Abstract
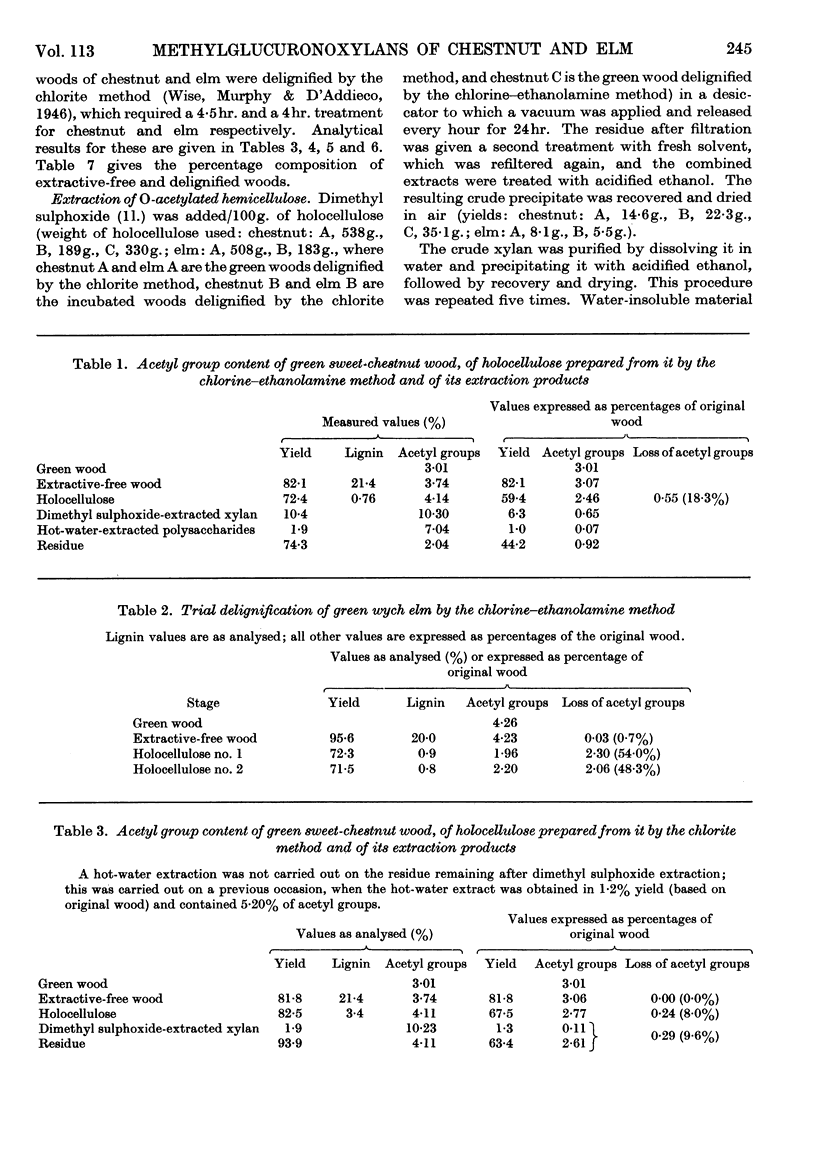
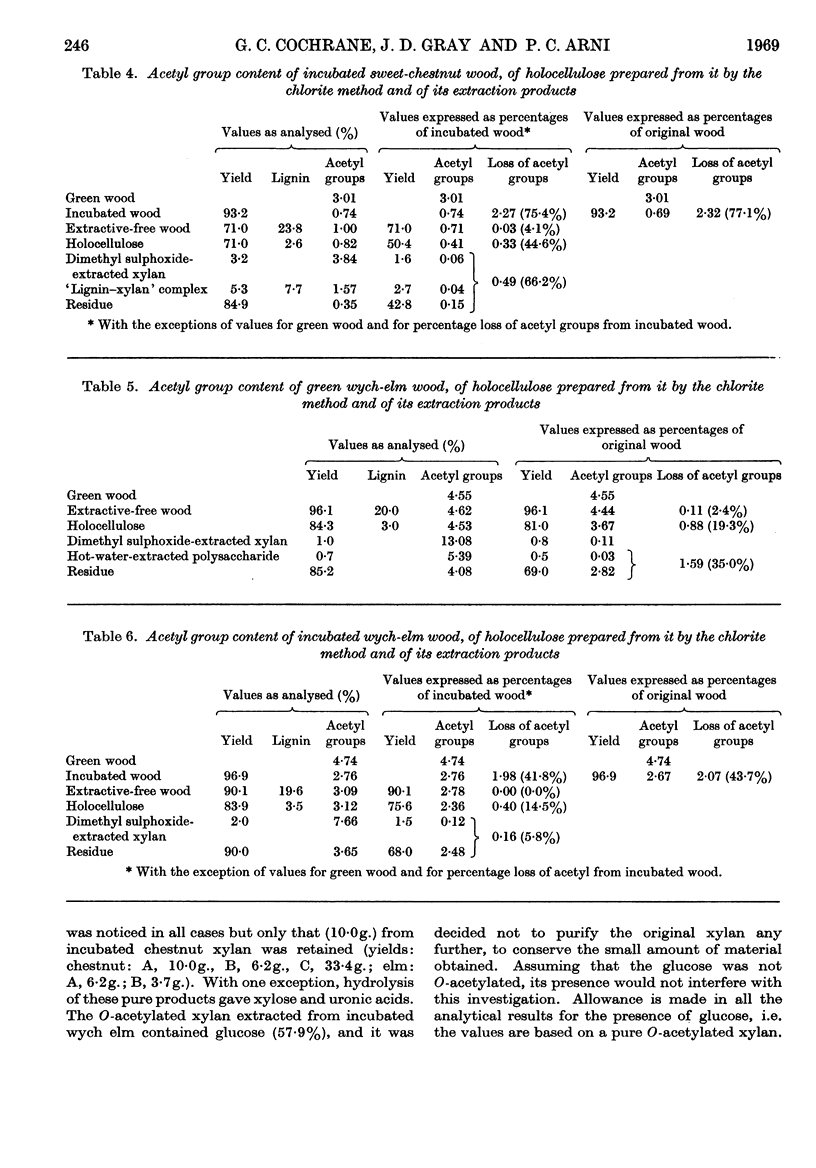
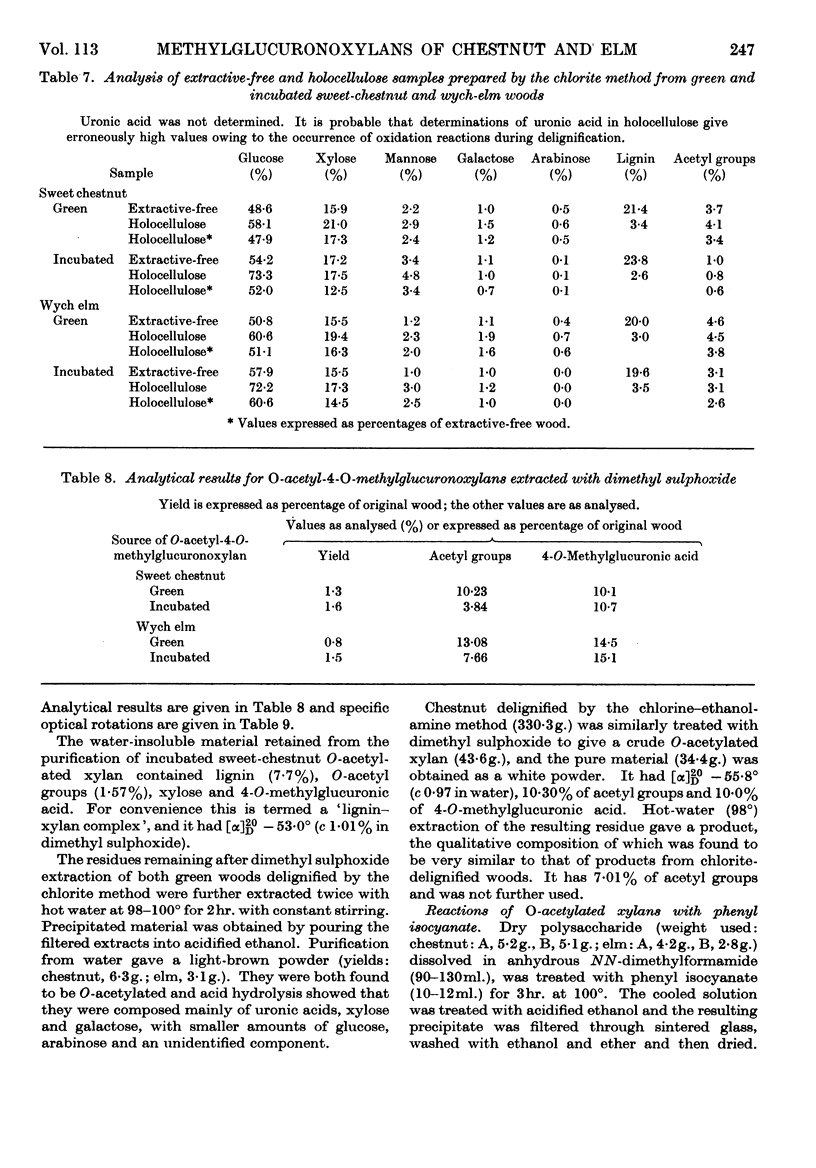
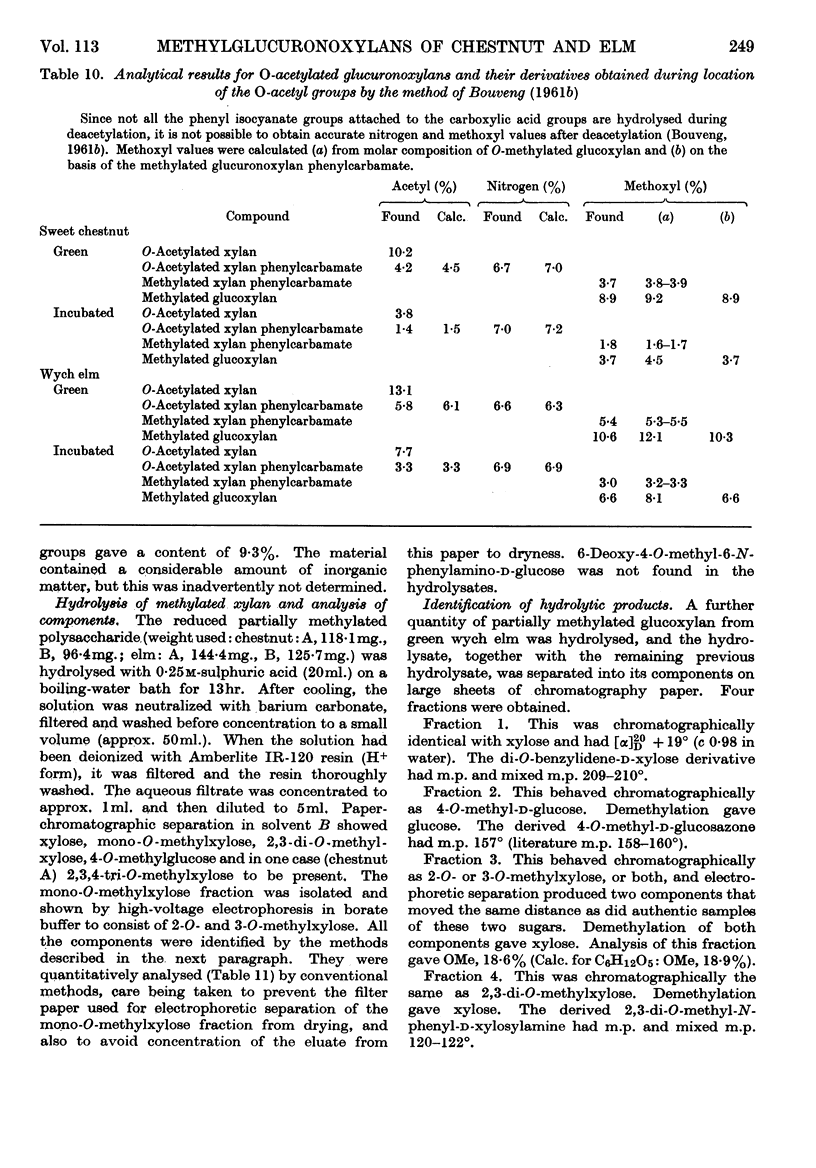
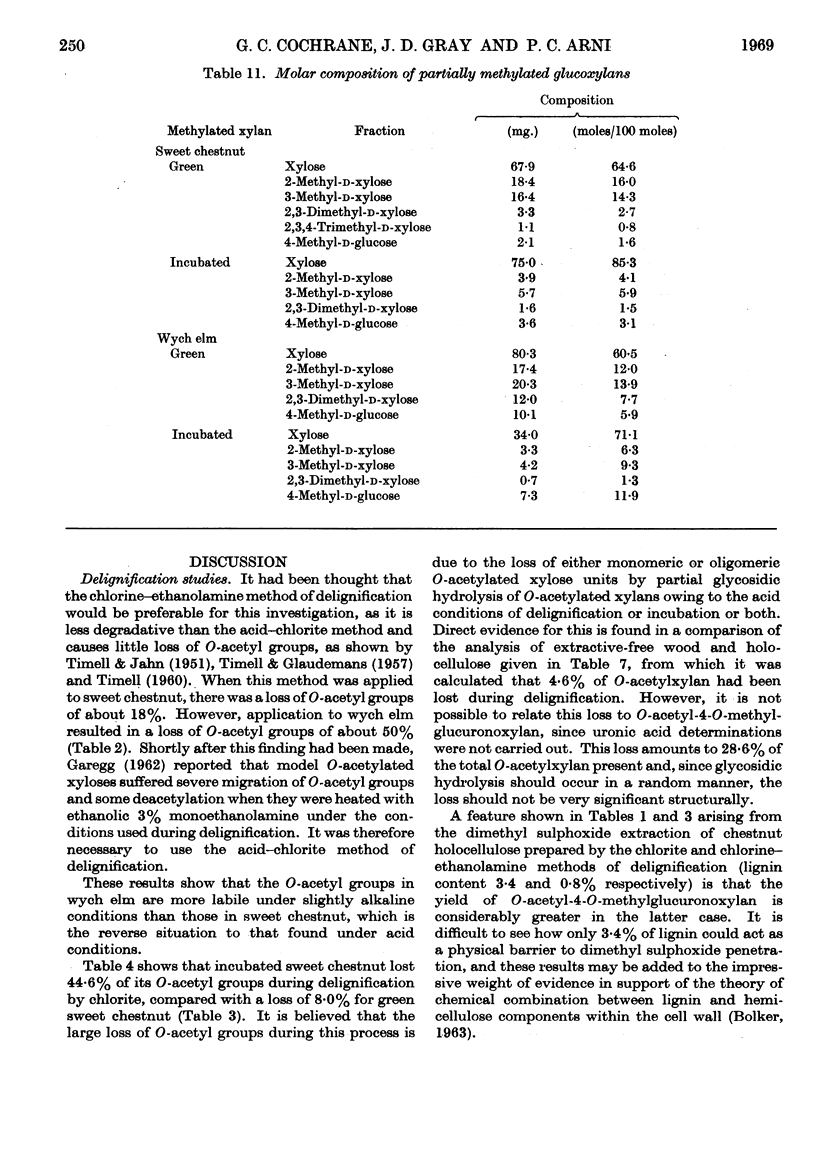
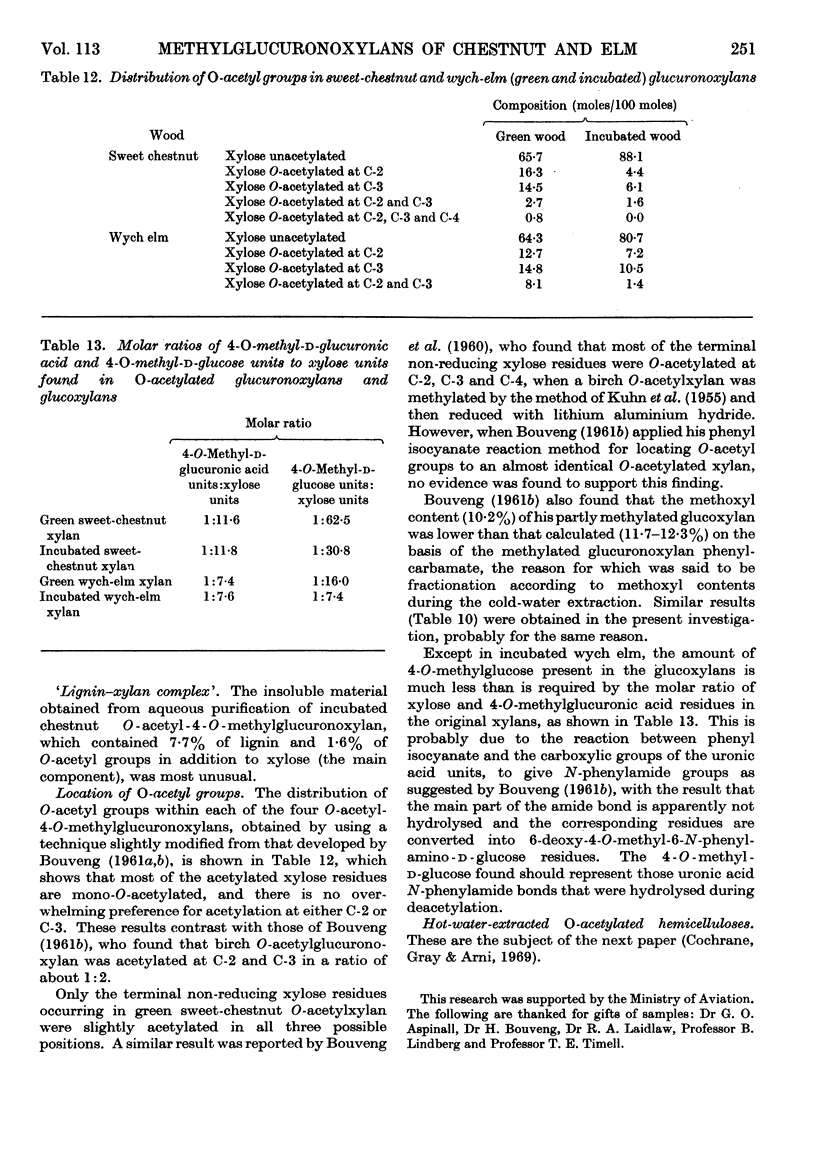
1. O-Acetylated 4-O-methylglucuronoxylans were isolated from sweet chestnut and wych elm, either green or incubated at 48° and 100% relative humidity for 36 weeks. 2. The chlorine–ethanolamine method of delignification resulted in a 50% loss of O-acetyl groups from green wych elm compared with an 18% loss from green sweet chestnut. 3. The acid–chlorite method gave an acceptable loss of O-acetyl groups in three cases, but incubated sweet chestnut showed a 44·6% loss. However, it is believed that this is due to the loss of simple O-acetylated xylose sugars resulting from glycosidic hydrolysis, rather than removal of O-acetyl groups by direct hydrolysis. Assuming that this occurs in a random manner, it is unlikely to have much structural significance. 4. Dimethyl sulphoxide extraction of chestnut holocellulose and elm holocellulose, green and incubated, yielded O-acetyl glucuronoxylans containing 10·2, 3·8, 13·1 and 7·7% O-acetyl groups respectively. 5. The location of these O-acetyl groups was determined by Bouveng's method in which phenyl isocyanate is used as a blocking group.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochrane G. C., Gray J. D., Arni P. C. The emision of corrosive vapours by wood. Hot-water-extracted O-acetylated hemicelluloses from sweet chestnut (Castnaea sativa) and wych elm (Ulmus glabrau) and a discussion of O-acetyl-group changes occurring in these woods during incubation at 48 degrees and 100 per cent relative humidity. Biochem J. 1969 Jun;113(2):253–257. doi: 10.1042/bj1130253. [DOI] [PMC free article] [PubMed] [Google Scholar]


