Abstract
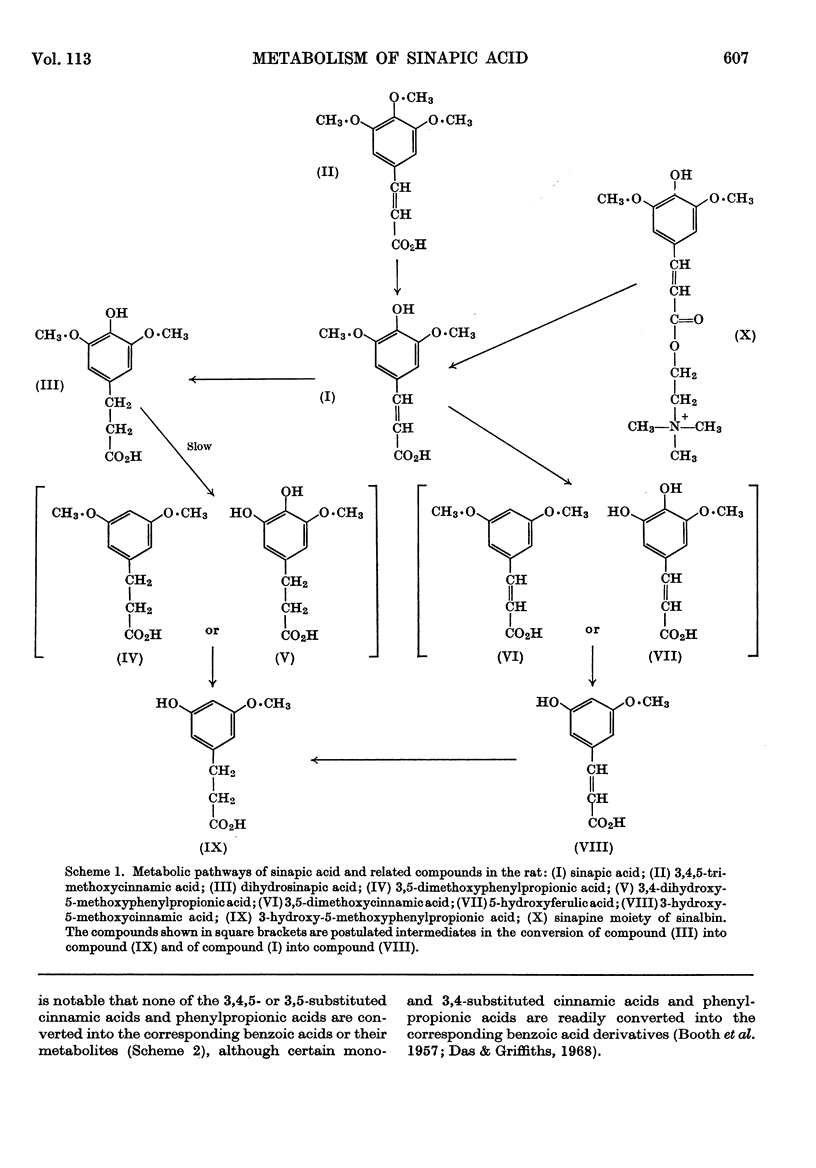
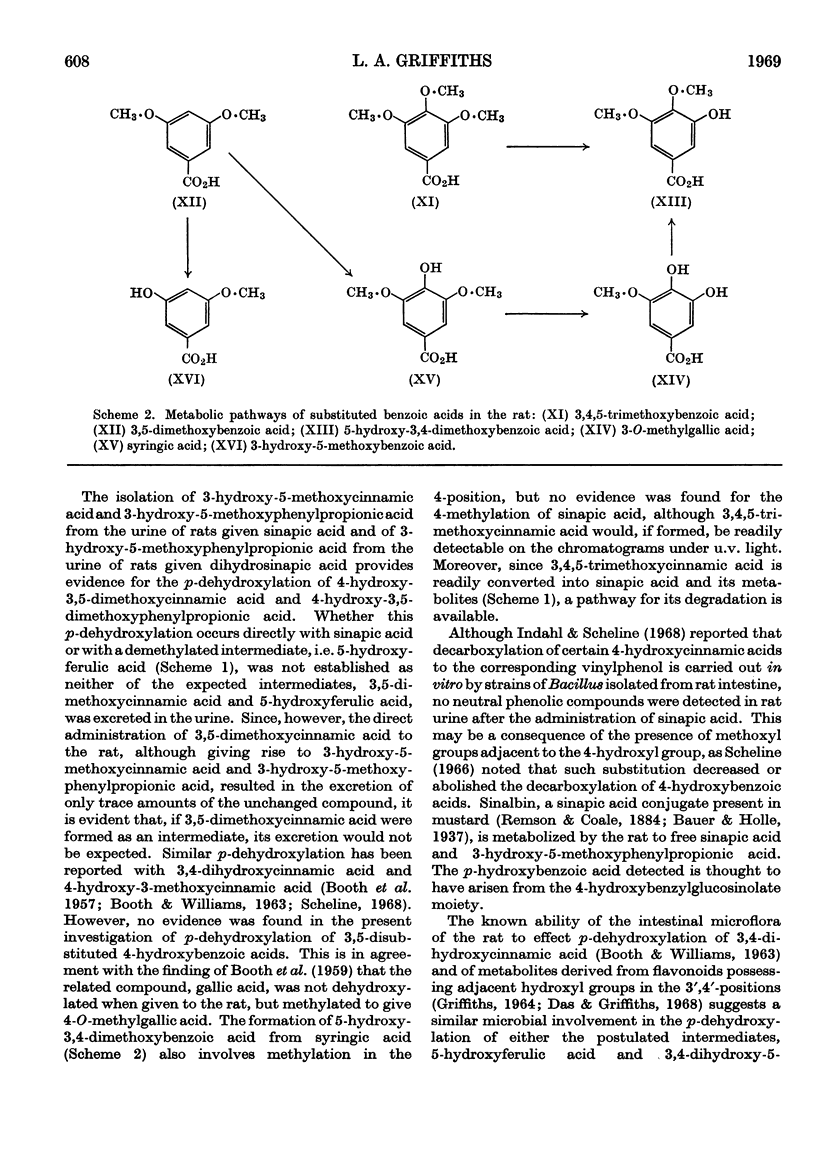
1. Administration of sinapic acid to the rat results in the excretion of 3-hydroxy-5-methoxyphenylpropionic acid, dihydrosinapic acid, 3-hydroxy-5-methoxycinnamic acid and unchanged sinapic acid in the urine. The sinapic acid conjugate sinalbin is also catabolized to free sinapic acid and 3-hydroxy-5-methoxyphenylpropionic acid in the rat. 2. 3,4,5-Trimethoxycinnamic acid is metabolized in part to sinapic acid and 3-hydroxy-5-methoxyphenylpropionic acid. 3. 3,5-Dimethoxycinnamic acid is metabolized to 3-hydroxy-5-methoxycinnamic acid and 3-hydroxy-5-methoxyphenylpropionic acid. 4. The metabolic interrelationships of these compounds were studied by the administration of intermediates and a metabolic pathway is proposed. 5. The metabolism of the corresponding benzoic acids was studied, but these compounds and their metabolites were shown not to be intermediates or products of the metabolism of the related cinnamic acids.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAKLEY E. R., SIMPSON F. J. THE MICROBIAL METABOLISM OF CINNAMIC ACID. Can J Microbiol. 1964 Apr;10:175–185. doi: 10.1139/m64-025. [DOI] [PubMed] [Google Scholar]
- BOOTH A. N., EMERSON O. H., JONES F. T., DEEDS F. Urinary metabolites of caffeic and chlorogenic acids. J Biol Chem. 1957 Nov;229(1):51–59. [PubMed] [Google Scholar]
- BOOTH A. N., MASRI M. S., ROBBINS D. J., EMERSON O. H., JONES F. T., DE EDS F. The metabolic fate of gallic acid and related compounds. J Biol Chem. 1959 Nov;234:3014–3016. [PubMed] [Google Scholar]
- BOOTH A. N., WILLIAMS R. T. Dehydroxylation of caffeic acid by rat and rabbit caecal contents and sheep rumen liquor. Nature. 1963 May 18;198:684–685. doi: 10.1038/198684a0. [DOI] [PubMed] [Google Scholar]
- Das N. P., Griffiths L. A. Studies on flavonoid metabolism. Metabolism of (+)-catechin in the guinea pig. Biochem J. 1968 Dec;110(3):449–456. doi: 10.1042/bj1100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT T. H., PARKE D. V., WILLIAMS R. T. Studies in detoxication. 79. The metabolism of cyclo [14C] hexane and its derivatives. Biochem J. 1959 Jun;72(2):193–200. doi: 10.1042/bj0720193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDHOFF A. J., GOLDSTEIN M. New developments in metabolism of mescaline and related amines. Ann N Y Acad Sci. 1962 Jan 13;96:5–13. doi: 10.1111/j.1749-6632.1962.tb50097.x. [DOI] [PubMed] [Google Scholar]
- Griffiths L. A. Studies on flavonoid metabolism. Identification of the metabolities of (+)-catechin in rat urine. Biochem J. 1964 Jul;92(1):173–179. doi: 10.1042/bj0920173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indahl S. R., Scheline R. R. Decarboxylation of 4-hydroxycinnamic acids by Bacillus strains isolated from rat intestine. Appl Microbiol. 1968 Apr;16(4):667–667. doi: 10.1128/am.16.4.667-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety S. S. Biochemistry and mental function. Nature. 1965 Dec 25;208(5017):1252–1257. doi: 10.1038/2081252a0. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA Y., SHETLAR M. R., WENDER S. H. SPECTRAL IDENTIFICATION STUDIES OF PHENOLIC ACIDS USING ALUMINUM CHLORIDE. Anal Biochem. 1964 Mar;7:374–378. doi: 10.1016/0003-2697(64)90146-0. [DOI] [PubMed] [Google Scholar]
- Scheline R. R. Decarboxylation and demethylation of some phenolic benzoic acid derivatives by rat caecal contents. J Pharm Pharmacol. 1966 Oct;18(10):664–669. doi: 10.1111/j.2042-7158.1966.tb07780.x. [DOI] [PubMed] [Google Scholar]
- Scheline R. R. Metabolism of phenolic acids by the rat intestinal microflora. Acta Pharmacol Toxicol (Copenh) 1968;26(2):189–205. doi: 10.1111/j.1600-0773.1968.tb00437.x. [DOI] [PubMed] [Google Scholar]


