-183, miR-203, and miR-let-7c-5p are involved in ischemic brain injury through the activation or phenotype switching of microglia.50,54–57 In the periphery, current studies on miRNAs primarily focus on their role as biomarkers for diagnosis and prognosis in animal models and patients with stroke.26,58 Only a few studies have explored miRNA regulation in stroke-induced immunosuppression (SIIS), which suppress the function of peripheral lymphocytes, increase the risk of infection, and exacerbate the neuro-deficits.40,42,59 We have demonstrated that the contraction of NK-cell numbers in the CNS and periphery is mediated by distinct transcriptional regulation after ischemic stroke, but the post-transcriptional mechanism remain unclear. 9 This study delves deeper into NK cells, providing new evidence implicating dysregulated miR-451a in the decline of NK cell efficacy and weakened immune defenses post-stroke. This advances our knowledge of the role and mechanism of miRNA in SIIS.
Acute ischemic stroke triggers profound systemic and local immunosuppression that increases the susceptibility to infections, especially stroke-associated pneumonia (SAP). 10 Owing to the lack of benefits from antibiotics in patients, a growing body of research have concentrated on immunomodulation therapy to improve host defense.4,6,7 Our group and others have reported that pharmacological blockade of the sympathetic pathway or HPA axis by β-blockers or glucocorticoid receptor antagonists can rescue lymphocyte activity, preserve IFN-γ production, and further reduce post-stroke infection in the periphery.9,42 Additionally, adoptive transfer of T or NK cells, injection of IFN-γ, or the specific activator α-galactosylceramide, has been demonstrated to boost peripheral immunity and reduce infection.60,61 As an important component of innate immunity, NK cell activation plays a vital role in host defense against pathogens by killing infected cells and producing cytokines (for example, TNF-α, IFN-γ).62,63 In our study, the miR-451a inhibitor rescued NK cell responses and preserved cytokines production (IFN-γ, TNF-α), resulting in a boosted antibacterial effect, which is consistent with previous findings. However, systemic immunomodulation strategies remain controversial because of potential detrimental effects on the brain or other side effects. For instance, it is suggested that elevated levels of circulating IFN-γ directly contributed to neurodegeneration after stroke. 64 Therefore, organ-specific immunomodulation represents a promising future direction for new preventive strategies for SAP.
Similar to the periphery, miR-451a inhibitor also augmented brain inflammation by preserving NK cell activity. However, this enhanced NK-cell response had no significant effect on stroke outcomes. We suggest that post-transcriptional regulation of NK cell activity by miR-451a in stroke exhibits temporal- and organ-specific features. In the pathological process of ischemic stroke, the formation of the infarct core primarily occurs within the first 24 hours of the acute phase.65,66 The cascade of inflammatory events occurs within minutes or hours of cerebral ischemia,8,67 and NK cells exert their deleterious effects in the ischemic brain predominantly within the initial 12 hours, partly through NK-derived perforin-mediated neuronal death. 19 In our study, during this critical period of NK-mediated neuronal injury and infarct volume formation, neither the infiltration of NK cells nor perforin production was influenced by miR-451a post-transcriptional regulation. Therefore miR-451a-mediated post-transcriptional regulation appears to have a limited effect on infarct volume during the acute phase. Subsequently, during the subacute phase of ischemic stroke (day 1 today 3), pathological processes focus on inflammatory edema in the peri-infarct core and systemic immune suppression.68,69 Therefore, miR-451a-transfected NK cells primarily affect cerebral inflammation and peripheral infection rather than the cerebral infarct core. Post-stroke immunosuppression persists until day 7 after ischemia,9,38 and the peak incidence of SAP occurs on day 3 after stroke. 70 Further studies investigating NK cell-targeted interventions should prioritize timeframes beyond the initial 12-hour window, particularly in mitigating infection risks during the subacute stages of stroke.
Mounting evidence has shown that the Akt-mTOR pathway in NK cells is important for their development, cellular proliferation, response to cytokine stimulation, and cytotoxic function. 45 According to our data, miR-451a was involved in NK cell modulation via the Akt-mTOR signaling in the brain and spleen after MCAO. It has been reported that miR-451 acts as the tumor suppressor by downregulating the Akt-mTOR pathway, which is consistent with our findings.71,72 Moreover, in recent studies, Akt-mTOR pathway activation has been widely proven to provide potential neuroprotective effects in the ischemic brain, primarily by reducing neuronal apoptosis, promoting cerebral angiogenesis, and improving axonal regeneration.43,44,73,74 Importantly, Guan and colleagues demonstrated that stroke impaired the PI3K/Akt pathway, contributing to SAP via PTEN activation. 75 This study supported our finding that miR-451a impaired NK cell function in the periphery via Akt-mTOR inhibition, which resulted in increased lung infection after MCAO. Overall, the Akt-mTOR pathway is a potential therapeutic target for neuroprotection and SAP in stroke.
There are also limitations in our study that warrant further investigation. Due to the limited lifetime of transfused cells, the alteration of NK cells and clinical outcomes are restricted to the subacute stage of stroke. In addition, SAP is a pathological process involving more than infections. Therefore, the lung pathology may not entirely result from increased bacterial burden. Lastly, although our results suggest that the inhibitory effects of miR-451a on NK cells are mediated by the Akt-mTOR signaling pathway, the precise targets need to be further elucidated.
In conclusion, our study presents novel evidence that miR-451a inhibits the phenotype and function of NK cells in the periphery and brain after ischemic stroke through the Akt-mTOR pathway, potentially dampening the capacity for bacterial clearance. This study revealed the inhibitory effect of miR-451a on NK cell phenotype and identified the Akt-mTOR pathway as a potential therapeutic target, providing new insight for organ-specific miRNA targeting in NK cells after ischemic stroke.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Science Foundation of China grant (82122021, 82371324).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: F.-D.S. and W.-N.J. formulated the study concept, designed the study, drafted the manuscript, and acquired funding for the study. Y.L., X.G and Z.-R.Z. performed experiments, analyzed and interpreted the data. Y.L., T.L., and M.L. assisted to edit the revised manuscript. Y.Z., M.L and S.-H.J. analyzed the data, assisted preparation of the manuscript, and edited the language of the manuscript.
ORCID iD: Wei-Na Jin https://orcid.org/0000-0002-2196-9078
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014; 129: 399–410. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet 2014; 383: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong S, Zhou Z, Zhou M, et al. Validation of risk scoring models for predicting stroke-associated pneumonia in patients with ischaemic stroke. Stroke Vasc Neurol 2016; 1: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwan J, Pickering RM, Kunkel D, et al. Impact of stroke-associated infection on long-term survival: a cohort study. J Neurol Neurosurg Psychiatry 2013; 84: 297–304. [DOI] [PubMed] [Google Scholar]
- 5.Westendorp WF, Nederkoorn PJ, Vermeij JD, et al. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol 2011; 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra L, Irshad S, Hodsoll J, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 2015; 386: 1835–1844. [DOI] [PubMed] [Google Scholar]
- 7.Westendorp WF, Vermeij J-D, Zock E, PASS investigators et al. The preventive antibiotics in stroke study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015; 385: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y, Liu Q, Anrather J, et al. Immune interventions in stroke. Nat Rev Neurol 2015; 11: 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Jin WN, Liu Y, et al. Brain ischemia suppresses immunity in the periphery and brain via different neurogenic innervations. Immunity 2017; 46: 474–487. [DOI] [PubMed] [Google Scholar]
- 10.Meisel C, Schwab JM, Prass K, et al. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci 2005; 6: 775–786. [DOI] [PubMed] [Google Scholar]
- 11.Kelly P, Lemmens R, Weimar C, et al. Long-term colchicine for the prevention of vascular recurrent events in non-cardioembolic stroke (CONVINCE): a randomised controlled trial. Lancet 2024; 404: 125–133. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Meng X, Shi FD, et al. Colchicine in patients with acute ischaemic stroke or transient ischaemic attack (CHANCE-3): multicentre, double blind, randomised, placebo controlled trial. BMJ 2024; 385: e079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz A, Stegmann KA, Riley EM. Activation of natural killer cells during microbial infections. Front Immunol 2011; 2: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huntington ND, Cursons J, Rautela J. The cancer-natural killer cell immunity cycle. Nat Rev Cancer 2020; 20: 437–454. [DOI] [PubMed] [Google Scholar]
- 15.Long EO, Kim HS, Liu D, et al. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2013; 31: 227–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi FD, Ljunggren HG, La Cava A, et al. Organ-specific features of natural killer cells. Nat Rev Immunol 2011; 11: 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Ai QD, Chu SF, et al. NK cells in cerebral ischemia. Biomed Pharmacother 2019; 109: 547–554. [DOI] [PubMed] [Google Scholar]
- 19.Gan Y, Liu Q, Wu W, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci U S A 2014; 111: 2704–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Gao Z, Wang D, et al. Accumulation of natural killer cells in ischemic brain tissues and the chemotactic effect of IP-10. J Neuroinflammation 2014; 11: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol 2011; 10: 471–480. [DOI] [PubMed] [Google Scholar]
- 22.Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol 2012; 19: 586–593. [DOI] [PubMed] [Google Scholar]
- 23.Stark A, Brennecke J, Bushati N, et al. Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3′UTR evolution. Cell 2005; 123: 1133–1146. [DOI] [PubMed] [Google Scholar]
- 24.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 25.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010; 11: 597–610. [DOI] [PubMed] [Google Scholar]
- 26.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 2008; 39: 959–966. [DOI] [PubMed] [Google Scholar]
- 27.Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab 2010; 30: 1564–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun P, Liu DZ, Jickling GC, et al. MicroRNA-based therapeutics in central nervous system injuries. J Cereb Blood Flow Metab 2018; 38: 1125–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong Y, Li S, Cheng X, et al. Brain ischemia significantly alters microRNA expression in human peripheral blood natural killer cells. Front Immunol 2020; 11: 759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Y, Liao S, Wei C, et al. Infiltration and persistence of lymphocytes during late-stage cerebral ischemia in middle cerebral artery occlusion and photothrombotic stroke models. J Neuroinflammation 2017; 14: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin WN, Gonzales R, Feng Y, et al. Brain ischemia induces diversified neuroantigen-specific T-cell responses that exacerbate brain injury. Stroke 2018; 49: 1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chresta CM, Davies BR, Hickson I, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res 2010; 70: 288–298. [DOI] [PubMed] [Google Scholar]
- 33.Kleinert M, Sylow L, Fazakerley DJ, et al. Acute mTOR inhibition induces insulin resistance and alters substrate utilization in vivo. Mol Metab 2014; 3: 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yates JWT, Holt SV, Logie A, et al. A pharmacokinetic-pharmacodynamic model predicting tumour growth inhibition after intermittent administration with the mTOR kinase inhibitor AZD8055. Br J Pharmacol 2017; 174: 2652–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You W, Wang Z, Li H, et al. Inhibition of mammalian target of rapamycin attenuates early brain injury through modulating microglial polarization after experimental subarachnoid hemorrhage in rats. J Neurol Sci 2016; 367: 224–231. [DOI] [PubMed] [Google Scholar]
- 36.Jin WN, Shi SX, Li Z, et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab 2017; 37: 2224–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Li M, Shi SX, et al. Brain transforms natural killer cells that exacerbate brain edema after intracerebral hemorrhage. J Exp Med 2020; 217: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin WN, Ducruet AF, Liu Q, et al. Activation of JAK/STAT3 restores NK-cell function and improves immune defense after brain ischemia. FASEB J 2018; 32: 2757–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Li M, Whiteaker P, et al. Attenuation in nicotinic acetylcholine receptor α9 and α10 subunit double knock-out mice of experimental autoimmune encephalomyelitis. Biomolecules 2019; 9: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Y, Li Y, Zhang Y, et al. miR-1224 contributes to ischemic stroke-mediated natural killer cell dysfunction by targeting Sp1 signaling. J Neuroinflammation 2021; 18: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Planas AM. Role of immune cells migrating to the ischemic brain. Stroke 2018; 49: 2261–2267. [DOI] [PubMed] [Google Scholar]
- 42.Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med 2003; 198: 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie R, Cheng M, Li M, et al. Akt isoforms differentially protect against stroke-induced neuronal injury by regulating mTOR activities. J Cereb Blood Flow Metab 2013; 33: 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol 2006; 34: 249–270. [DOI] [PubMed] [Google Scholar]
- 45.Ali AK, Nandagopal N, Lee SH. IL-15-PI3K-AKT-mTOR: a critical pathway in the life journey of natural killer cells. Front Immunol 2015; 6: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao Y, van Hoef V, Zhang X, et al. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood 2016; 128: 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W, Sinha B, Li Y, et al. Monogenic, polygenic, and MicroRNA markers for ischemic stroke. Mol Neurobiol 2019; 56: 1330–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khoshnam SE, Winlow W, Farbood Y, et al. Emerging roles of microRNAs in ischemic stroke: As possible therapeutic agents. J Stroke 2017; 19: 166–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li G, Morris-Blanco KC, Lopez MS, et al. Impact of microRNAs on ischemic stroke: from pre- to post-disease. Prog Neurobiol 2018; 163–164: 59–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lian L, Zhang Y, Liu L, et al. Neuroinflammation in ischemic stroke: focus on MicroRNA-mediated polarization of microglia. Front Mol Neurosci 2020; 13: 612439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edbauer D, Neilson JR, Foster KA, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 2010; 65: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harraz MM, Eacker SM, Wang X, et al. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc Natl Acad Sci U S A 2012; 109: 18962–18967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang ZB, Zhang Z, Li TB, et al. Up-regulation of brain-enriched miR-107 promotes excitatory neurotoxicity through down-regulation of glutamate transporter-1 expression following ischaemic stroke. Clin Sci (Lond) 2014; 127: 679–689. [DOI] [PubMed] [Google Scholar]
- 54.Hamzei Taj S, Kho W, Riou A, et al. MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 2016; 91: 151–165. [DOI] [PubMed] [Google Scholar]
- 55.Song Y, Li Z, He T, et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019; 9: 2910–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang B, Zhong P, Fang L, et al. miR-183 inhibits microglia activation and expression of inflammatory factors in rats with cerebral ischemia reperfusion via NF-kappaB signaling pathway. Exp Ther Med 2019; 18: 2540–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni J, Wang X, Chen S, et al. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behav Immun 2015; 49: 75–85. [DOI] [PubMed] [Google Scholar]
- 58.Laterza OF, Lim L, Garrett-Engele PW, et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem 2009; 55: 1977–1983. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Lu G, Wang D, et al. MicroRNA-4443 regulates monocyte activation by targeting tumor necrosis factor receptor associated factor 4 in stroke-induced immunosuppression. Eur J Neurol 2020; 27: 1625–1637. [DOI] [PubMed] [Google Scholar]
- 60.Mracsko E, Liesz A, Karcher S, et al. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav Immun 2014; 41: 200–209. [DOI] [PubMed] [Google Scholar]
- 61.Wong CH, Jenne CN, Lee WY, et al. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 2011; 334: 101–105. [DOI] [PubMed] [Google Scholar]
- 62.Iwasaki A, Foxman EF, Molony RD. Early local immune defences in the respiratory tract. Nat Rev Immunol 2017; 17: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mujal AM, Delconte RB, Sun JC. Natural killer cells: from innate to adaptive features. Annu Rev Immunol 2021; 39: 417–447. [DOI] [PubMed] [Google Scholar]
- 64.Seifert HA, Collier LA, Chapman CB, et al. Pro-Inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol 2014; 9: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Y, Zhang X, Chen X, et al. Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (review). Int J Mol Med 2022; 49: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mao R, Zong N, Hu Y, et al. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci Bull 2022; 38: 1229–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Endres M, Moro MA, Nolte CH, et al. Immune pathways in etiology, acute phase, and chronic sequelae of ischemic stroke. Circ Res 2022; 130: 1167–1186. [DOI] [PubMed] [Google Scholar]
- 68.Faura J, Bustamante A, Miró-Mur F, et al. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation 2021; 18: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi K, Wood K, Shi FD, et al. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc Neurol 2018; 3: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Jonge JC, van de Beek D, Lyden P, et al. Temporal profile of pneumonia after stroke. Stroke 2022; 53: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Streleckiene G, Inciuraite R, Juzenas S, et al. miR-20b and miR-451a are involved in gastric carcinogenesis through the PI3K/AKT/mTOR signaling pathway: Data from gastric cancer patients, cell lines and Ins-Gas mouse model. Int J Mol Sci 2020; 21: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian Y, Nan Y, Han L, et al. MicroRNA miR-451 downregulates the PI3K/AKT pathway through CAB39 in human glioma. Int J Oncol 2012; 40: 1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J, Zhang X, Liu X, et al. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/akt/mTOR signaling pathway in ischemic mice. Eur J Pharmacol 2019; 856: 172418. [DOI] [PubMed] [Google Scholar]
- 74.Hou Y, Wang K, Wan W, et al. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis 2018; 5: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan YT, Mao LL, Jia J, et al. Postischemic administration of a potent PTEN inhibitor reduces spontaneous lung infection following experimental stroke. CNS Neurosci Ther 2013; 19: 990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.