Abstract
Guanylate-binding proteins (GBPs) are interferon-inducible GTPases that confer protective immunity against a variety of intracellular pathogens. GBP2 is one of the two highly inducible GBPs, yet the precise mechanisms underlying the activation and regulation of GBP2, in particular the nucleotide-induced conformational changes in GBP2, remain poorly understood. In this study, we elucidate the structural plasticity of GBP2 upon nucleotide binding through crystallographic analysis. By determining the crystal structures of GBP2 G domain (GBP2GD) in complex with GDP and nucleotide-free full-length GBP2 with K51A mutation (GBP2K51A), we unveil distinct conformational states adopted by the nucleotide-binding pocket and distal regions of the protein. Comparison between the nucleotide-free full-length GBP2K51A structure with homologous structures reveals notable movement in the C-terminal helical region, along with conformational changes in the G domain. Through comparative analysis, we identify subtle but critical differences in the nucleotide-bound states of GBP2, providing insights into the molecular basis of its dimer-monomer transition and enzymatic activity. These findings pave the way for future investigations aimed at elucidating the precise molecular mechanisms underlying GBP2’s role in the immune response and open avenues for exploring how the unique functions of GBPs could be leveraged to combat pathogen invasion.
Subject terms: X-ray crystallography, Innate immunity
Using biophysical and crystallographic studies, the authors provide insight into the nucleotide-induced conformational plasticity of the interferon-induced guanylate-binding protein 2. These findings pave the way towards new therapeutic strategies to overcome pathogen invasion.
Introduction
Cell-autonomous immunity is the collective effector mechanisms that provide protection to both immune and non-immune cells and often induced by proinflammatory cytokines, especially interferons1. Among the thousands of genes induced by interferons is a prominent family of GTPases, which accounts for twenty percent of the total population of interferon-stimulated genes2,3. Interferon-inducible GTPases comprise four subfamilies: the 65 kDa guanylate-binding proteins (GBPs), the 47 kDa immunity-related GTPases (IRGs), the 72–82 kDa Myxovirus resistant (Mx) proteins, and the 200–285 kDa very large inducible GTPases (VLIGs/GVINs)2,3.
GBP proteins are widely spread in eukaryotes. A recent hidden Markov modeling revealed that there are 132 intact GBP genes across 32 taxa4. The human GBP family consists of seven members, GBP1-7, all located on chromosome 1. GBPs are composed of a G domain at the N-terminus and a helical region at the C-terminus. Prenylation of certain GBPs at their C-termini CaaX motif brings them to the membrane structures in cell5,6. GBPs are most closely related to dynamin family of GTPases that play critical roles in membrane remodeling2,3,7. Dynamin family GTPases undergo guanine nucleotide driven self-assembly to form large homotypic complexes8. Like dynamins, GBPs can homodimerize via their G domains, including both the small GTPase like region and the intermediate region, in a parallel fashion9–11. GBPs possess distinctive features, for example, GBP1 binds GTP, GDP, and GMP with similar affinities, yet it cannot hydrolyze external GDP to GMP12,13. However, the physiological importance of this nucleotide preference is unclear. Once GTP bound, GBP1 exhibits high intrinsic rates of GTPase and GDPase activity that occurs in a structurally conserved two-step reaction9. Interestingly, the GBP1-mediated GMP production is necessary for inflammasome activation, but dispensable for the restriction of Chlamydia trachomatis growth14. GBP1-mediated GMP production is also essential for its anti-HCV activity15.
Following interferon induction, GBPs confer immunity against a wide range of pathogens. Antibacterial defense was the first function tested across the complete mouse Gbp family16. Subsequent work in the following years established the role of GBPs in restricting apicomplexan parasites17 and certain viruses including human immunodeficiency virus (HIV) and Zika virus18, though the range of antiviral activity is still under characterization. Studies to identify the structures recognized by GBPs to target the pathogen niche have led to the discoveries that GBP5 in mouse macrophages recognizes lipid A moiety of Gram-negative LPS and human GBP1 recognizes LPS O antigen in lung epithelia infected by Shigella or Burkholderia4. GBP1 can encapsulate several cytosol invasive Gram-negative19–21 and Gram-positive pathogens, and has a non-selective nature regarding LPS detection16,22,23. Recently, it was shown that GBP2 can also recognize LPS, form LPS aggregates and lead to LPS dependent caspase-4 activation23. Altered self-ligands in the form of liberated intraluminal host ligands could also serve as proxies of infection, apart from conserved microbial structures24.
GBPs mediate host defense by initiating the assembly of platforms for caspase-4 recruitment and activation25,26, promoting the rupture of Salmonella-containing vacuoles27, and disrupting the structural integrity of bacteria, either alone or together with other interferon-inducible GTPases19,28,29, all of which lead to pyroptosis within the cell30. Out of the three members which are prenylated in the cell, GBP1, 2, and 5, GBP1 received the maximum attention in the last decade and is the most extensively studied, followed by GBP5. Available structures include full-length GBP1 in nucleotide-free and GMPPNP-bound forms31,32, GBP1 G domain (GBP1GD) in GMPPNP-, GDP∙AlF3-, GMP∙AlF4--, and GMP-bound forms9, full-length nucleotide-free GBP1 with farnesyl modification33, a truncated nucleotide-free GBP1 in complex with Shigella flexneri effector IpaH 9.833, a full-length GDP-bound GBP1 in complex with IpaH 9.834, nucleotide-free and GDP∙AlF3-bound forms of truncated GBP5, and GDP∙AlF3-bound form of GBP5GD10. Recently, the other members of the GBP family, including GBP2, 3, and 4, started gaining importance, when it was shown that GBPs form supramolecular complexes around bacterial surface25,26. GBP2 is one of the two most highly induced GBPs35 yet it is not well studied as GBP1 or GBP5. The only reported GBP2 structure is the full-length, nucleotide-free GBP2 structure published in 202110. Though human GBP1 and GBP2 share sequence identity of 76.3% and similarity of 91.1% (Supplementary Fig. 1), they differ in several noticeable aspects. GBP1 hydrolyzes GTP to GDP and then GMP in two quick successive steps9. While GBP2 is able to produce GMP as the end product, the efficiency is much less. Over 75% of GBP2 hydrolysis products is GDP36,37. This low efficiency has been attributed to sequence differences in GBP2 GTP-binding region37. GBP1 binds to GTP, GDP, and GMP in comparable affinities, but GBP2 binding to GMP is ten times lower than that of GTP or GDP36. Furthermore, GDP is a potent inhibitor of GTP hydrolysis by GBP2 but not GBP136. It is also unclear how GTP hydrolysis couples with GBP dimerization or resolves after GDP formation and release. Here, we employed crystallographic and biochemical tools to reveal the structural features that underlie the inhibited form of GBP2 and the reason that GBP2 shifts from dimer to monomer upon completion of GTP hydrolysis. Comparative analysis of GBP2 in different nucleotide-bound forms further illustrates the effect of nucleotide binding on not only the G domain, but also the C-terminal helical region.
Results
GBP2 forms dimer in solution upon GTP hydrolysis
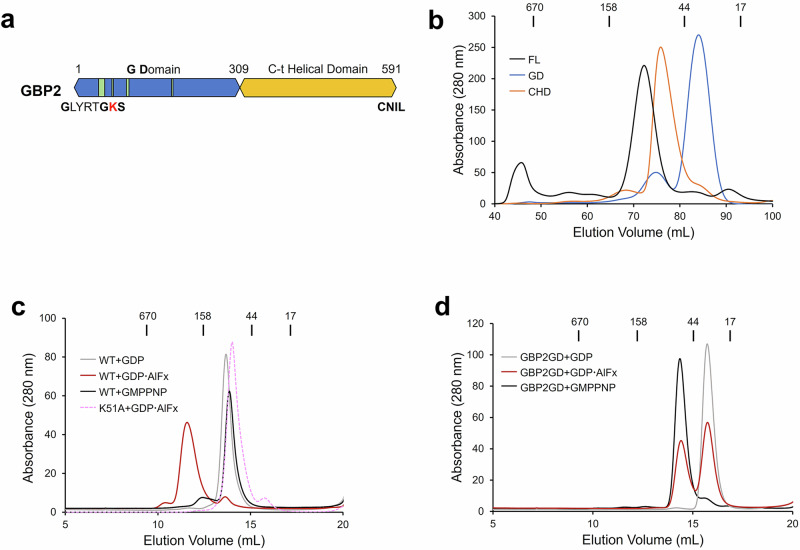
We expressed full-length human GBP2 (1–591) and GBP2 G domain (GBP2GD, 1-309) (Fig. 1a) in E. coli and purified the proteins to homogeneity. Judging from size-exclusion chromatography, nucleotide-free full-length GBP2 eluted exclusively as a monomer (Fig. 1b). Similarly, nucleotide-free GBP2GD eluted mostly as a monomer, but a noticeable population eluted at the position consistent with dimer formation (Fig. 1b). Since GBP1 and GBP5 had been shown to form dimers in solution38, we proceeded to examine whether and how GBP2 changes oligomeric status when bound to different guanine nucleotides. Binding to the nonhydrolyzable GTP analog GMPPNP or GDP retained GBP2 in monomeric form; however, binding to GDP∙AlFx, the GTP hydrolysis transition state mimic, shifted full-length GBP2 completely to an earlier, presumably dimer elution position (Fig. 1c, Suppl. Fig. 2a). We further confirmed the oligomeric status of GBP2 in different nucleotide binding states via multi-angle light scattering (MALS). Consistent with size-exclusion chromatography results, MALS showed that GBP2 was dimeric only in the presence of GDP∙AlFx, but not in GMPPNP-binding form or in nucleotide free form (Suppl. Fig. 2b-e). Dimerization depends on the nucleotide binding capability and GTPase activity of GBP2, as an active site mutant, K51A, remained monomeric when incubated with GDP∙AlFx (Fig. 1c). We then examined whether GBP2GD followed the same pattern of nucleotide binding and dimerization. Interestingly, GMPPNP binding induced full dimer formation of GBP2GD, yet more than half of GBP2GD remained monomeric even in the presence of excessive amount of GDP∙AlFx (Fig. 1d). The difference in dimerization patterns suggests that in full-length GBP2 the C-terminal helical domain either contributes additional dimerization interface or allosterically stabilizes GTP hydrolysis-induced GBP2 dimerization at the transition state.
Fig. 1. GBP2 forms dimer upon GTP hydrolysis in solution.
a Domain organization of GBP2. G domain is colored blue and C-terminal helical domain is colored orange. G motifs are represented by green vertical bars. G1 motif sequence is spelt out with active site lysine (K) colored in red. The last four residues CNIL in GBP2 constitute an isoprenylation site. b Size exclusion chromatography (SEC) profiles of full-length GBP2 (FL, black), GBP2 G domain (GD, blue) and C-terminal helical domain (CHD, orange) from a HiLoad 16/600 Superdex200 column. c SEC profiles of wild-type (WT) GBP2 bound to GDP (gray), GDP·AlFx (red), GMPPNP (black), and GBP2 K51A mutant incubated with GDP·AlFx (pink dash) from a Superdex200 10/300 column. d SEC profiles of GBP2GD incubated with GDP (gray), GDP·AlFx (red), and GMPPNP (black) from a Superdex200 10/300 column. In b–d, elution positions of protein standards with known MW are marked at the top.
Crystal structure of GBP2GD in complex with GDP reveals a closed active site
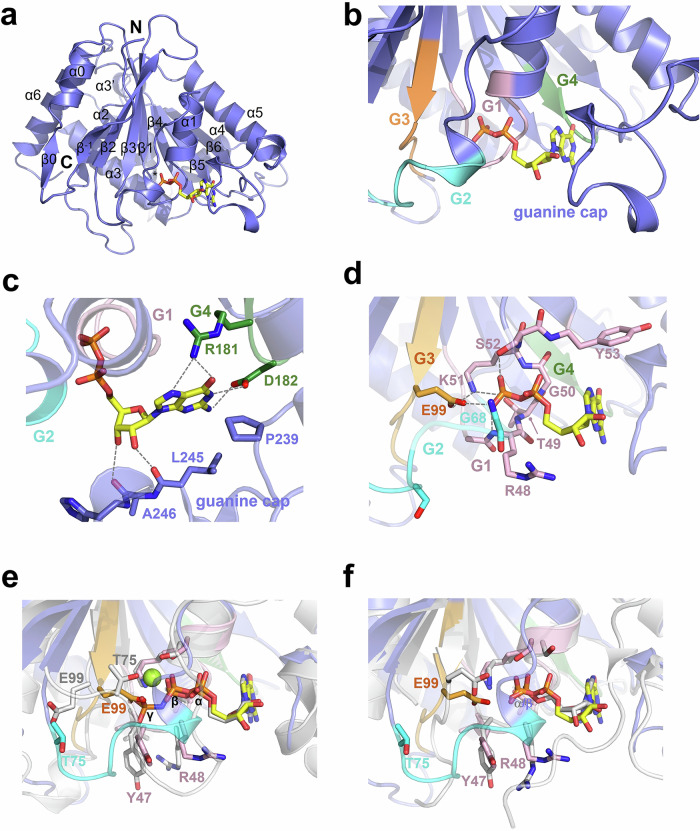
To gain molecular insight into GBP2 activity and dimerization, we determined a 2.1 Å crystal structure of GBP2GD in complex with GDP, which represents the post-hydrolysis, product bound state of GBP2 (Table 1). The overall structure of GBP2GD contains a mixture of seven beta sheets and seven alpha helices typical of the small GTPases with insertions belonging to the GBP family (Fig. 2a and Supplementary Fig. 1). The guanine base and ribose are enclosed in the space formed by the G1 motif, the G4/RD motif, and the guanine cap (aa 236–255) (Fig. 2b and Supplementary Fig. 1). The G4/RD motif interacts with the guanine moiety via hydrogen bonding. The O6 and N7 groups on the guanine base form dual hydrogen bonds with R181, and N1 and 2’-NH2 are both hydrogen-bonded to D182. P239 adds hydrophobic interaction to the pyrimidine side of the guanine rings. The 2’- and 3’-OH groups of the ribose are held in position by carbonyl oxygens of L245 and A246 in the guanine cap (Fig. 2c). At the other end of GDP, the diphosphate group fits snugly into the pocket formed by G1/P-loop, G2/Switch I, and G3/Switch II motifs. Aside from hydrogen bonding with mainchain amines of R48, T49, G50, K51, and S52, the β-phosphate is further elaborately coordinated by side chains of K51, S52, and interestingly, E99 (Fig. 2d, Supplementary Fig. 3). The E99 sidechain is 3.1 Å away from the K51 sidechain, forming a moderate hydrogen bond; in contrast, with a 4.6 Å distance, the two residues do not interact with each other in the nucleotide-free full-length GBP2 structure10. The interactions between the diphosphate group and GBP2 are further reinforced by the hydrogen bonds between the mainchain amines of Y53 and G68 and the α-phosphate (Fig. 2d). The nucleotide-binding pocket wraps so tightly around the diphosphate moiety that there is no space to accommodate a magnesium ion even when abundant magnesium was included during crystallization.
Table 1.
Crystallography data collection and refinement statistics
| GBP2GD·GDP | GBP2FLK51A | |
|---|---|---|
| Data collection | ||
| Space group | P 41 21 2 | C 2 2 21 |
| Unit cell (a, b, c, Å) (α, β, γ, °) |
87.951 87.951 105.279 90 90 90 |
59.631 140.569 240.079 90 90 90 |
| Resolution range (Å) | 45.17–2.105 (2.181–2.105)* | 45.27–2.91 (3.02–2.91)* |
| Multiplicity | 16.7 (10.4) | 9.4 (7.5) |
| Completeness (%) | 99.31 (93.11) | 98.81 (89.49) |
| Mean I/sigma(I) | 24.61 (1.42) | 12.16 (1.21) |
| R-merge | 0.244 (1.334) | 0.173 (1.594) |
| R-meas | 0.251 (1.404) | 0.182 (1.694) |
| Refinement | ||
| Reflections used in refinement | 24389 (2231) | 22383 (1984) |
| Reflections used for R-free | 2000 (183) | 1995 (178) |
| R-work | 0.2275 (0.3213) | 0.2451 (0.4230) |
| R-free | 0.2390 (0.3348) | 0.2736 (0.4278) |
| Number of non-hydrogen atoms | 2552 | 4729 |
| Macromolecules | 2394 | 4608 |
| Ligands | 28 | 5 |
| Solvent | 130 | 116 |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.017 | 0.011 |
| Bond angles, ° | 1.31 | 1.54 |
| Average B-factor | 54.09 | 100.55 |
| Macromolecules | 55.49 | 99.89 |
| Ligands | 46.08 | 112.01 |
| Solvent | 30.00 | 126.24 |
Values in parentheses are for the highest resolution shell. * one crystal for each structure.
Fig. 2. GBP2GD adopts a closed active site conformation when bound to GDP.
a Cartoon representation of GBP2GD·GDP structure with GDP shown as sticks. N- and C-termini of GD and the secondary structure elements are labeled. * indicates helix α4’ at the back of the structure. b Close-up view of GBP2GD active site when bound to GDP. G1, G2, G3, and G4 motifs are colored in pink, cyan, orange, and green, respectively. c Close-up view of guanine-ribose-binding space. Critical residues in G4 motif and guanine cap are shown as sticks and hydrogen bonds are represented by dashed gray lines. For clarity, cartoon representation of the rest part of the protein is partially transparent. d Close-up view of diphosphate-binding pocket. Crucial residues in G1, G2, and G3 motifs are shown as sticks, and hydrogen bonds with side chains are represented by gray dashed lines. G2 motif laying in front of β-phosphate is shown as transparent for clarity purpose. e Close-up view of superposed nucleotide binding pockets in GBP2GD·GDP structure and GBP1·GMPPNP structure (PDB ID: 1F5N). The residues that display large scale conformational change are shown as sticks with GBP2 residues colored as in (b) and GBP1 residues in gray. The magnesium ion in GBP1 structure is represented by a green sphere. f Close-up view of superposed nucleotide binding pockets in GBP2GD·GDP structure and GBP1GD·GMP structure (PDB ID: 2D4H, gray).
GBP2GD·GDP structure superposes well with that of homologous GBP1 in complex with GMPPNP (GBP1·GMPPNP, PDB ID 1F5N), and the GDP molecule occupies exactly the same space as the GDP portion in GMPPNP (Fig. 2e). Nonetheless, we noticed substantial conformational changes in the G2/Switch I region and a distal region on the opposite side of GD (Fig. 2e and Supplementary Fig. 4a), highlighting the two distinct states of GBP2 before and after GTP hydrolysis. In the GBP2GD·GDP structure, G2/Switch I region swings out prominently, with some Cαs moving more than 10 Å away from the bound nucleotide (Supplementary Fig. 4a)9,21,32. On the opposite side, the most prominent difference lies in the α3-α3’ region. While α3 remains in the same position but moves about 3 Å as a whole, α3’ swings ~40° from its equivalent position in the GBP1·GMPPNP structure. The N-terminal half of α4’ also rotates ~20°, resulting in 5–7 Å movement of corresponding residues. The last α helix in GD, α6, also shows slight movement at its C-terminus (Supplementary Fig. 4a).
G1 and G3 motifs remain relatively immobile at first look, but closer examination revealed large scale changes in side-chain orientations that result in the substantial difference in phosphate binding pocket. In the G1 motif, the side chains of Y47 and R48 swings about 70° from their outward orientations in GBP1·GMPPNP structure, pushing away the G2 motif to its current position in the GBP2GD·GDP structure (Fig. 2e). E99 in the G3 motif flips 120° inward, occupying the γ-phosphate position in the GMPPNP-bound structure (Fig. 2d, e). The negative charge of E99 side chain is only 2.6 Å away from the β-phosphate in GDP and 3.1 Å away from the G1 motif residue K51 (Fig. 2d, e). Movement of the G2/Switch I motif disrupts the conformation that is essential for magnesium coordination. In the GBP1·GMPPNP structure, the side chains of S52 in G1 motif and T75 in G2 motif hold the magnesium ion in place to coordinate with β- and γ- phosphates. In GBP2GD·GDP, T75 moves 7 Å away due to the prominent movement of G2 motif, no longer capable to coordinate magnesium ion together with S52 (Fig. 2e). Together with the swinging in of E99, the GDP-binding pocket can no longer accommodate a magnesium ion around diphosphate.
While GBP1 quickly hydrolyzes GTP to the final product of GMP, the major product of GBP2-mediated hydrolysis is GDP36,37. We then compared GBP2GD·GDP and GBP1GD·GMP structures to examine whether the product-bound structures of GBP1 and GBP2 share more similarities. Though the residues Y47, R48, and E99 display very similar orientations in the two structures (Fig. 2f, Supplementary Fig. 4b), G2 motif and guanine cap display substantial movement. In GBP2GD·GDP structure, the active site is more closed. G2 and the region immediately preceding it are ordered and closely packed into the diphosphate moiety. In the GBP1GD·GMP structure, the same region swings outwards and towards ribose moiety, not making contacts with the α-phosphate, which occupy almost the same position as the β-phosphate in the GBP2GD·GDP structure. Part of the G2 motif even becomes disordered and is not visible in the GBP1GD·GMP structure. At the opposite side, α3, α3’, and α4’ all show movement in the two structures, recapitulating the differences we observed between GBP2GD·GDP and GBP1·GMPPNP structures (Supplementary Fig. 4b).
GBP2 GD is monomeric when bound to GDP and partially dimeric when bound to GDP∙AlFx (Fig. 1d). We then set out to identify the structural features that account for the monomeric nature of GBP2GD·GDP. Superposing the GBP2GD·GDP structure onto the GBP1GD dimer structure (GBP1GD·GDP·AlF3, PDB ID 2B92) reveals conformations that are incompatible with dimer formation of GBP2GD in the GDP-binding state (Supplementary Fig. 4c). In the GDP-bound form, the region immediately following switch II (the region connecting β3 and α2) will clash with the N-terminus of α3 of the other protomer (Supplementary Fig. 4d). In addition, the G2/switch I region moves away from the dimer interface (Supplementary Fig. 4e), losing contacts with the loop between β5 and α4 of the other protomer. Together, conformational rearrangements in switch I and switch II regions dictate that once GTP is hydrolyzed to GDP, the majority of GBP2 can no longer form dimer.
Mutation of G motif residues abrogates GTPase activity of GBP2
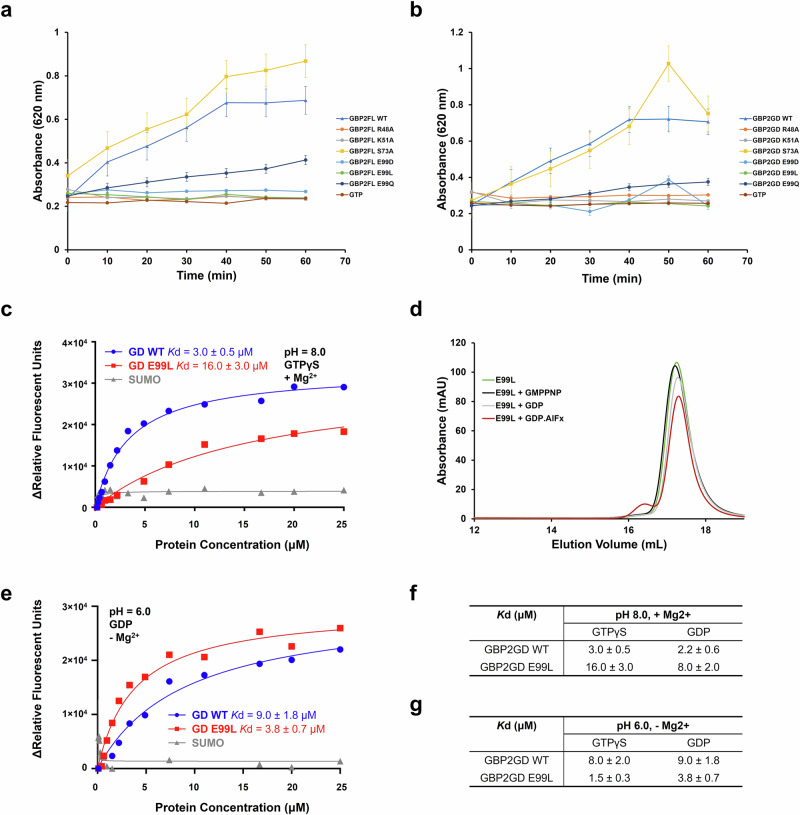
In the GBP2GD ∙ GDP crystal structure, several conserved G1 and G3 residues interact with the diphosphate moiety, including R48, K51, and E99 (Fig. 2d). To validate our observation in the crystal, we mutated the residues in both GBP2FL and GBP2GD and evaluated the activity of the mutants in comparison to wild-type proteins using the colorimetric malachite green assay. All proteins used for activity assays are tag-free. Individual mutation of G1 motif residues R48 or K51 to alanine (R48A and K51A) completely abrogated the GTPase activity of both GBP2FL and GBP2GD (Fig. 3a, b). As a control, mutation of a G2 residue S73 that points away from GDP in the structure (S73A) did not reduce the GTPase activity in GBP2 (Fig. 3a, b)9,10,39. We are particularly interested in the G3 motif residue E99 since its negative charge is positioned right next to the β-phosphate in the crystal structure (Fig. 2d). Moreover, E99 is conserved in the GBP family but not in other dynamin family GTPases (Supplementary Figs. 1 and 5a). Mutation of E99 to glutamine (E99Q) reduced GTPase activity to ~ 40% of the wild type but did not completely abolish it (Fig. 3a, b), suggesting that the negative charges on E99 are optimal for GTPase activity, but hydrogen bonding still functions at a lower level. Mutation of E99 to leucine or aspartic acid (E99L and E99D) both abrogated GBP2 activity (Fig. 3a, b), suggesting the negative charges of E99 must be precisely positioned in the active site for GTPase activity. We further investigated the GBP2GD E99L mutant to probe whether the mutation affects nucleotide binding. Using fluorescently labeled GTP and GDP analogs, we found that the E99L mutation reduced both GTP and GDP binding by 4–5 folds (Fig. 3c, Supplementary Fig. 5b). Interestingly, in the absence of Mg2+, the WT protein bound to GTP or GDP very weakly, yet the E99L mutant maintained a low-level affinity to both GTP and GDP, stronger than the WT (Supplementary Fig. 5c, d), indicating that E99 is important in the proper positioning of Mg2+ for high affinity binding of the guanine nucleotides. The E99L mutant did not dimerize in the presence of GMPPNP or the transition state mimic GDP·AlFx (Fig. 3d), suggesting deficiency in catalysis as well. Collectively, our results demonstrate that E99 is important in GTP binding and formation of the catalytic center.
Fig. 3. Mutation of critical G motif residues abrogates GTPase activity of GBP2.
a Comparison of the GTPase activities of wild-type (WT) and mutant full-length GBP2. b Comparison of GTPase activities of wild-type (WT) and mutant GBP2GD. The graphs shown in a and b are representatives of at least two independent experiments. The value plotted is an average of duplicate measurements in the same experiment. c Comparison of GTP binding affinities of GBP2GD WT (blue) and E99L mutant (red). The graph shown in c is representative of at least three independent experiments. d SEC profiles of GBP2GD E99L nucleotide free (green), or in the presence of GMPPNP (black), GDP (gray), or GDP·AlFx (red) on a Superdex 200 10/300 column. e Comparison of GDP binding affinities of GBP2GD WT and E99L mutant at pH 6.0, in the absence of magnesium ions. The graph shown in e is representatives of at least three independent experiments. f Table summarizing the GTPγS and GDP binding affinities of GBP2GD WT and E99L mutant at pH 8.0 in the presence of magnesium ions. g Table summarizing the GTPγS and GDP binding affinities of GBP2GD WT and E99L mutant at pH 6.0 in the absence of magnesium ions.
It is perplexing that Mg2+ is critical for GTP or GDP binding (Supplementary Fig. 5c, d), yet we do not observe any Mg2+ in the GBP2GD·GDP crystal structure (Fig. 2b, d). GBP2GD·GDP crystallized at pH ~6.0 (Supplementary Fig. 5e), prompting us to examine the behaviors of GBP2GD under acidic conditions. In the presence of Mg2+, GBP2GD WT binding affinities to GTP and GDP at pH 6.0 are comparable to those at pH 8.0 (Fig. 3c, f, Supplementary Fig. 5f, g, i). The E99L mutant, however, bound GTP and GDP with elevated affinities at pH 6.0 than at pH 8.0 (Fig. 3c, f, Supplementary Fig. 5f, g, i). While Mg2+ is essential for stable GTP and GDP binding at pH 8.0, at pH 6.0, both GBP2GD WT and E99L bound guanine nucleotides with detectable affinities even in the absence of Mg2+ (Fig. 3e, g, Supplementary Fig. 5h). The new set of nucleotide binding results clearly demonstrated that in solution, GBP2GD can bind to GDP in the absence of Mg2+ under acidic conditions, consistent with our observation in the crystal structure. At pH 8.0 and 6.0, GBP2GD WT binding to GDP is weaker in the absence of Mg2+ than in the presence of Mg2+ (Fig. 3e, f, Supplementary Fig. 5b, d, g, i), indicating removal of Mg2+ weakens the interaction between GBP2GD and GDP. In contrast, the E99L mutant binds to GDP with comparable affinities with or without Mg2+ at pH 6.0 (Fig. 3e, g, Supplementary Fig. 5g, i). The differences between WT and E99L proteins in GDP binding in the absence of Mg2+ suggest that E99 promotes GBP2 activity by facilitating GDP release after Mg2+ leaving the catalytic site.
When conducting GTPase assays, we noticed that at the same concentration, GBP2GD displayed slightly higher activity than GBP2FL (Supplementary Fig. 6a), a phenomenon also observed for GBP19. We then investigated whether the C-terminal helical domain (CHD) affects GD activity in trans. GBP2GD activity remained the same in the presence of increasing concentration of GBP2CHD (Supplementary Fig. 6b), suggesting that the isolated GBP2CHD does not interact with the GBP2GD to affect the GTPase activity in trans37.
Crystal structure of nucleotide-free full-length GBP2 with K51A mutation reveals structural plasticity of CHD
GBP2FL and GBP2GD manifested different dimerization patterns upon nucleotide binding but no drastic differences in GTP hydrolysis (Fig. 1c, d, and Supplementary Fig. 6a). We then structurally characterized GBP2FL to uncover any structural basis that accounts for such difference, in particular the effect of the C-terminal helical domain on the G domain. The expression level of full-length GBP2 was low, making structural studies difficult. However, mutation of the active site lysine to alanine (K51A) substantially increased GBP2 yield. We successfully crystallized the full-length GBP2 K51A mutant (GBP2K51A) and determined and refined the structure to 2.9 Å (Table 1). Though abundant GDP and magnesium ions were present during crystallization, no GDP or magnesium ion density was observed in the structure. The structure thus likely represents the nucleotide-free form of full-length GBP2. The GBP2K51A structure assumes an elongated shape, with the globular GTPase domain at one end and the C-terminal helical domain extending out for about 90 Å (Fig. 4a). The C-terminal helical domain is composed of seven α helices α7–α13 (Fig. 4a and Supplementary Fig. 1). α7, α8, and the N-terminal half of α9 loosely form one three-helix bundle, and the C-terminal half of α9, α10, and α11 form another three-helix bundle. The region consisting of α7 to α11, also known as the middle domain (MD)31, does not contact the G domain at all. The second-to-last alpha helix α12 folds back towards the GTPase domain, and the last helix α13 makes another about turn to reach towards the center of the helical region40,41. Together, the C-terminal ~15 residues of α12 and α13 make contacts with α3’ and α4’ on the GTPase domain, which localize on the opposite side of the nucleotide binding site. The GD:CHD interface is mostly hydrophilic. The most prominent electrostatic interaction is formed between E554 and E561 on α12 and R225 on α4’, E561 and K205 at the end of α4, and E554 and R231 on β6 (Fig. 4b, c, Supplementary Fig. 7a). In addition, E543 on α12 forms hydrogen bonds with the mainchain NH groups of G282 and G283 between the last two helices (α5 and α6) of the G domain, and the sidechain NH group of K551 on α12 forms a hydrogen bond with the mainchain carbonyl oxygen of A167 on α3’ (Supplementary Fig. 7b). A second patch of interaction is at the junction of GD and CHD, between α6 on the GD and the N-terminus of α7. In particular, E321 on α7 forms salt bridges with R290 on α6 (Supplementary Fig. 7c).
Fig. 4. Full-length GBP2 structure displays flexibility between the G domain (GD) and the C-terminal helical domain (CHD).
a Cartoon representation of full-length GBP2K51A structure. The G domain is colored blue as in Fig. 2. The C-terminal helical domain is colored yellow. Secondary structure elements in CHD are labeled. b The interactions between GD and CHD are mainly electrostatic. α12-α13 helices are shown as cartoon, while the GD is shown as the electrostatic surface. c A close-up view of the GD:CHD interface showing the major electrostatic interactions. Key residues are labeled. d Superposition of nucleotide-free GBP2K51A structure (blue and yellow) with nucleotide-free GBP1 structure (PDB ID: 1DG3, gray). Orientation of the molecules are rotated by 40 degrees compared to a to highlight the movement. The red arrow marks the movement of the far end of the CHD.
Overall, the GBP2K51A structure highly resembles the structure of wild-type GBP2 in the nucleotide-free form (PDB ID 7E58)10. Superposition of the two structures reveals an RMSD of 0.772 Å over 477 Cα (out of 545 common Cαs) (Supplementary Fig. 8a). The two GD domains superpose with an RMSD of 0.488 Å over 243 Cα (out of 276 common Cαs)10. The major deviation comes from the active site (Supplementary Fig. 9a). While the G1 motif displays modest structural variations, the G2/switch I region, G3/switch II region, and the guanine cap all show large scale movement (Supplementary Fig. 9a). Similarly, the GD domain in the GBP2K51A structure is highly similar to the GD structures in the two available full-length GBP1 structures, with RMSD values of 0.543 Å over 232 Cα (out of 268 common Cαs with nucleotide-free GBP1, PDB ID: 1DG3) and 0.628 Å over 238 Cα (out of 296 common Cαs, GMPPNP-bound GBP1, PDB ID: 1F5N). Structural deviations in the GD domains cluster in the G motifs and the guanine cap (Supplementary Fig. 9b, c), consistent with a flexible active site in the absence of substrates or products. The C-terminal helical domain, however, displays remarkable swinging movement. Compared to the nucleotide-free GBP1 structure, the distal end of the C-terminal helical region moves a striking 18 Å (Fig. 4d). While the N-terminal half of α7, the C-terminal third of α12, and the whole α13 superpose well with their counterparts in GBP1, tilting of the C-terminal helical region is collectively mediated by the varied bending in the middle of α7, tilting angle of the N-terminus of α9, and the bending in α12 around T530 (Fig. 4d). Indeed, the large-scale swinging movement of the C-terminal helical domain has been observed for full-length GBPs and GBP proteins lacking the last two α helices (Supplementary Fig. 8a–f)31–33. Superposing the G domain structure in GBP2K51A to the GBP2GD·GDP structure reveals conformational changes in the nucleotide binding pocket, in particular the G2/switch I region, the loop following the G3 motif, and the guanine cap (Supplementary Fig. 10a). In the GBP2K51A structure, the N-terminal portion of the G2 motif moves toward the guanine cap, pushing it outward. The G1 motifs in the two structures adopt very similar mainchain conformations, though the sidechains of Y47 and R48 point outward in the GBP2K51A structure (Supplementary Fig. 10b). The G2/switch I region, however, displays drastic rearrangement. In the nucleotide free GBP2K51A structure, the G2 motif moves outward, no longer able to support GDP binding (Supplementary Fig. 10c). Similarly, the G3 motif also slightly moves away from the nucleotide binding pocket (Supplementary Fig. 10d). Collectively, the nucleotide binding pocket in the GBP2K51A structure is more open than in the GBP2GD·GDP structure. On the opposite side, comparison of the two GBP2 structures reveals conformational changes in the α3-α3’ linker and α4’ region (Supplementary Fig. 10e), which likely contribute to the movement of the C-terminal helical domain.
Discussion
In this study, we overexpressed and purified human GBP2 from E. coli for structural and biochemical studies. The low yield of wild-type full-length GBP2 from E. coli may be the result of LPS-induced aggregation of GBP2, leading to its low solubility. Using recombinant proteins and guanine nucleotide mimics, we showed that GBP2FL is monomeric in both nucleotide-free and substrate-binding forms. It dimerizes at the transition state of GTP hydrolysis, then returns to the monomeric form when only GDP, the product, remains bound (Fig. 1c). GBP2GD, however, displays a different profile. GBP2GD is readily dimeric when bound to the substrate mimic GMPPNP but shows a ~50:50 split between dimers and monomers at the transition state (Fig. 1d). As GBP2GD and GBP2FL displayed comparable GTPase activity but contrasted in the dimer formation upon GTP hydrolysis (Fig. 1c, d, Supplementary Fig. 6a), we reason that the C-terminal helical domain does not allosterically regulate GD activity. Instead, the C-terminal helical domain further stabilizes the dimer mediated by GD, likely through a secondary dimerization interface. Indeed, a truncated dimeric GBP5 structure shows additional dimerization interfaces in the CHD when bound to GDP∙AlF310.
We did not observe any magnesium ion in the GBP2GD ∙ GDP structure even though 5 mM magnesium ions were present during crystallization. Magnesium ions only moderately increased GTP or GDP binding affinity to GBP2GD at acidic pH (Fig. 3e, g, Supplementary Fig. 5f–i); the crystal lattice may have further stabilized the magnesium-free environment, explaining the absence of magnesium ions in the active site in the crystal structure. As magnesium is essential for GBP2 activity (Supplementary Fig. 6c), we propose that the captured crystal structure represents the second to last step in GTP hydrolysis—magnesium ion already released, but GDP remains binding (Fig. 5a). The presence of GDP in the active site likely induces a low-energy conformation that precludes rebinding of magnesium ions. Such binding modes may explain the inability of GBP2 to hydrolyze GDP, even when GBP2 is capable of directly hydrolyzing a small portion of GTP to GMP36. Any released GDP intermediate will function more as an inhibitor than a substrate. GBP1 cannot utilize GDP as a substrate either, but GDP does not inhibit the hydrolysis of GTP to GMP, likely because GTP binds to GBP1 with higher affinity and is quickly hydrolyzed to GMP without releasing the GDP intermediate or the magnesium ion.
Fig. 5. Diagram of GBP2 conformational changes through a complete GTP hydrolysis cycle in solution.
a Conformational changes at the catalytic site through states 1–4. G1–4 motifs are colored pink, cyan, orange, and dark green as in Fig. 2. Key residues R48, K51, T75, and E99 are shown as sticks. Based on E99 orientations, the states are also labeled as E99 “in” or “out”. Guanine nucleotides and magnesium ions are represented as sticks and green spheres, respectively. The structures used are 7M1S (nucleotide free), 1F5N (GMPPNP-bound), 2B92 (GDP·AlFx-bound), and 6VKJ (GDP-bound). b Proposed full-length GBP2 conformational changes through GTPase cycle. GTP binding to the G domain (GD, blue sphere) in nucleotide-free GBP1 (state 1) prepares the dissociation of α12 from GD in the GMPPNP-bound form (state 2). GTP hydrolysis leads to GBP “cross-over” dimerization at the transition state. α12–α13 may assume orientations ranging from contact with the GD in the other protomer to extending out (represented by dashed outlines) (state 3). Release of the inorganic phosphate returns GBP2 to monomeric form (state 4). After GDP dissociation, GBP2 returns to the nucleotide-free state 1 for the next round of GTP binding and hydrolysis.
GBP2 belongs to the family of p67 GTPases with all the signature GTP binding motifs (Supplementary Fig. 1). Residue K51 resides in the G1 motif, also known as the P-loop (Supplementary Fig. 1), which contributes to nucleotide binding and positioning42,43. Mutation of lysine 51 to alanine (K51A) neutralized the GTP hydrolysis enzymatic activity and disrupted the nucleotide dependent oligomerization of GBP2 (Figs. 1c and 3a, b). R48, conserved in all GBP members, serves the purpose of the catalytic arginine finger, which usually is provided in trans by the GTPase activating proteins (GAPs) as in the case of Ras44. R48A mutation abolished the enzymatic activity of GBP2, in accordance with what has been observed for GBP1 previously45. In the crystal structure, the negatively charged side chain of G3 motif residue E99 points towards the β-phosphate of GDP, also negatively charged, seemingly creating a charge incompatibility. E99 also interacts with K51 through electrostatic interaction, likely weakening the interaction between K51 and the β-phosphate. We argue that this repulsion between the negative charges and weakening of K51 interaction both facilitate the release of GDP and prepares GBP2 for the next round of GTP hydrolysis. Indeed, mutating E99 to neutral glutamine reduced GBP2 activity by 60%, while the E99D or E99L mutation completely abolished GBP2 activity (Fig. 3a, b). The differential behaviors of WT and E99L proteins in binding GDP in the presence and absence of magnesium ions further corroborate the hypothesis that after the magnesium ion leaves the catalytic site, the charge repulsion between E99 sidechain and the β-phosphate favors the release of GDP (Fig. 3e–g, Supplementary Fig. 5b, d, g, i). Interestingly, when bound to its guanine nucleotide exchange factor (GEF) SOS, a Ras G3 motif glutamate is remodeled to interact with the G1 motif lysine. Other GEFs insert an acidic residue “glutamate finger” to the nucleotide binding pocket to destabilize magnesium ion and GDP46. E99 is conserved in all human GBPs, and in GBP1GD ∙ GMP structure E99 points toward the α-phosphate in GMP and K51 sidechain (Fig. 2f, Supplementary Fig. 4b). It is tempting to postulate that the conserved glutamate residue in GBP proteins assumes the “glutamate finger” role usually provided by GEFs in trans. The presence of R48 and E99 will then explain the intrinsically high GTPase activity of GBPs in the absence of GAPs or GEFs. However, our data showed that mutation of E99 affected both guanine nucleotide binding and formation of the transition state. More detailed experiments are needed to further clarify its role in GTP hydrolysis. We also noticed GBP2GD appeared to be slightly more active than full-length GBP2, yet the C-terminal helical domain (CHD) did not affect GBP2GD activity when added in trans (Supplementary Fig. 6a–c). However, given the salt bridge network between GD and CHD in both GBP1 and GBP240,41, it is likely that CHD has a regulatory role only in full-length GBP proteins47. Carefully conducted GTPase activity assays and nucleotide binding assays are necessary to further elucidate the role of CHD. Structures of full-length GBP2 bound to various guanine nucleotides or functional mutants are needed to validate our observations of the GBP2 G domain and to fully elucidate the conformational changes occurring through the GTP hydrolysis cycle.
Overall, our findings suggest a working model that GBP2 undergoes large scale conformational changes during GTP hydrolysis cycle (Fig. 5a, states 1–4, Supplementary Video 1). GTP binding and hydrolysis induces conformational changes in the G domain (GD), both in the nucleotide binding region and the α3, α3’, and α4 region on the opposite side of the nucleotide binding region (Fig. 5a, b). The K51-E99 interaction is closely linked to the repositioning of the switch I loop. When K51 and E99 interact, the switch I loop shifts outward, displacing T75 in the G2 motif away from the catalytic site. Disruption of this interaction allows the switch I loop to close in, positioning T75 to coordinate the magnesium ion essential for GTP binding and hydrolysis. Conformational changes in the GD enable GD dimerization. They are further transmitted to the CHD via the α3-α3’ linker, leading to α12 moving away from the GD (Fig. 5a, b). Dissociation of α12 from GD substantially extends the range of CHD movement, which favors the formation of a cross-over structure in the transition state10. A previous model suggested a possible conformation of full-length GBP2 dimer in the transition state, where α12 and α13 of CHD of one protomer interacts with the GD of the other protomer10 (Fig. 5b, state 3). However, no full-length dimeric GBP structure is available to substantiate such a closed dimer conformation. Recent studies showed that farnesylated GBP1 can attain an open conformation in the presence of the transition state analog, where the protein assumes an elongated shape with α12 and α13 stretching out instead of interacting with the GD (Fig. 5b, state 3)20,21. Such elongated structures are necessary for coating bacterial membranes as has been shown for GBP120–22. It is unclear whether farnesylation and interaction with bacterial membrane are essential for the formation of such elongated structures. We propose that in solution, α12 and α13 are capable of assuming any possible conformation in dimeric GBP2, from closely interacting with the GD of the other protomer to fully stretching out (Fig. 5b). Alternatively, the intrinsic flexibility of GBP proteins, in particular the α12-α13 region, may allow the dimer to adopt a more open dimer conformation mediated by the GD and the α13 helix, as shown by a recent integrative structural analysis on GBP148. Once the bound GTP is hydrolyzed, the inorganic phosphate is released, leading to a GDP-bound conformation that dissociates the GD-mediated GBP2 dimer. The final step of GDP release returns GBP2 to the nucleotide-free form, completes one cycle of GTP hydrolysis, and prepares GBP2 for the next round of GTP binding and hydrolysis (Fig. 5a, b, state 1).
Materials and methods
Molecular cloning and site-directed mutagenesis
Constructs of full-length human GBP2 (GBP2FL, 1–591), GBP2 G domain (GBP2GD, 1–309), GBP2 C-terminal helical domain (GBP2CHD, 310–591) were amplified from the cDNA (Accession number: BC073163) using standard polymerase chain reaction, digested with BamHI and SalI, and subcloned into a modified pRSFDuet-1 vector containing an N-terminal His6-SUMO tag. All mutants of GBP2FL and GBP2GD were generated by Agilent QuikChange Site-Directed Mutagenesis Kit following the manufacturer’s protocol. The primers used for cloning and mutagenesis are listed in Supplementary Table 1. GBP2GD mutants were generated using the same primers for the respective GBP2FL mutants. All constructs were sequenced (Eurofins) to ensure the correct sequences and open-reading frames.
Protein expression and purification
GBP2FL was overexpressed in Escherichia coli BL21-CodonPlus (DE3)-RIPL cells (Agilent). The cells were grown in LB broth containing 34 µg/ml chloramphenicol and 50 µg/ml kanamycin at 37 °C. At OD600 of 0.7, the cells were induced with 0.4 mM isopropyl β-d-1-thiogalactopyranoside (IPTG, GoldBio). Post induction, the cells were grown at 20 °C for 16–20 h. After incubation, the cells were harvested and resuspended in Lysis Buffer (50 mM sodium phosphate pH 7.4, 300 mM NaCl, 20 mM imidazole, 10% (v/v) glycerol, and 5 mM β-mercaptoethanol). The resuspended cell pellet was sonicated and centrifuged at 31,000×g at 4 °C for 45 min. The supernatant was loaded onto a gravity column containing Ni-NTA beads (Qiagen) equilibrated with Lysis Buffer. Ni-NTA beads were then washed with 10 column volumes of Wash Buffer (50 mM sodium phosphate pH 7.4, 300 mM NaCl, 30 mM imidazole, and 5 mM β-mercaptoethanol). Bound proteins on the beads were eluted with Elution Buffer (50 mM sodium phosphate pH 6.0, 300 mM NaCl, 10% (v/v) glycerol, and 300 mM imidazole). The eluted protein fractions were pooled and treated with the Ulp1 protease at 4 °C overnight to cleave the His6-SUMO tag. The overnight treatment also included 25 mM EDTA to remove any endogenous magnesium ion and guanine nucleotides. Concomitant with Ulp1 cleavage, the eluted proteins were dialyzed against Dialysis Buffer (20 mM Tris pH 8.0, 150 mM NaCl, and 5 mM β-mercaptoethanol) to remove imidazole. The cleaved His6-SUMO tag and uncleaved His6-SUMO-GBP2 were separated from tag free GBP2 by passing the solution over a His-Trap column (GE Healthcare/Cytiva). Tag free GBP2 in the flow-through was concentrated and subjected to size exclusion chromatography on a HiLoad16/600 Superdex 200 pg column (GE Healthcare/Cytiva) using the running buffer (20 mM Tris pH 8.0, 150 mM NaCl, and 5 mM β-mercaptoethanol). Peak fractions corresponding to the protein were pooled, concentrated, aliquoted, flash frozen in liquid nitrogen, and stored at −80 °C. GBP2GD and all mutants were expressed and purified in the same way.
Analytical size exclusion chromatography
Oligomerization states of GBP2FL, GBP2GD, and GBP2FLK51A were analyzed by size exclusion chromatography using a Superdex 200 increase 10/300 GL column (GE Healthcare/Cytiva). 20 µM protein was incubated at 4 °C overnight with 200 µM of the corresponding nucleotide. For the transition state intermediate, 300 µM AlCl3 (Sigma) and 10 mM NaF (Sigma) were added along with 200 µM GDP (guanosine 5’-diphosphate sodium salt, Sigma). The column was equilibrated with the running buffer containing 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 5 mM MgCl2 and 2 mM Tris (2-carboxyethyl) phosphine (TCEP, GoldBio).
Crystallization, structural determination, and refinement
Crystallization screening was carried out using a Crystal Gryphon Crystallization Robot (Art Robbins Instruments) with 96-well intelli 3 well crystallization plate (Hampton Research). 0.3 µl protein solution was mixed with 0.3 µl reservoir solution and was set up as a sitting drop with 100 µl reservoir solution at 16 °C. For crystal optimization, 1 µl of protein was mixed with 1 µl of reservoir solution. Both GBP2GD·GDP and GBP2FLK51A crystals were obtained by hanging drop vapor diffusion method at 16 °C. GBP2GD at 13 mg/ml was incubated overnight with 10-fold molar excess of GDP and 5 mM MgCl2 before mixing with the reservoir solution of 0.04 M KH2PO4, 12% PEG 8000, and 18% glycerol. GBP2FLK51A at 12.1 mg/ml was mixed with the reservoir solution of 0.7 M NaH2PO4, 1.05 M K2HPO4, and 0.1 M sodium acetate pH 3.8. Crystals were flash frozen in respective reservoir solution supplemented with glycerol to the final concentration of 30% (v/v) as the cryoprotectant.
Diffraction data were collected at National Synchrotron Light Source II (NSLS II) beamline AMX 17-ID-1 at the wavelength of 0.920126 Å. Diffraction data were indexed, integrated, and scaled using HKL200049. Both GBP2GD·GDP and GBP2FLK51A structures were solved by molecular replacement using Phenix50. The search model for GBP2GD·GDP is the structure of GBP1GD·GMP (PDB ID 2D4H), while the search model for GBP2FLK51A is the nucleotide free GBP1 structure (PDB ID 1DG3). Further iterative modeling building and refinement were carried out using Phenix and Coot50,51. The favored, allowed, and outliers in the GBP2GD·GDP Ramachandran plot are 97.03, 2.97, and 0.00%. The favored, allowed, and outliers in the GBP2FLK51A Ramachandran plot are 93.91, 5.91, and 0.17%. The RMSD values of the aligned α carbon atoms (Cαs) in the two structures were automatically calculated by The PyMOL Molecular Graphics System, Version 2.5 Schrödinger, LLC.
GTPase activity assay
For measuring the GTPase activity, malachite green phosphate detection kit (Sigma, Cat No. MAK307) was used. Unless otherwise specified, 500 nM GBP2FL or 300 nM GBP2GD protein (wild type or mutant) was mixed with 200 µM GTP (Sigma, Cat No. G8877) in a reaction buffer containing 20 mM Tris pH 8.0, 150 mM NaCl, 8 mM MgCl2 and 1 mM EDTA at room temperature. Aliquots were taken out at indicated time points up to 60 min and the GTP hydrolysis reaction was stopped by 25 mM EDTA. Reagent A and Reagent B from the kit were mixed in 100:1 ratio. 20 μL of the prepared reagent was mixed with 80 μL of EDTA-stopped reaction mixture, added to 96 well clear plate, and incubated at room temperature for 30 min for malachite green color change to develop. Absorbance was read in the plate reader (SpectraMax iD5, Molecular Devices) at 620 nm.
Multi-angle light scattering (MALS)
MALS experiments were performed at SIBYLS beamline 12.3.1 at the Advanced Light Source. Separation of analytes was accomplished by size-exclusion chromatography (SEC) using an Agilent 1260 series HPLC with a Shodex 830 analytical column at a flow rate of 0.65 ml/min using 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 5 mM MgCl2 and 2 mM Tris (2-carboxyethyl) phosphine (TCEP, GoldBio). The eluent from SEC was measured in line with MALS. The experiments used an 18-angle DAWN HELEOS II light scattering detector connected with an Optilab refractive index concentration detector (Wyatt). A 55 μl sample of 7 mg ml−1 BSA monomer in the buffer noted above, and a refractive index increment (dn/dc) value of 0.18, was used for system normalization and calibration. We used light scattering experiments to perform analytical scale chromatographic separations for MW determination of the principal peaks in the SEC analysis. UV, MALS, and the differential refractive index data were analyzed using the Wyatt Astra 8 software package to monitor the homogeneity of the sample across the elution peak.
Guanine nucleotide binding assay
The 2’/3’-O-(N-methylanthraniloyl (mant-) nucleotides mant-GDP and mant-GTPγS (Jena Bioscience) were used as fluorescent probes for determining the binding affinity of GBP2GD WT and E99L mutant. SUMO was included as a negative control. Different concentrations of proteins were incubated with mant-nucleotides in the binding buffer containing 20 mM Tris-HCl pH 8.0, 150 mM NaCl, and 5 mM β-ME (pH 8.0) or 50 mM MES pH 6.0, 150 mM NaCl, and 5 mM β-ME (pH 6.0) at 25 °C. In binding assays containing Mg2+, the buffers also include 5 mM MgCl2. The mant-nucleotides were excited at 355 nm and the fluorescence was measured at 448 nm. The binding of the mant-nucleotides to the proteins was monitored as increase in fluorescence using a microplate reader (TECAN). The increase in fluorescence was plotted against the protein concentrations and the dissociation constant was obtained by fitting the data to one-site specific binding model in GraphPad Prism 9.0 (GraphPad Software, San Diego, CA).
Statistics and reproducibility
For nucleotide binding and malachite green-based GTPase activity assays, the experiments are repeated at least three times with different batches of proteins to ensure reproducibility.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank the beamline scientists at NSLSII AMX 17-ID-1 AMX and Dr. T. “Soma” Somasundaram at Institute of Molecular Biophysics, Florida State University for assistance in X-ray crystallographic diffraction data collection. We thank the beamline scientists at SIBYLS beamline 12.3.1 at the Advanced Light Source for multi-angle light scattering data collection. We thank Dr. Peter Randolph at the Protein Biochemistry Facility at FSU for guidance on the usage of the plate readers and Dr. Gwimoon Seo at the Protein Expression Facility at FSU for competent cells and the protease Ulp1. We appreciate the assistance from Taiwo S. Adewole, Cristina Dabrowski, and Andrew Brasington in protein expression and purification. This work was supported by FSU startup funds and National Institutes of Health grants R00AI108793 and R01AI146330 to Q. Y. We thank members of Yin group for critical readings of the manuscript and helpful discussions.
Author contributions
Y.T. and Q.Y. conceived the initial experimental plan. S.R., Y.T., K.R., M.B., and S.W. expressed and purified the proteins. S.R., B.W., Y.T., Q.Y. crystallized the proteins and determined the structures. S.R. and K.R. carried out GTPase activity assays and nucleotide binding assays. S.R. and M.B. prepared samples for MALS experiments and carried out analysis. S.R. and Q.Y. drafted the manuscript and all authors edited the paper.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Joanna Timmins and Tobias Goris. A peer review file is available.
Data availability
Coordinates and structural factors for human GBP2GD·GDP and human GBP2FLK51A structures have been deposited in the Protein Data Bank with the accession codes 6VKJ and 7M1S, respectively. All other data supporting the findings of this study are available within the paper and its Supplementary Information.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sayantan Roy, Bing Wang, Krittika Roy.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-025-07727-3.
References
- 1.Randow, F., MacMicking, J. D. & James, L. C. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science340, 701–706 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim, B. H., Shenoy, A. R., Kumar, P., Bradfield, C. J. & MacMicking, J. D. IFN-inducible GTPases in host cell defense. Cell Host Microbe12, 432–444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martens, S. & Howard, J. The interferon-inducible GTPases. Annu. Rev. Cell Dev. Biol.22, 559–589 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Tretina, K., Park, E. S., Maminska, A. & MacMicking, J. D. Interferon-induced guanylate-binding proteins: guardians of host defense in health and disease. J. Exp. Med.216, 482–500 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stickney, J. T. & Buss, J. E. Murine guanylate-binding protein: incomplete geranylgeranyl isoprenoid modification of an interferon-gamma-inducible guanosine triphosphate-binding protein. Mol. Biol. Cell11, 2191–2200 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nantais, D. E., Schwemmle, M., Stickney, J. T., Vestal, D. J. & Buss, J. E. Prenylation of an interferon-gamma-induced GTP-binding protein: the human guanylate binding protein, huGBP1. J. Leukoc. Biol.60, 423–431 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Daumke, O. & Praefcke, G. J. Invited review: mechanisms of GTP hydrolysis and conformational transitions in the dynamin superfamily. Biopolymers105, 580–593 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Praefcke, G. J. & McMahon, H. T. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol.5, 133–147 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Ghosh, A., Praefcke, G. J., Renault, L., Wittinghofer, A. & Herrmann, C. How guanylate-binding proteins achieve assembly-stimulated processive cleavage of GTP to GMP. Nature440, 101–104 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Cui, W. et al. Structural basis for GTP-induced dimerization and antiviral function of guanylate-binding proteins. Proc. Natl Acad. Sci. USA118 (2021). [DOI] [PMC free article] [PubMed]
- 11.Abdullah, N., Balakumari, M. & Sau, A. K. Dimerization and its role in GMP formation by human guanylate binding proteins. Biophys. J.99, 2235–2244 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Praefcke, G. J., Geyer, M., Schwemmle, M., Robert Kalbitzer, H. & Herrmann, C. Nucleotide-binding characteristics of human guanylate-binding protein 1 (hGBP1) and identification of the third GTP-binding motif. J. Mol. Biol.292, 321–332 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Schwemmle, M. & Staeheli, P. The interferon-induced 67-kDa guanylate-binding protein (hGBP1) is a GTPase that converts GTP to GMP. J. Biol. Chem.269, 11299–11305 (1994). [PubMed] [Google Scholar]
- 14.Xavier, A., Al-Zeer, M. A., Meyer, T. F. & Daumke, O. hGBP1 coordinates chlamydia restriction and inflammasome activation through sequential GTP hydrolysis. Cell Rep.31, 107667 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Pandita, E. et al. Tetrameric assembly of hGBP1 is crucial for both stimulated GMP formation and antiviral activity. Biochem. J.473, 1745–1757 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Kim, B. H. et al. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science332, 717–721 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Fisch, D. et al. Human GBP1 differentially targets Salmonella and Toxoplasma to LIcense Recognition of Microbial Ligands and Caspase-mediated Death. Cell Rep.32, 108008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun, E. et al. Guanylate-binding proteins 2 and 5 exert broad antiviral activity by inhibiting furin-mediated processing of viral envelope proteins. Cell Rep.27, 2092–2104 e2010 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Kutsch, M. et al. Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J.39, e104926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhm, T. et al. Structural basis of membrane targeting and coatomer assembly by human GBP1. bioRxiv 2023.2003.2028.534355 (2023).
- 21.Weismehl, M. et al. Structural insights into the activation mechanism of antimicrobial GBP1. EMBO J.43, 615–636 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu, S. et al. Native architecture of a human GBP1 defense complex for cell-autonomous immunity to infection. Science383, eabm9903 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickinson, M. S. et al. LPS-aggregating proteins GBP1 and GBP2 are each sufficient to enhance caspase-4 activation both in cellulo and in vitro. Proc. Natl Acad. Sci. USA120, e2216028120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, S., Meng, Q., Maminska, A. & MacMicking, J. D. Cell-autonomous immunity by IFN-induced GBPs in animals and plants. Curr. Opin. Immunol.60, 71–80 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wandel, M. P. et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat. Immunol.21, 880–891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos, J. C. et al. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun.11, 3276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meunier, E. et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature509, 366–370 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Man, S. M. et al. IRGB10 liberates bacterial ligands for sensing by the AIM2 and Caspase-11-NLRP3 inflammasomes. Cell167, 382–396.e317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng, S. et al. Pathogen-selective killing by guanylate-binding proteins as a molecular mechanism leading to inflammasome signaling. Nat. Commun.13, 4395 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilla, D. M. et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl Acad. Sci. USA111, 6046–6051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prakash, B., Praefcke, G. J., Renault, L., Wittinghofer, A. & Herrmann, C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature403, 567–571 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Prakash, B., Renault, L., Praefcke, G. J., Herrmann, C. & Wittinghofer, A. Triphosphate structure of guanylate-binding protein 1 and implications for nucleotide binding and GTPase mechanism. EMBO J.19, 4555–4564 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji, C. et al. Structural mechanism for guanylate-binding proteins (GBPs) targeting by the Shigella E3 ligase IpaH9.8. PLoS Pathog.15, e1007876 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye, Y., Xiong, Y. & Huang, H. Substrate-binding destabilizes the hydrophobic cluster to relieve the autoinhibition of bacterial ubiquitin ligase IpaH9.8. Commun. Biol.3, 752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boehm, U. et al. Two families of GTPases dominate the complex cellular response to IFN-gamma. J. Immunol.161, 6715–6723 (1998). [PubMed] [Google Scholar]
- 36.Neun, R., Richter, M. F., Staeheli, P. & Schwemmle, M. GTPase properties of the interferon-induced human guanylate-binding protein 2. FEBS Lett.390, 69–72 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Rajan, S., Pandita, E., Mittal, M. & Sau, A. K. Understanding the lower GMP formation in large GTPase hGBP-2 and role of its individual domains in regulation of GTP hydrolysis. FEBS J.286, 4103–4121 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Kutsch, M., Ince, S. & Herrmann, C. Homo and hetero dimerisation of the human guanylate-binding proteins hGBP-1 and hGBP-5 characterised by affinities and kinetics. FEBS J.285, 2019–2036 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Mittal, M., Kausar, T., Rajan, S., Rashmi, D. & Sau, A. K. Difference in catalytic loop repositioning leads to GMP variation between two human GBP homologues. Biochemistry62, 1509–1526 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Ince, S. et al. Catalytic activity of human guanylate-binding protein 1 coupled to the release of structural restraints imposed by the C-terminal domain. FEBS J.288, 582–599 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Vopel, T. et al. Mechanism of GTPase-activity-induced self-assembly of human guanylate binding protein 1. J. Mol. Biol.400, 63–70 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Wittinghofer, A. & Vetter, I. R. Structure-function relationships of the G domain, a canonical switch motif. Annu. Rev. Biochem.80, 943–971 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Walker, J. E., Saraste, M., Runswick, M. J. & Gay, N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J.1, 945–951 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheffzek, K. et al. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science277, 333–338 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Praefcke, G. J. et al. Identification of residues in the human guanylate-binding protein 1 critical for nucleotide binding and cooperative GTP hydrolysis. J. Mol. Biol.344, 257–269 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Cherfils, J. & Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev.93, 269–309 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Abdullah, N., Srinivasan, B., Modiano, N., Cresswell, P. & Sau, A. K. Role of individual domains and identification of internal gap in human guanylate binding protein-1. J. Mol. Biol.386, 690–703 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Peulen, T. O. et al. Integrative dynamic structural biology unveils conformers essential for the oligomerization of a large GTPase. eLife10.7554/eLife.79565 (2023). [DOI] [PMC free article] [PubMed]
- 49.Otwinowski, Z. M. W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol.276, 307–326 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr.66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Coordinates and structural factors for human GBP2GD·GDP and human GBP2FLK51A structures have been deposited in the Protein Data Bank with the accession codes 6VKJ and 7M1S, respectively. All other data supporting the findings of this study are available within the paper and its Supplementary Information.