Abstract
1. The pH-dependence of the pepsin-catalysed hydrolysis of three peptide substrates was studied by using a method for the continuous monitoring of the formation of ninhydrin-positive products. 2. Two peptide acid substrates, N-acetyl-l-phenylalanyl-l-phenylalanine and N-acetyl-l-phenylalanyl-l-phenylalanyl-glycine, show apparent pKa values of 1·1 and 3·5 in the plots of k0/Km versus pH. By contrast a neutral substrate, N-acetyl-l-phenylalanyl-l-phenylalanine amide, shows apparent pKa values of 1·0 and 4·7. 3. Together with the data of the preceding paper (Knowles, Sharp & Greenwell, 1969), these results are taken to indicate that the rate of pepsin-catalysed hydrolysis is controlled by the ionization of two groups, which on the free enzyme have apparent pKa values of 1·0 and 4·7. It is apparent that the anions of peptide acid substrates are not perceptibly bound to the enzyme, resulting in apparent pKa values of 3·5 for the dependence of k0/Km for these materials.
Full text
PDF




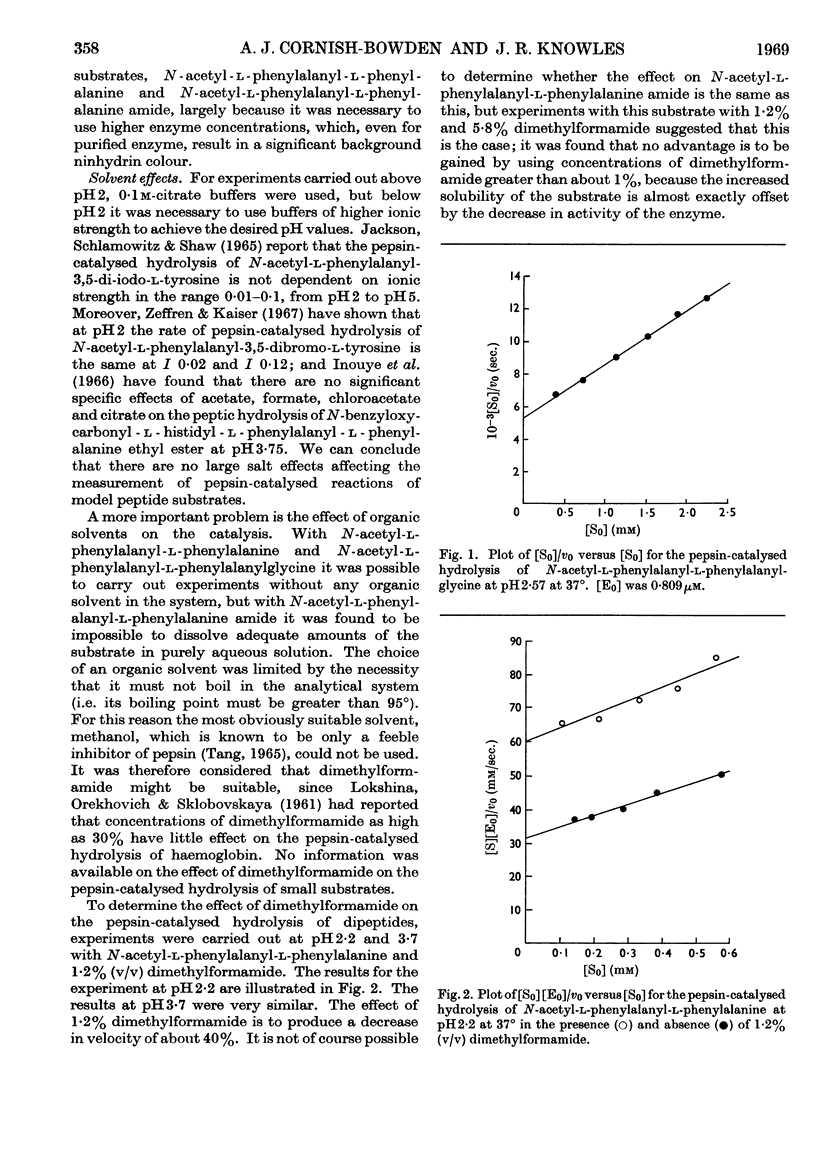
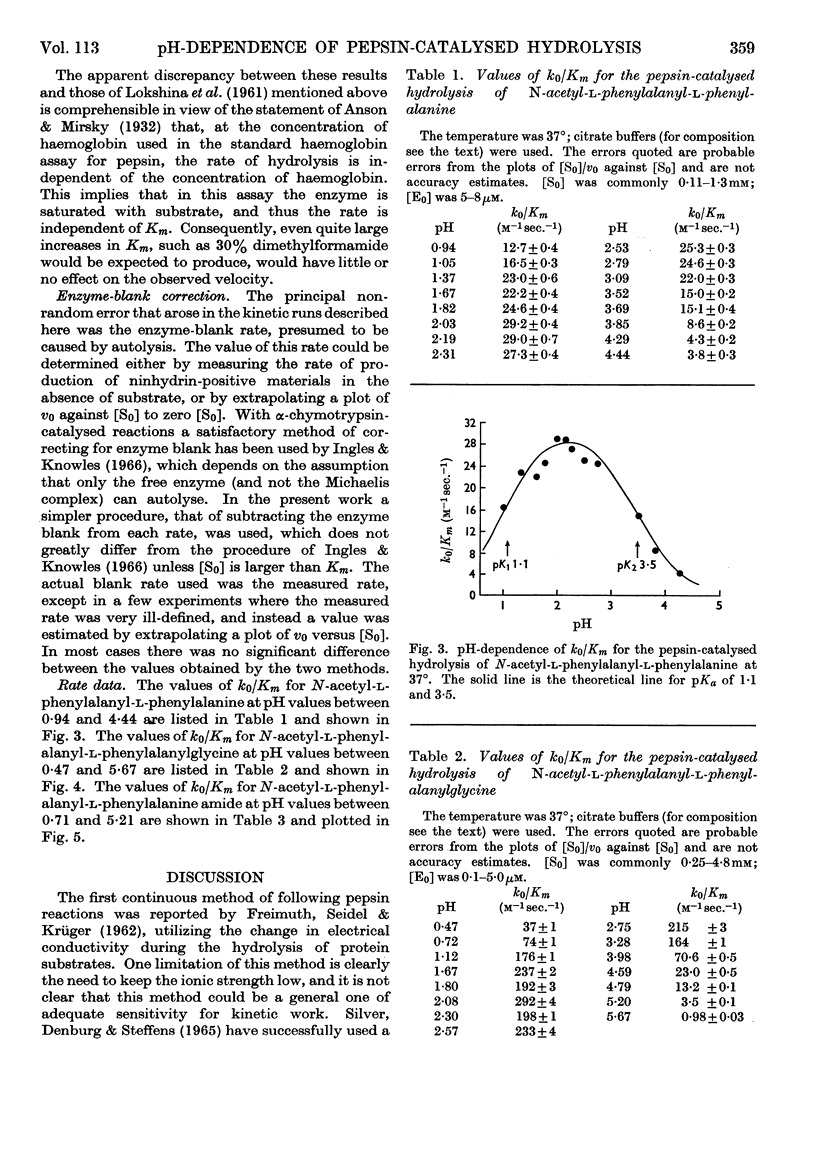
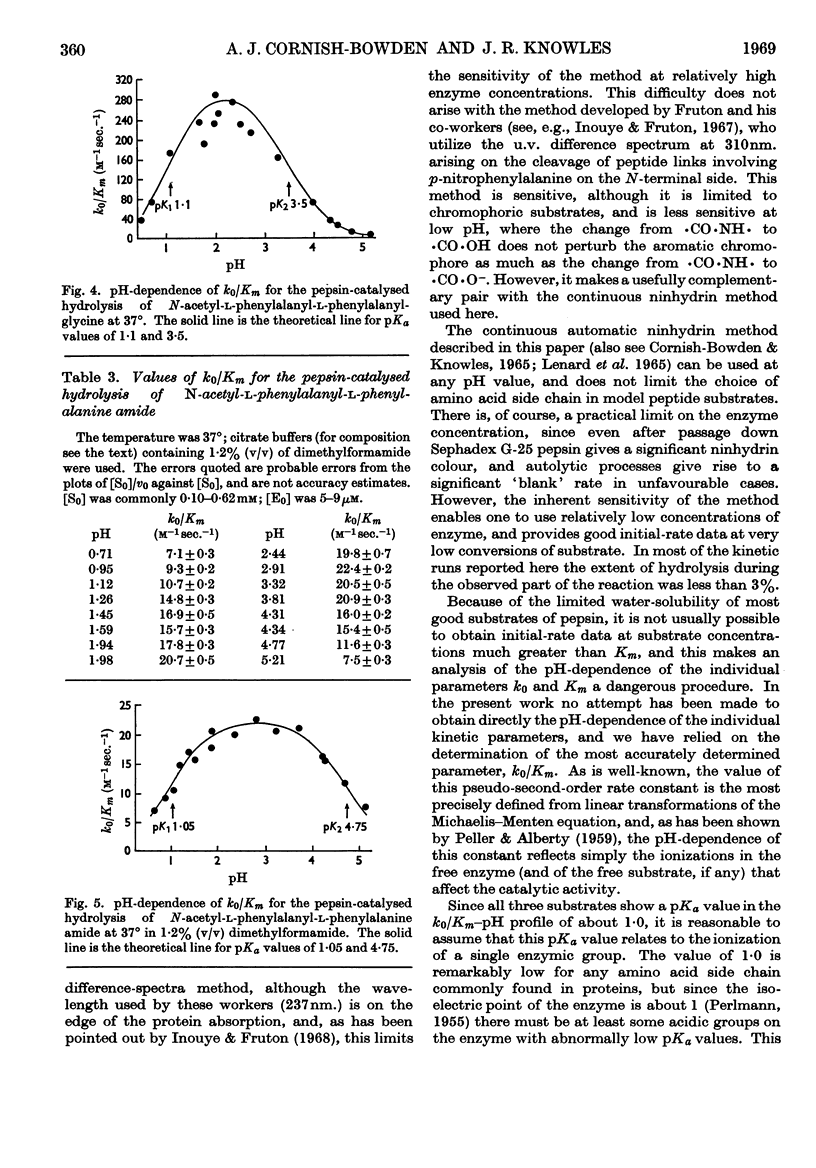


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMENFELD O. O., LEONIS J., PERLMANN G. E. The effect of guanidine hydrochloride on crystalline pepsin. J Biol Chem. 1960 Feb;235:379–382. [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Denburg J. L., Nelson R., Silver M. S. The effect of pH on the rates of hydrolysis of three acylated dipeptides by pepsin. J Am Chem Soc. 1968 Jan 17;90(2):479–486. doi: 10.1021/ja01004a046. [DOI] [PubMed] [Google Scholar]
- Ingles D. W., Knowles J. R. The alpha-chymotryptic ydrolysis of glycine esters. Biochem J. 1966 May;99(2):275–282. doi: 10.1042/bj0990275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye K., Fruton J. S. Studies on the specificity of pepsin. Biochemistry. 1967 Jun;6(6):1765–1777. doi: 10.1021/bi00858a027. [DOI] [PubMed] [Google Scholar]
- Inouye K., Fruton J. S. The inhibition of pepsin action. Biochemistry. 1968 May;7(5):1611–1615. doi: 10.1021/bi00845a001. [DOI] [PubMed] [Google Scholar]
- Inouye K., Voynick I. M., Delpierre G. R., Fruton J. S. New synthetic substrates for pepsin. Biochemistry. 1966 Jul;5(7):2473–2483. doi: 10.1021/bi00871a044. [DOI] [PubMed] [Google Scholar]
- Jackson W. T., Schlamowitz M., Shaw A. Kinetics of the pepsin-catalyzed hydrolysis of N-acetyl-L-phenylalanyl-L-diiodotyrosine. Biochemistry. 1965 Aug;4(8):1537–1543. doi: 10.1021/bi00884a012. [DOI] [PubMed] [Google Scholar]
- KNOWLES J. R. THE ROLE OF METHIONINE IN ALPHA-CHYMOTRYPSIN-CATALYSED REACTIONS. Biochem J. 1965 Apr;95:180–190. doi: 10.1042/bj0950180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. R., Sharp H., Greenwell P. The pH-dependence of the binding of competitive inhibitors to pepsin. Biochem J. 1969 Jun;113(2):343–351. doi: 10.1042/bj1130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENARD J., JOHNSON S. L., HYMAN R. W., HESS G. P. A CONTINUOUS, AUTOMATIC METHOD FOR THE STUDY OF RATE OF HYDROLYSIS OF PEPTIDES AND AMIDES. Anal Biochem. 1965 Apr;11:30–41. doi: 10.1016/0003-2697(65)90039-4. [DOI] [PubMed] [Google Scholar]
- Lee D., Ryle A. P. Pepsin D. A minor component of commercial pepsin preparations. Biochem J. 1967 Sep;104(3):742–748. doi: 10.1042/bj1040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko N. G., Ginodman L. M., Orekhovich V. N. Opredelenie konstant ionizatsii funktsional'nykh grupp aktivnogo tsentra pepsina. Biokhimiia. 1967 Mar-Apr;32(2):223–227. [PubMed] [Google Scholar]
- Oppenheimer H. L., Labouesse B., Hess G. P. Implication of an ionizing group in the control of conformation and activity of chymotrypsin. J Biol Chem. 1966 Jun 10;241(11):2720–2730. [PubMed] [Google Scholar]
- PERLMANN G. E. The nature of phosphorus linkages in phosphoproteins. Adv Protein Chem. 1955;10:1–30. doi: 10.1016/s0065-3233(08)60103-5. [DOI] [PubMed] [Google Scholar]
- SCHLAMOWITZ M., PETERSON L. U. Studies on the optimum pH for the action of pepsin on "native" and denatured bovine serum albumin and bovine hemoglobin. J Biol Chem. 1959 Dec;234:3137–3145. [PubMed] [Google Scholar]
- SILVER M. S., DENBURG J. L., STEFFENS J. J. SPECTROPHOTOMETRIC DETERMINATION OF THE KINETICS OF THE PEPSIN-CATALYZED HYDROLYSIS OF CERTAIN DIPEPTIDE SUBSTRATES. J Am Chem Soc. 1965 Feb 20;87:886–889. doi: 10.1021/ja01082a032. [DOI] [PubMed] [Google Scholar]
- SMITH E. L., SPACKMAN D. H. Leucine aminopeptidase. V. Activation, specificity, and mechanism of action. J Biol Chem. 1955 Jan;212(1):271–299. [PubMed] [Google Scholar]
- Tang J. Competitive inhibition of pepsin by aliphatic alcohols. J Biol Chem. 1965 Oct;240(10):3810–3815. [PubMed] [Google Scholar]
- Zeffren E., Kaiser E. T. The pH dependence of the pepsin-catalyzed hydrolysis of N-acetyl-L-phenylalanyl-L-3,5-dibromotyrosine. J Am Chem Soc. 1967 Aug 2;89(16):4204–4208. doi: 10.1021/ja00992a039. [DOI] [PubMed] [Google Scholar]


