Abstract
1. The pH-dependence of the binding to pepsin of four dipeptide competitive inhibitors is reported. Values of Ki obtained from equilibrium-dialysis experiments agree closely with those from kinetic measurements. 2. The binding of uncharged N-acyl-dipeptide amides to pepsin is essentially independent of pH from 0·2 to 5·8. Values of Ki for the corresponding N-acyl-dipeptide acids rise rapidly above pH3·5, and depend on the ionization of a group of apparent pKa 3·6. 3. The data indicate that pepsin does not undergo any gross conformation change (at least none that affects binding) over the whole pH range of its catalytic activity. The pH-dependence of the dipeptide acid inhibitors indicates that the acid anions do not bind to pepsin, presumably because of electrostatic repulsion between the inhibitor anion and a negative centre at or near the active site of the enzyme. 4. The binding of all four stereoisomers of N-acetylphenylalanylphenylalanine, of the depside analogues of the l–l- and d–l-compounds and of N-acetylglycyl-l-phenylalanine and N-acetyl-l-phenylalanylglycine was studied at pH2·2. 5. These results throw further light on the binding specificity of pepsin and on the charge nature of the active site of this enzyme.
Full text
PDF



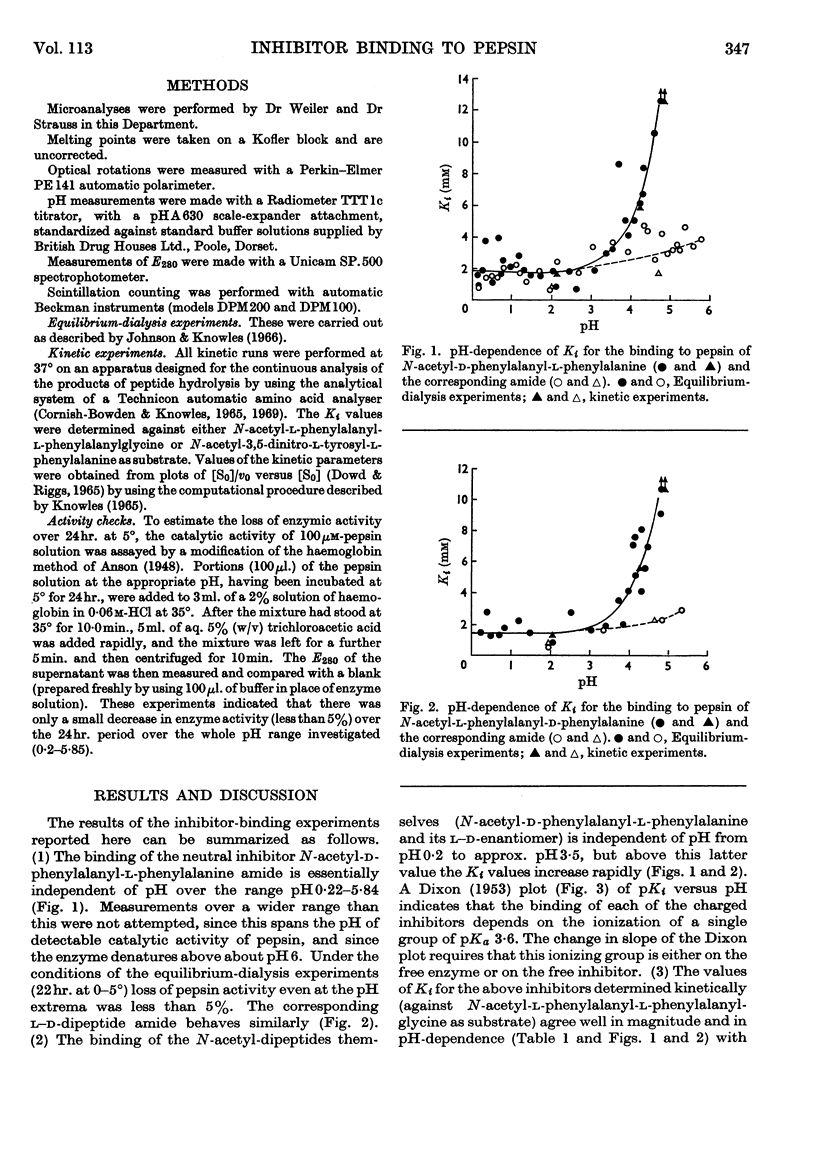
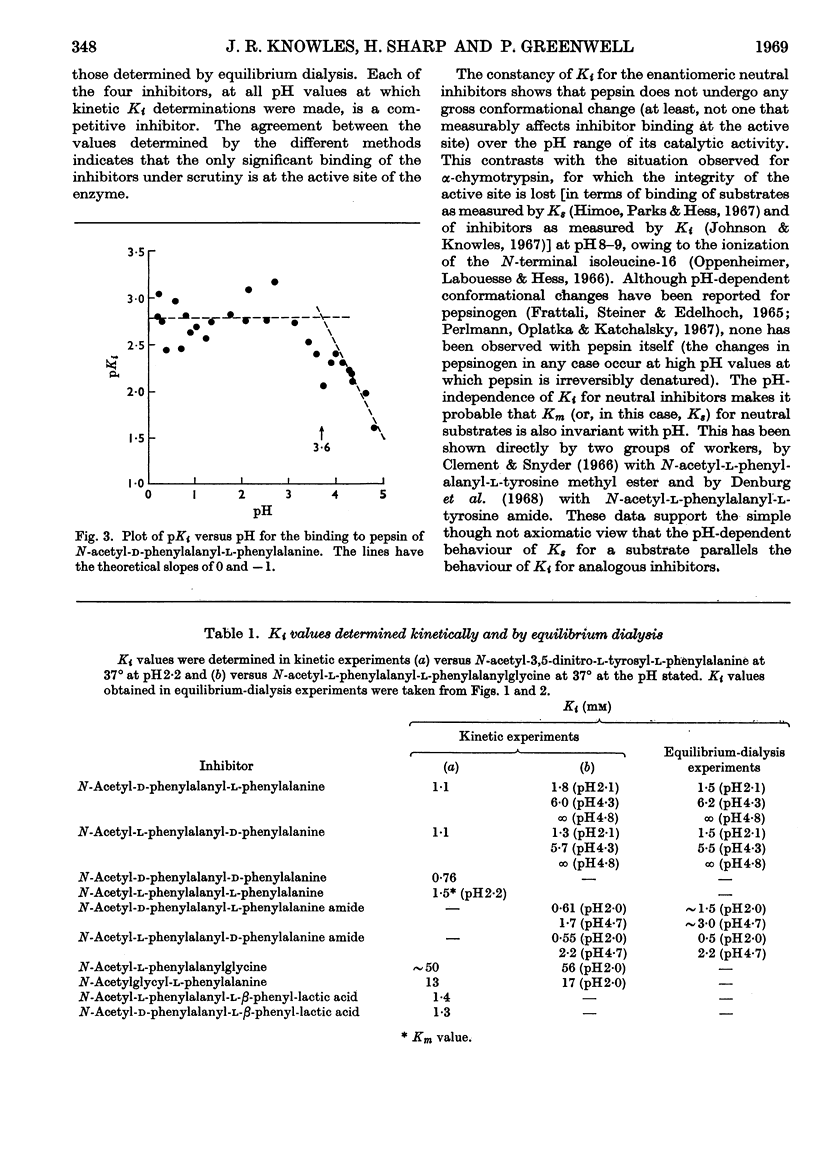
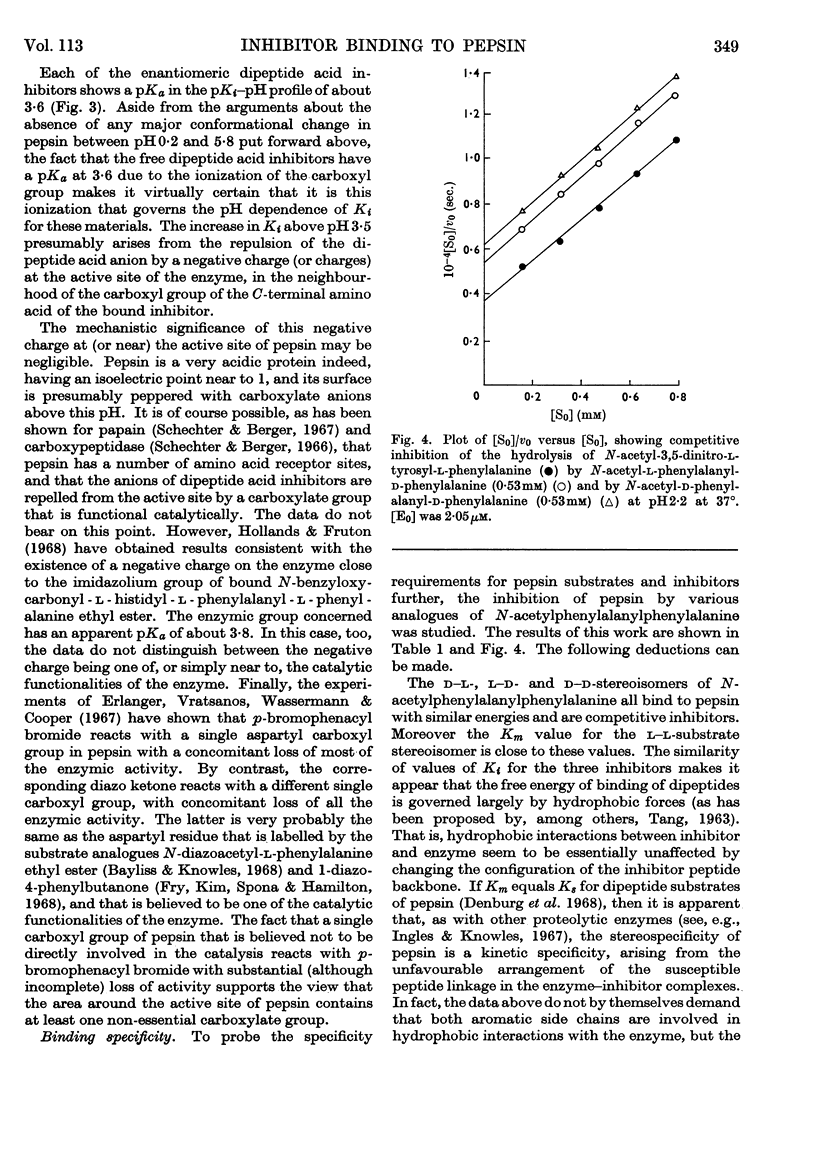


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornish-Bowden A. J., Knowles J. R. The pH-dependence of pepsin-catalysed reactions. Biochem J. 1969 Jun;113(2):353–362. doi: 10.1042/bj1130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J. 1953 Aug;55(1):161–170. doi: 10.1042/bj0550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Denburg J. L., Nelson R., Silver M. S. The effect of pH on the rates of hydrolysis of three acylated dipeptides by pepsin. J Am Chem Soc. 1968 Jan 17;90(2):479–486. doi: 10.1021/ja01004a046. [DOI] [PubMed] [Google Scholar]
- Erlanger B. F., Vratsanos S. M., Wassermann N., Cooper A. G. Stereochemical investigation of the active center of pepsin using a new inactivator. Biochem Biophys Res Commun. 1967 Jul 21;28(2):203–208. doi: 10.1016/0006-291x(67)90430-5. [DOI] [PubMed] [Google Scholar]
- FRATTALI V., STEINER R. F., EDELHOCH H. NATIVE AND UNFOLDED STATES OF PEPSINOGEN. I. THE MOLECULAR CONFORMATION IN WATER AND IN UREA. J Biol Chem. 1965 Jan;240:112–121. [PubMed] [Google Scholar]
- Fry K. T., Kim O. K., Kettering C. F., Spona J., Hamilton G. A. A reactive aspartyl residue of pepsin. Biochem Biophys Res Commun. 1968 Mar 12;30(5):489–495. doi: 10.1016/0006-291x(68)90078-8. [DOI] [PubMed] [Google Scholar]
- Himoe A., Parks P. C., Hess G. P. Investigations of the chymotrypsin-catalyzed hydrolysis of specific substrates. I. The pH dependence of the catalytic hydrolysis of N-acetyl-L-tryptophanamide by three forms of the enzyme at alkaline pH. J Biol Chem. 1967 Mar 10;242(5):919–929. [PubMed] [Google Scholar]
- Hollands T. R., Fruton J. S. Kinetics of the hydrolysis of synthetic substrates by pepsin and by acetyl-pepsin. Biochemistry. 1968 Jun;7(6):2045–2053. doi: 10.1021/bi00846a005. [DOI] [PubMed] [Google Scholar]
- Ingles D. W., Knowles J. R. Specificity and stereospecificity of alpha-chymotrypsin. Biochem J. 1967 Aug;104(2):369–377. doi: 10.1042/bj1040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye K., Fruton J. S. Pepsin as an esterase. J Am Chem Soc. 1967 Jan 4;89(1):187–188. doi: 10.1021/ja00977a062. [DOI] [PubMed] [Google Scholar]
- Jackson W. T., Schlamowitz M., Shaw A. Kinetics of the pepsin-catalyzed hydrolysis of N-acetyl-L-phenylalanyl-L-diiodotyrosine. Biochemistry. 1965 Aug;4(8):1537–1543. doi: 10.1021/bi00884a012. [DOI] [PubMed] [Google Scholar]
- Johnson C. H., Knowles J. R. The binding of inhibitors to alpha-chymotrypsin at alkaline pH. Biochem J. 1967 May;103(2):428–430. doi: 10.1042/bj1030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. H., Knowles J. R. The binding of inhibitors to alpha-chymotrypsin. Biochem J. 1966 Oct;101(1):56–62. doi: 10.1042/bj1010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOWLES J. R. THE ROLE OF METHIONINE IN ALPHA-CHYMOTRYPSIN-CATALYSED REACTIONS. Biochem J. 1965 Apr;95:180–190. doi: 10.1042/bj0950180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOKSHINA L. A., OREKHOVICH V. N., SKLYANKINA V. A. ESTERASE ACTIVITY OF PEPSIN. Nature. 1964 Nov 7;204:580–580. doi: 10.1038/204580a0. [DOI] [PubMed] [Google Scholar]
- Oppenheimer H. L., Labouesse B., Hess G. P. Implication of an ionizing group in the control of conformation and activity of chymotrypsin. J Biol Chem. 1966 Jun 10;241(11):2720–2730. [PubMed] [Google Scholar]
- Perlmann G. E., Oplatka A., Katchalsky A. Potentiometric titration and conformational change in pepsinogen. J Biol Chem. 1967 Nov 25;242(22):5163–5168. [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- TANG J. SPECIFICITY OF PEPSIN AND ITS DEPENDENCE ON A POSSIBLE 'HYDROPHOBICBINDING SITE'. Nature. 1963 Sep 14;199:1094–1095. doi: 10.1038/1991094a0. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M., Jr Separation and detection of organic acids on silica gel. Anal Biochem. 1965 Sep;12(3):571–578. doi: 10.1016/0003-2697(65)90224-1. [DOI] [PubMed] [Google Scholar]



