Abstract
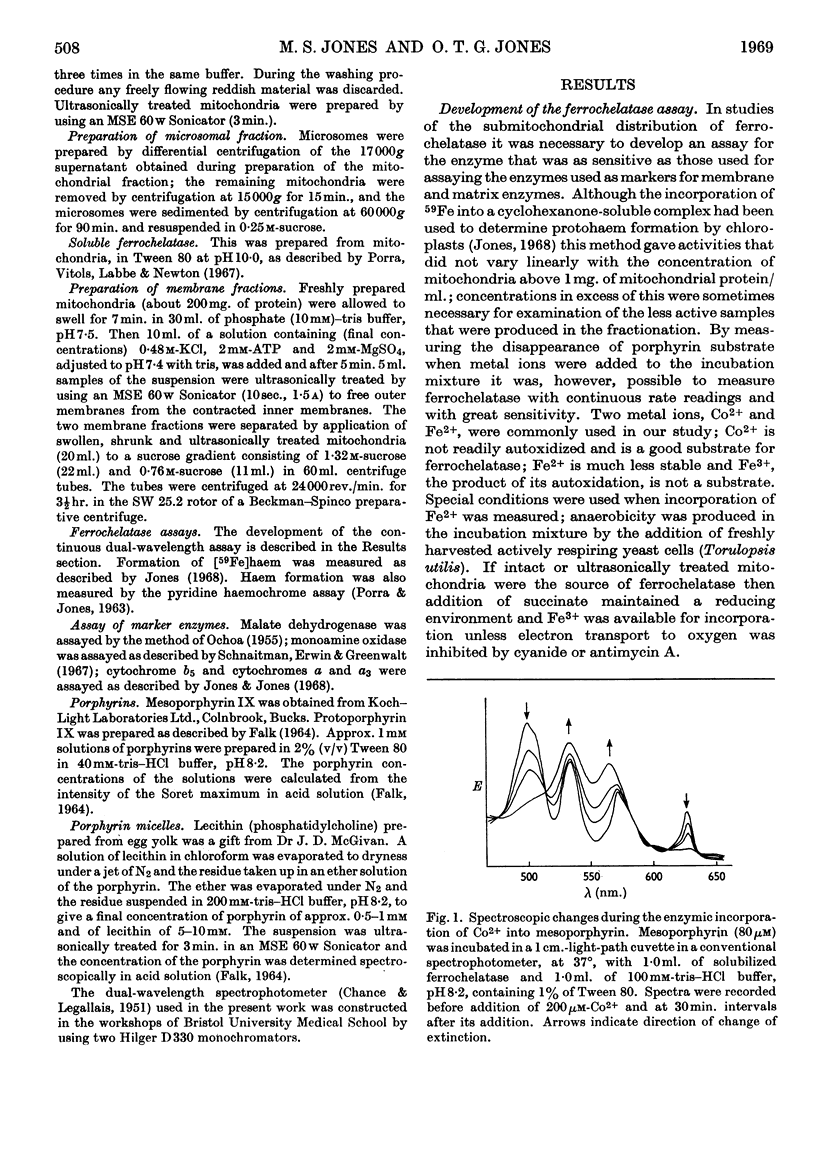
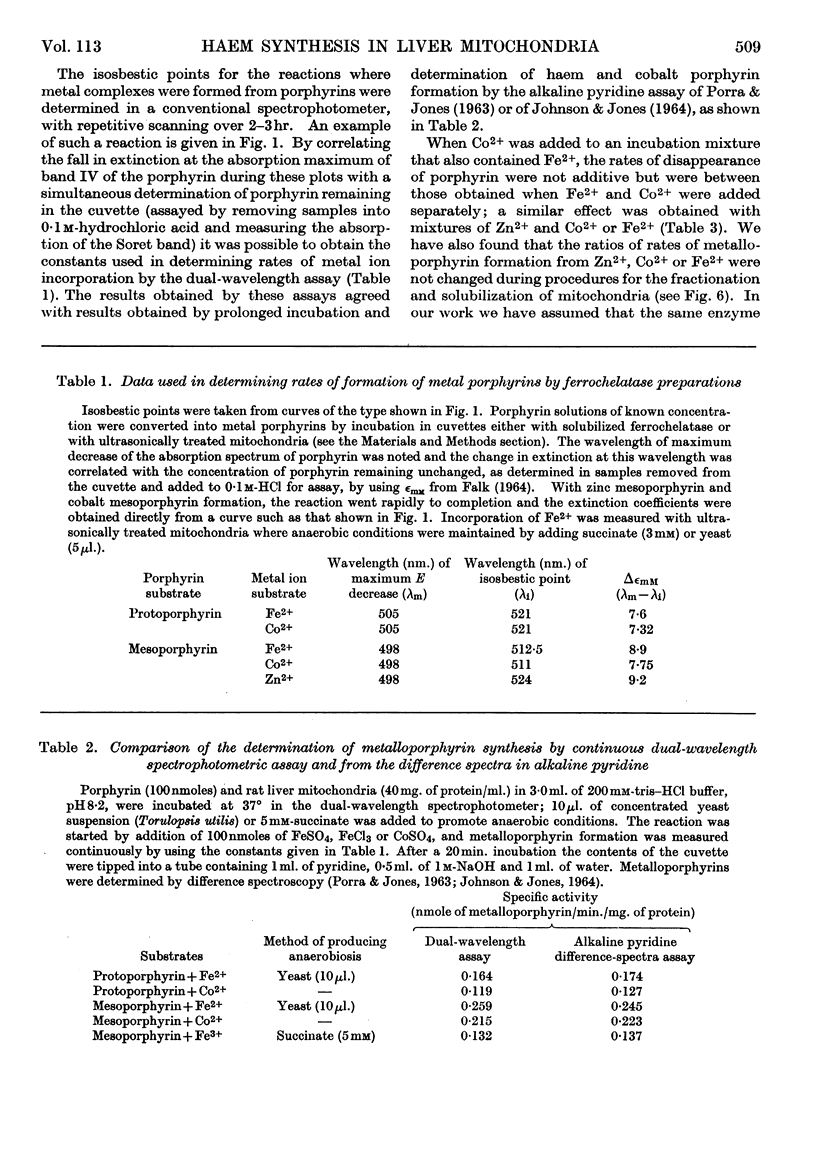
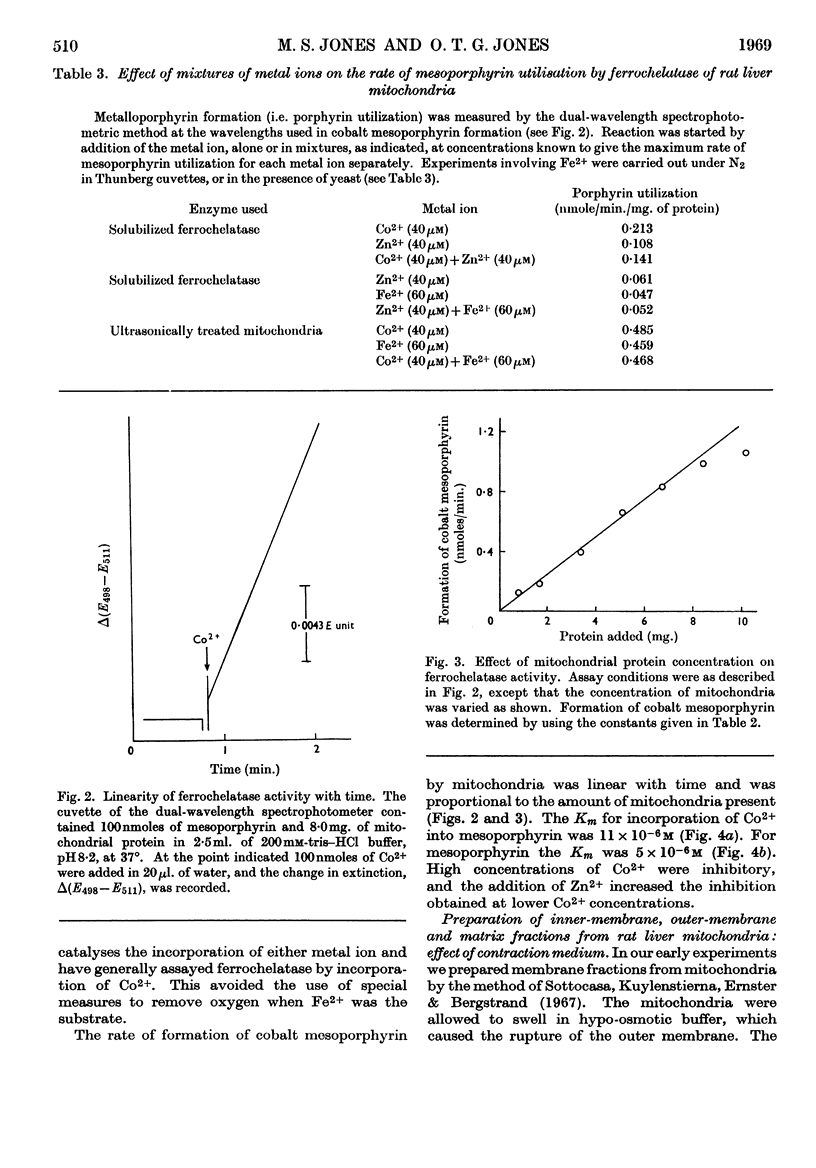
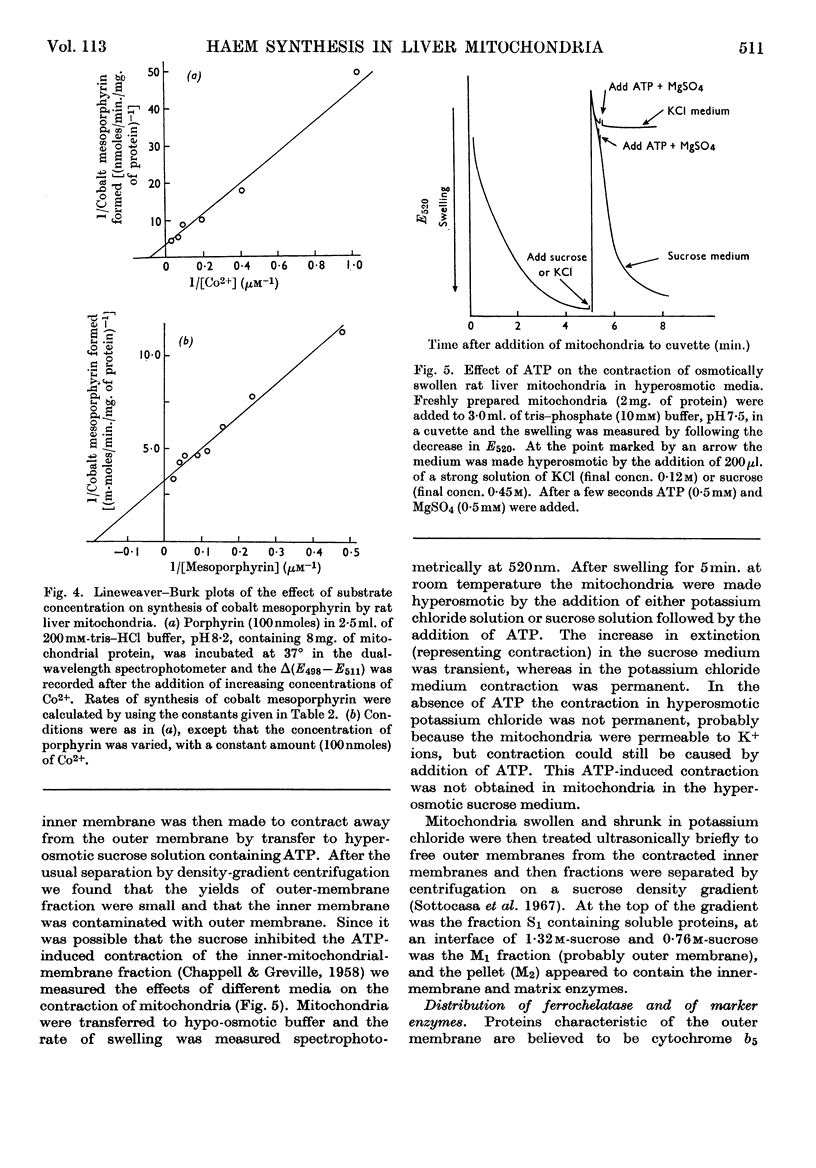
1. Anaerobic conditions are normally necessary for incorporation of iron into haems and only ferrous iron is used. After addition of succinate to an incubation mixture containing intact or ultrasonically treated mitochondria, Fe3+ is used, but only if no inhibitors prevent the transfer of electrons from the mitochondrial respiratory chain to oxygen. 2. A dual-wavelength spectrophotometric assay for ferrochelatase is described that has been used for the continuous assay of incorporation of metal ions into porphyrins. Constants are given for the determination of rates of formation of protohaem and cobalt protoporphyrin, mesohaem, cobalt mesoporphyrin and zinc mesoporphyrin. For cobalt mesoporphyrin formation the Km for Co2+ is 11×10−6m and that for mesoporphyrin is 5×10−6m. 3. An improved method for the separation of inner and outer membranes of mitochondria is described. Mitochondria swollen in hypo-osmotic media were contracted in hyperosmotic potassium chloride solution containing ATP and the outer membranes detached by mild ultrasonic treatment. Sucrose inhibited the ATP-induced contraction and decreased the yield of outer membranes. 4. Ferrochelatase is associated with cytochrome oxidase, which is used as a marker for inner mitochondrial membranes. 5. By using as substrate porphyrin dissolved in phospholipid micelles, ferrochelatase activity of intact mitochondria was shown to be latent, and to be liberated by ultrasonic treatment. 6. No ferrochelatase was detectable in microsomes or soluble cell components.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey E., Taylor C. B., Bartley W. Turnover of mitochondrial components of normal and essential fatty acid-deficient rats. Biochem J. 1967 Sep;104(3):1026–1032. doi: 10.1042/bj1041026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLETCHER M. J., SANADI D. R. Turnover of rat-liver mitochondria. Biochim Biophys Acta. 1961 Aug 5;51:356–360. doi: 10.1016/0006-3002(61)90177-9. [DOI] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- González-Cadavid N. F., Campbell P. N. The biosynthesis of cytochrome c. Sequence of incorporation in vivo of [14C]lysine into cytochrome c and total proteins of rat-liver subcellular fractions. Biochem J. 1967 Nov;105(2):443–450. doi: 10.1042/bj1050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON A., JONES O. G. ENZYMIC FORMATION OF HAEMS AND OTHER METALLOPORPHYRINS. Biochim Biophys Acta. 1964 Oct 9;93:171–173. doi: 10.1016/0304-4165(64)90273-9. [DOI] [PubMed] [Google Scholar]
- Jones O. T. Ferrochelatase of spinach chloroplasts. Biochem J. 1968 Mar;107(1):113–119. doi: 10.1042/bj1070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T., Yoda B., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. 3. Effects of triiodothyronine and hydrocortisone on the induction process. Arch Biochem Biophys. 1968 Aug;126(2):530–538. doi: 10.1016/0003-9861(68)90438-4. [DOI] [PubMed] [Google Scholar]
- NISHIDA G., LABBE R. F. Heme biosynthesis; on the incorporation of iron into protoporphyrin. Biochim Biophys Acta. 1959 Feb;31(2):519–524. doi: 10.1016/0006-3002(59)90028-9. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R. J., Vitols K. S., Labbe R. F., Newton N. A. Studies on ferrochelatase. The effects of thiols and other factors on the determination of activity. Biochem J. 1967 Aug;104(2):321–327. doi: 10.1042/bj1040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., SUTTIE J. W., WORK T. S. Protein synthesis in mitochondria. 2. Rate of incorporation in vitro of radioactive amino acids into soluble proteins in the mitochondrial fraction, including catalase, malic dehydrogenase and cytochrome c. Biochem J. 1962 Apr;83:29–40. doi: 10.1042/bj0830029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S., Nanzyo N., Rimington C. Synthesis of porphyrin c-type compounds from protoporphyrinogen. Biochem J. 1964 Nov;93(2):270–280. doi: 10.1042/bj0930270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]



