Abstract
1. Pseudomonas F2 isolated by enrichment culture on 2-furoic acid and grown with it as carbon source oxidized the compound with a Qo2 of 170μl./mg. dry wt./hr. and the overall consumption of 2·5μmoles of oxygen/μmole of substrate. 2. In the presence of 1mm-sodium arsenite, oxygen uptake was restricted to 0·54μmole/μmole of 2-furoate oxidized, with the formation of 0·86μmole of 2-oxoglutarate/μmole of 2-furoate. 3. Cell suspensions, disrupted in a French pressure cell and centrifuged at 27000g, yielded supernatants capable of catalysing the slow oxidation of 2-furoate (0·17μmole/mg. of protein/hr.). 4. Fractionation of 27000g supernatants at 200000g yielded a soluble enzyme fraction capable of catalysing the oxidation of 2-furoate only in the presence of added 200000g pellet or of Methylene Blue. 5. The 2-furoate-stimulated uptake of oxygen or the anaerobic reduction of Methylene Blue by dialysed 27000g supernatant required the addition of ATP and CoA, and the rate of oxygen uptake was further enhanced by the addition of magnesium chloride and NAD+. 6. The role of ATP and CoA in the formation of 2-furoyl-CoA was demonstrated by the accumulation of 2-furoylhydroxamic acid in the presence of hydroxylamine. 7. Dialysed 200000g supernatant, treated with Dowex 1, required the addition of ATP, CoA and Methylene Blue before it could oxidize 2-furoate to 2-oxoglutarate, which was trapped in unitary stoicheiometric yield as its phenylhydrazone. Magnesium chloride and NAD+ were not stimulatory in this system. The oxidation of 2-furoate to 2-oxoglutarate was not inhibited by substrate analogues, metal ion-chelating agents, thiol-active compounds or inhibitors of cytochrome-mediated electron transport. 8. No evidence was obtained for the intervention of 2,5-dioxovalerate as an intermediate in 2-oxoglutarate formation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANTRENNE H. The requirement for coenzyme A in the enzymatic synthesis of hippuric acid. J Biol Chem. 1951 Mar;189(1):227–233. [PubMed] [Google Scholar]
- Conrad H. E., Lieb K., Gunsalus I. C. Mixed function oxidation. 3. An electron transport complex in camphor ketolactonization. J Biol Chem. 1965 Oct;240(10):4029–4037. [PubMed] [Google Scholar]
- DAGLEY S., CHAPMAN P. J., GIBSON D. T., WOOD J. M. DEGRADATION OF THE BENZENE NUCLEUS BY BACTERIA. Nature. 1964 May 23;202:775–778. doi: 10.1038/202775a0. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., FEWSTER E., HAPPOLD F. C. The bacterial oxidation of phenylacetic acid. J Bacteriol. 1952 Mar;63(3):327–336. doi: 10.1128/jb.63.3.327-336.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., JOHNSON P. A. MICROBIAL OXIDATION OF KYNURENIC, XANTHURENIC AND PICOLINIC ACIDS. Biochim Biophys Acta. 1963 Dec 13;78:577–587. doi: 10.1016/0006-3002(63)91023-0. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., TRUDGILL P. W. THE METABOLISM OF GALACTARATE, D-GLUCARATE AND VARIOUS PENTOSES BY SPECIES OF PSEUDOMONAS. Biochem J. 1965 Apr;95:48–58. doi: 10.1042/bj0950048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. C. THE MICROBIOLOGICAL DEGRADATION OF AROMATIC COMPOUNDS. J Gen Microbiol. 1963 Aug;32:177–184. doi: 10.1099/00221287-32-2-177. [DOI] [PubMed] [Google Scholar]
- Freudenberg K. Lignin: Its Constitution and Formation from p-Hydroxycinnamyl Alcohols: Lignin is duplicated by dehydrogenation of these alcohols; intermediates explain formation and structure. Science. 1965 Apr 30;148(3670):595–600. doi: 10.1126/science.148.3670.595. [DOI] [PubMed] [Google Scholar]
- Gibson D. T. Microbial degradation of aromatic compounds. Science. 1967 Sep 13;161(3846):1093–1097. [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- HUNT A. L., HUGHES D. E., LOWENSTEIN J. M. The hydroxylation of nicotinic acid by Pseudomonas fluorescens. Biochem J. 1958 Jun;69(2):170–173. doi: 10.1042/bj0690170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson B., McGarry J. D. A direct pathway for the metabolism of propionate in cell extracts from Moraxella lwoffi. Biochem J. 1968 Mar;107(1):19–28. doi: 10.1042/bj1070019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKINUMA A., YAMATODANI S. L-GLUTAMIC ACID FORMATION FROM 2-FUROIC ACID BY SOIL BACTERIA. Nature. 1964 Jan 25;201:420–421. doi: 10.1038/201420a0. [DOI] [PubMed] [Google Scholar]
- KATAGIRI M., MAENO H., YAMAMOTO S., HAYAISHI O., KITAO T., OAE S. SALICYLATE HYDROXYLASE, A MONOOXYGENASE REQUIRING FLAVIN ADENINE DINUCLEOTIDE. II. THE MECHANISM OF SALICYLATE HYDROXYLATION TO CATECHOL. J Biol Chem. 1965 Aug;240:3414–3417. [PubMed] [Google Scholar]
- KORNBERG H. L. The metabolism of C2 compounds in micro-organisms. I. The incorporation of [2-14C] acetate by Pseudomonas fluorescens, and by a Corynebacterium, grown on ammonium acetate. Biochem J. 1958 Mar;68(3):535–542. doi: 10.1042/bj0680535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNER H. W., LAWRENCE N. S., FRENCH C. S. Colloidal dispersion of chloroplast material. Science. 1950 Jun 9;111(2893):633–634. doi: 10.1126/science.111.2893.633. [DOI] [PubMed] [Google Scholar]
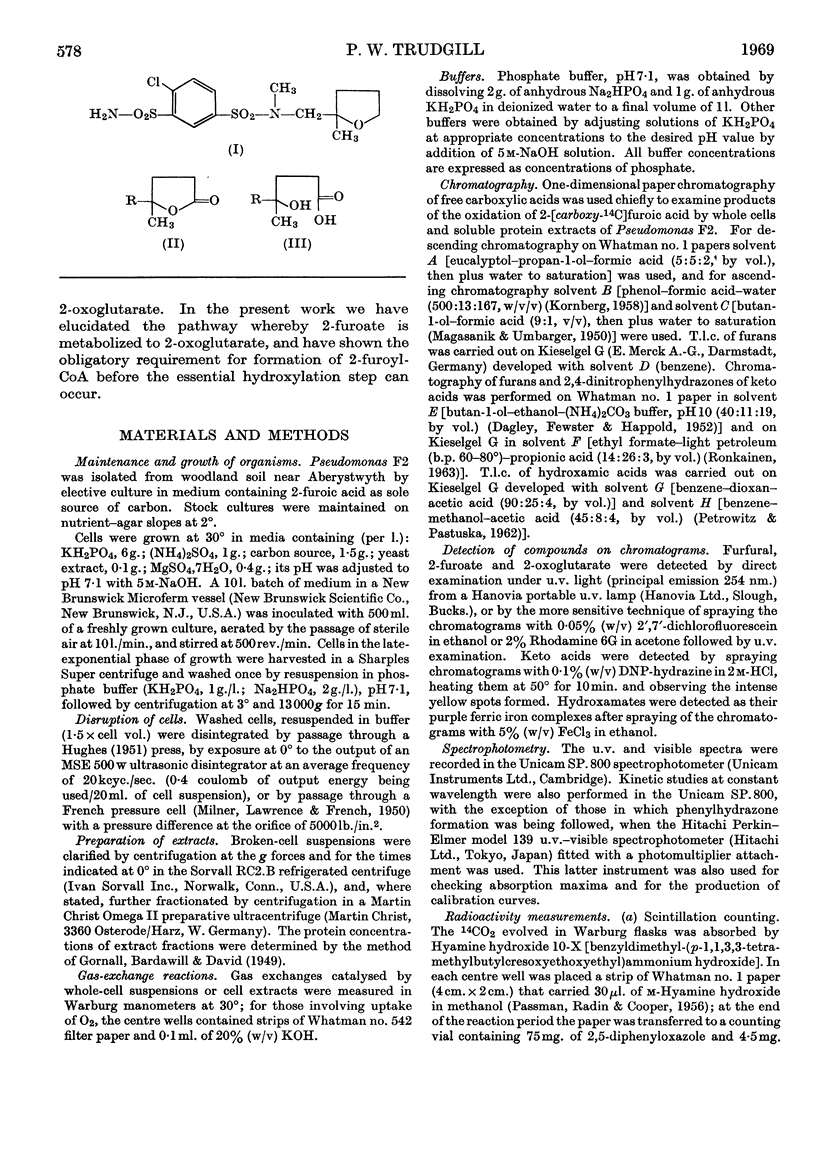
- Meng K., Kroneberg G. Pharmakologie von N-(4'-Chlor-3'-sulfamoyl-benzolsulfonyl)-N-methyl-2-aminomethyl-2-methyl-tetrahydrofuran, einer neuen diuretisch wirkenden Verbindung. Arzneimittelforschung. 1967 Jun;17(6):659–671. [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- RONKAINEN P. [Thin-layer chromatography of keto acids]. J Chromatogr. 1963 Jun;11:228–237. doi: 10.1016/s0021-9673(01)80897-9. [DOI] [PubMed] [Google Scholar]
- ROSENBERG E., HOLMES P. OXIDATION OF SECONDARY ALCOHOLS BY EXTRACTS OF A CORYNEBACTERIUM. J Bacteriol. 1965 May;89:1212–1216. doi: 10.1128/jb.89.5.1212-1216.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH R. M., ADAMS E. ALPHA-KETOGLUTARIC SEMIALDEHYDE: A METABOLIC INTERMEDIATE. Science. 1964 Apr 3;144(3614):67–68. doi: 10.1126/science.144.3614.67. [DOI] [PubMed] [Google Scholar]
- Singh R. M., Adams E. Isolation and identification of 2,5-dioxovalerate, an intermediate in the bacterial oxidation of hydroxyproline. J Biol Chem. 1965 Nov;240(11):4352–4356. [PubMed] [Google Scholar]
- Trudgill P. W., DuBus R., Gunsalus I. C. Mixed function oxidation. VI. Purification of a tightly coupled electron transport complex in camphor lactonization. J Biol Chem. 1966 Sep 25;241(18):4288–4290. [PubMed] [Google Scholar]


