Abstract
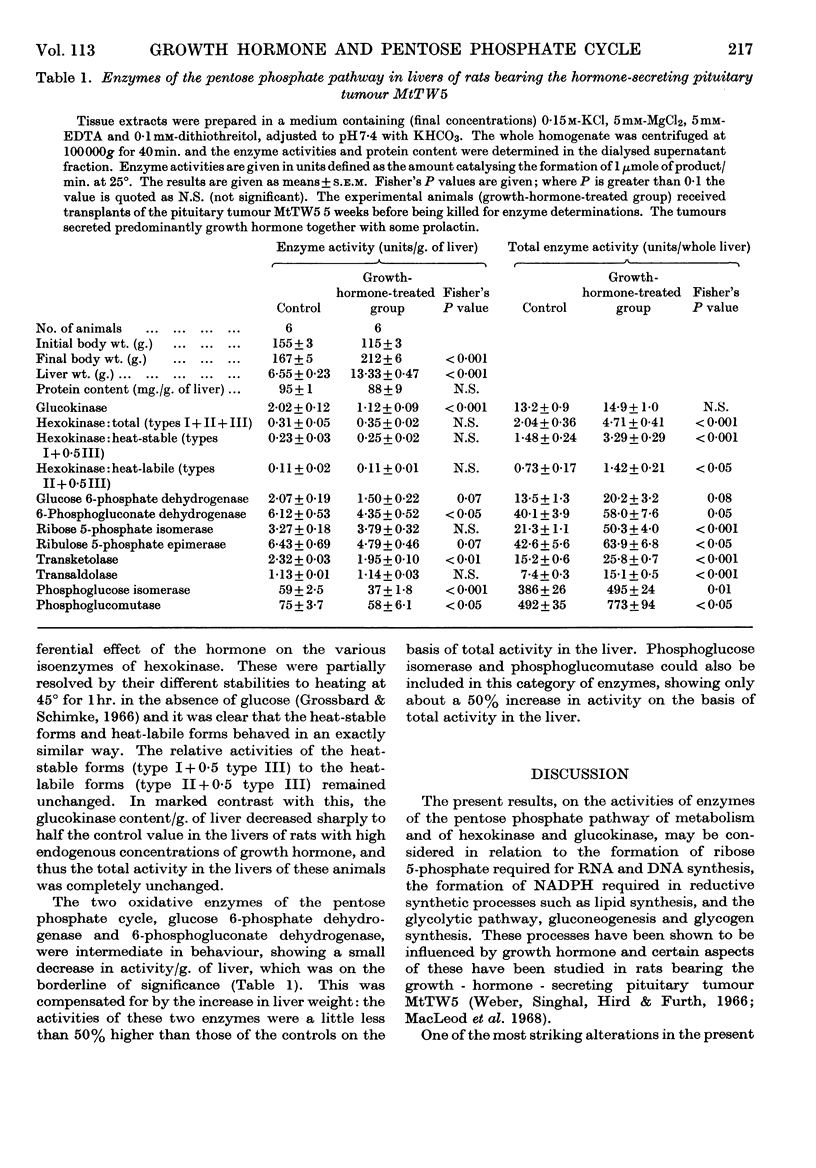
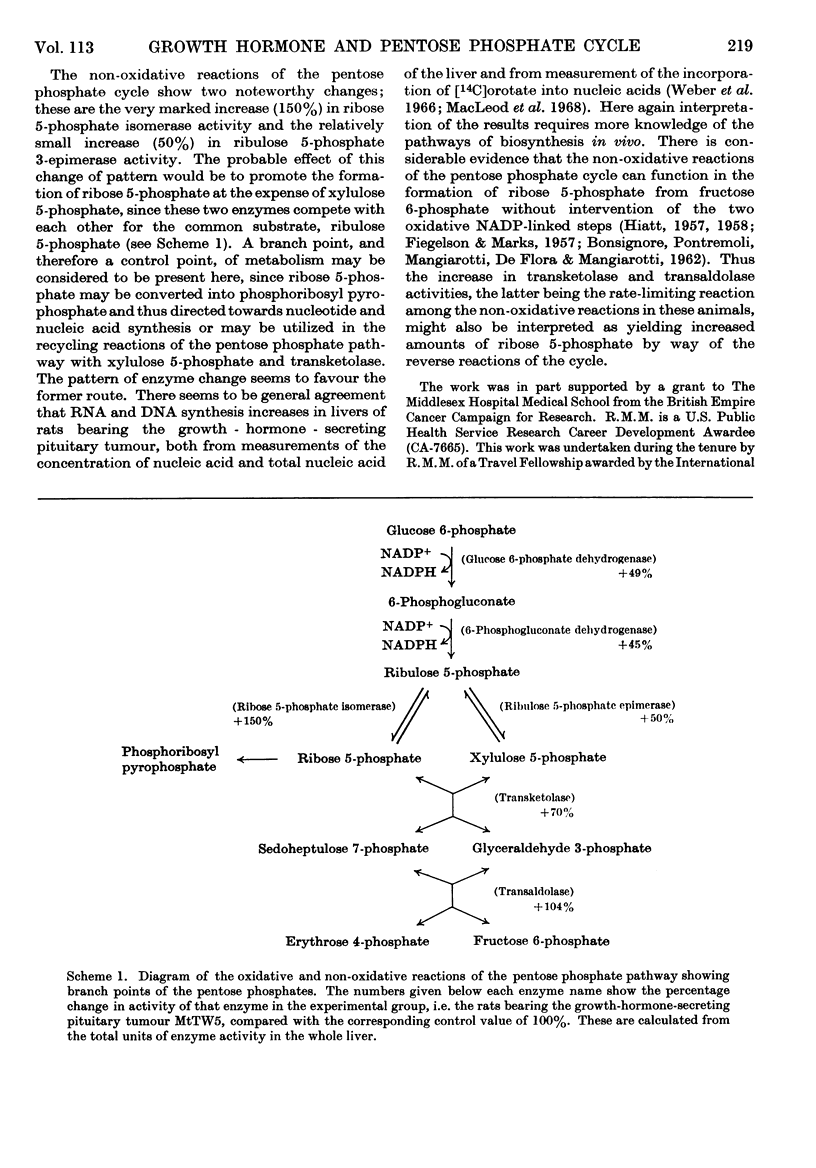
1. Measurements were made of the activities of enzymes of the pentose phosphate cycle, glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, ribose 5-phosphate isomerase, ribulose 5-phosphate epimerase, transketolase and transaldolase, as well as of the related or competing enzymes glucokinase, hexokinase, phosphoglucose isomerase and phosphoglucomutase, in control rats and in rats bearing the growth-hormone- and prolactin-secreting pituitary tumour MtTW5, to study the effect of high endogenous concentrations of growth hormone on this pathway in liver. 2. There was a twofold increase in liver weight. Glucokinase activity/g. of liver decreased to half the control value in the experimental group, although on a total liver basis it remained unchanged. Hexokinase activity increased in parallel with the liver weight, so that the total activity was doubled in rats with a high endogenous concentration of growth hormone. No differences in response were found between heat-stable and heat-labile forms of hexokinase. 3. The activity/g. of liver of the two oxidative enzymes of the pathway decreased slightly in the experimental group, but this was offset by the increase in liver weight, and the resultant effect was a 50% increase in the total activity. 4. Of the non-oxidative enzymes of the cycle the most marked increase on a total liver basis was in ribose 5-phosphate isomerase activity, to 2·5 times the control value. Ribulose 5-phosphate epimerase activity showed the smallest increase. Transketolase and transaldolase activities were also increased. The latter is the rate-limiting enzyme of the non-oxidative reactions of the cycle in these animals. 5. The results are discussed in relation to the glycolytic pathway and synthesis of glycogen, and more particularly to the increased requirement for ribose 5-phosphate for RNA synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENEVENGA N. J., STIELAU W. J., FREEDLAND R. A. FACTORS AFFECTING THE ACTIVITY OF PENTOSE PHOSPHATE-METABOLIZING ENZYMES IN RAT LIVER. J Nutr. 1964 Dec;84:345–350. doi: 10.1093/jn/84.4.345. [DOI] [PubMed] [Google Scholar]
- BLUMENTHAL M. D., ABRAHAM S., CHAIKOFF I. L. ADAPTIVE BEHAVIOR OF HEPATIC GLUCOKINASE IN THE ALLOXAN-DIABETIC RAT. Arch Biochem Biophys. 1964 Feb;104:225–230. doi: 10.1016/s0003-9861(64)80007-2. [DOI] [PubMed] [Google Scholar]
- BRIN M., YONEMOTO R. H. Stimulation of the glucose oxidative pathway in human erythrocytes by methylene blue. J Biol Chem. 1958 Jan;230(1):307–317. [PubMed] [Google Scholar]
- Bates R. W., Scow R. O., Lacy P. E. Induction of permanent diabetes in rats by pituitary hormones from a transplantable mammotropic tumor. Concomitant changes in organ weights and the effect of adrenalectomy. Endocrinology. 1966 Apr;78(4):826–836. doi: 10.1210/endo-78-4-826. [DOI] [PubMed] [Google Scholar]
- CAHILL G. F., Jr, HASTINGS A. B., ASHMORE J., ZOTTU S. Studies on carbohydrate metabolism in rat liver slices. X. Factors in the regulation of pathways of glucose metabolism. J Biol Chem. 1958 Jan;230(1):125–135. [PubMed] [Google Scholar]
- DIPIETRO D. L., WEINHOUSE S. Hepatic glucokinase in the fed, fasted, and alloxan-diabetic rat. J Biol Chem. 1960 Sep;235:2542–2545. [PubMed] [Google Scholar]
- FEIGELSON P., MARKS P. A. Biosynthesis of liver and tumor RNA ribose in the tumor-bearing rat. Proc Soc Exp Biol Med. 1957 Jun;95(2):376–377. doi: 10.3181/00379727-95-23227. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. A preliminary investigation of the hormonal control of the hexose monophosphate oxidative pathway. Biochem J. 1955 Nov;61(3):390–397. doi: 10.1042/bj0610390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- HIATT H. H. Studies of ribose metabolism. II. A method for the study of ribose synthesis in vivo. J Biol Chem. 1957 Dec;229(2):725–730. [PubMed] [Google Scholar]
- HIATT H. H. Studies of ribose metabolism. V. Factors influencing in vivo ribose synthesis in the rat. J Clin Invest. 1958 Oct;37(10):1453–1460. doi: 10.1172/JCI103736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGINS C., YAO F. O. Influence of hormones on liver. I. Effects of steroids and thyroxine on pyridine nucleotide-linked dehydrogenases. J Exp Med. 1959 Dec 1;110:899–919. doi: 10.1084/jem.110.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACLEOD R. M., ALLEN M. S., HOLLANDER V. P. STUDIES ON THE INLUENCE OF MAMMO-SOMATOTROPIC TUMOR (MTTW5) ON THE METABOLISM OF MAMMARY TUMOR (MTW9) AND ADIPOSE TISSUE. Endocrinology. 1964 Aug;75:259–285. doi: 10.1210/endo-75-2-259. [DOI] [PubMed] [Google Scholar]
- MACLEOD R. M., SHANNONALLEN M., HOLLANDER V. P. HORMONAL REQUIREMENTS FOR THE GROWTH OF MAMMARY ADENOCARCINOMA (MTW9) IN RATS. Endocrinology. 1964 Aug;75:249–258. doi: 10.1210/endo-75-2-249. [DOI] [PubMed] [Google Scholar]
- MARSHALL N. B., ENGEL F. L. Growth hormone and carbohydrate tolerance in adrenalectomized rat. Proc Soc Exp Biol Med. 1960 Apr;103:743–745. doi: 10.3181/00379727-103-25656. [DOI] [PubMed] [Google Scholar]
- MacLeod R. M., Bass M. B., Huang S. C., Smith M. C. Intermediary metabolism in the liver and adipose tissue of rats with hormone-secreting pituitary tumors. Endocrinology. 1968 Feb;82(2):253–265. doi: 10.1210/endo-82-2-253. [DOI] [PubMed] [Google Scholar]
- McCann S. M., Dhariwal P. S., Porter J. C. Regulation of the adenohypophysis. Annu Rev Physiol. 1968;30:589–640. doi: 10.1146/annurev.ph.30.030168.003105. [DOI] [PubMed] [Google Scholar]
- McLean P., Brown J. Activities of some enzymes concerned with citrate and glucose metabolism in transplanted rat hepatomas. Biochem J. 1966 Mar;98(3):874–882. doi: 10.1042/bj0980874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P., Brown J., Walters E., Greenslade K. Effect of alloxan-diabetes on multiple forms of hexokinase in adipose tissue and lung. Biochem J. 1967 Dec;105(3):1301–1305. doi: 10.1042/bj1051301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEMEYER H., CLARK-TURRI L., GARCES E., VERGARA F. E. Selective response of liver enzymes to the administration of different diets after fasting. Arch Biochem Biophys. 1962 Jul;98:77–85. doi: 10.1016/0003-9861(62)90147-9. [DOI] [PubMed] [Google Scholar]
- Novello F., Gumaa J. A., McLean P. The pentose phosphate pathway of glucose metabolism. Hormonal and dietary control of the oxidative and non-oxidative reactions of the cycle in liver. Biochem J. 1969 Mar;111(5):713–725. doi: 10.1042/bj1110713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novello F., McLean P. The pentose phosphate pathway of glucose metabolism. Measurement of the non-oxidative reactions of the cycle. Biochem J. 1968 May;107(6):775–791. doi: 10.1042/bj1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARMA C., MANJESHWAR R., WEINHOUSE S. EFFECTS OF DIET AND INSULIN ON GLUCOSE-ADENOSINE TRIPHOSPHATE PHOSPHOTRANSFERASES OF RAT LIVER. J Biol Chem. 1963 Dec;238:3840–3845. [PubMed] [Google Scholar]
- Taketa K., Pogell B. M. The effect of palmityl coenzyme A on glucose 6-phosphate dehydrogenase and other enzymes. J Biol Chem. 1966 Feb 10;241(3):720–726. [PubMed] [Google Scholar]
- WENNER C. E. Oxidation of reduced triphosphopyridine nucleotide by ascites tumor cells. J Biol Chem. 1959 Sep;234:2472–2479. [PubMed] [Google Scholar]
- Walker D. G., Rao S. The role of glucokinase in the phosphorylation of glucose by rat liver. Biochem J. 1964 Feb;90(2):360–368. doi: 10.1042/bj0900360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Singhal R. L., Hird H. J., Furth J. Hepatic gluconeogenic enzymes, RNA metabolism and amino acid levels in rats carrying transplantable growth hormone-secreting pituitary tumors. Endocrinology. 1966 Nov;79(5):865–870. doi: 10.1210/endo-79-5-865. [DOI] [PubMed] [Google Scholar]


