Abstract
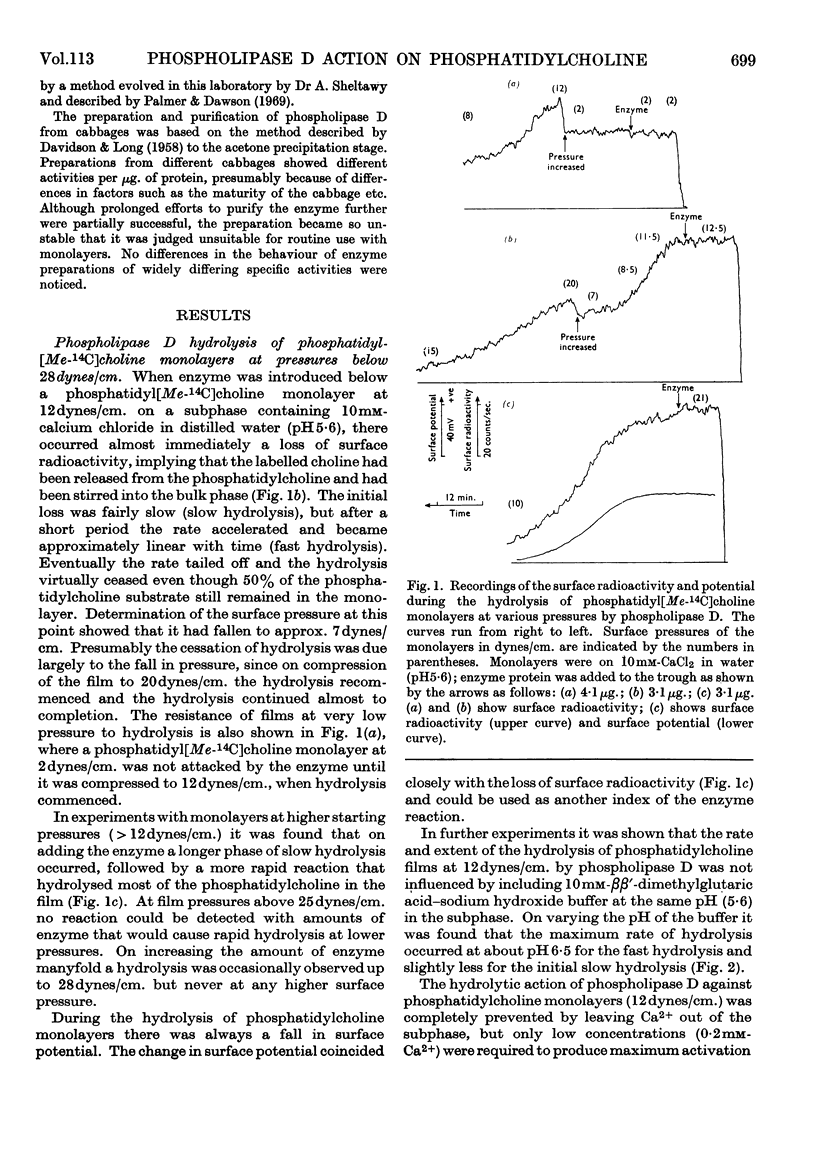
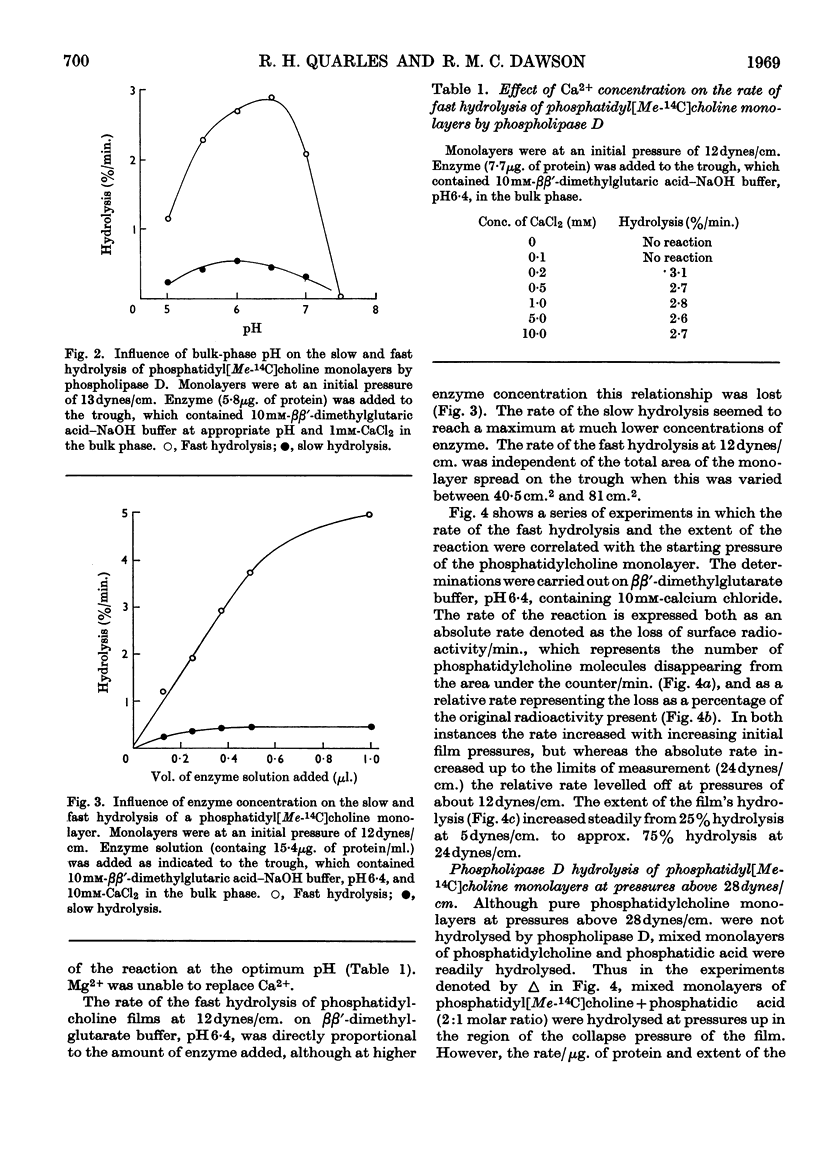
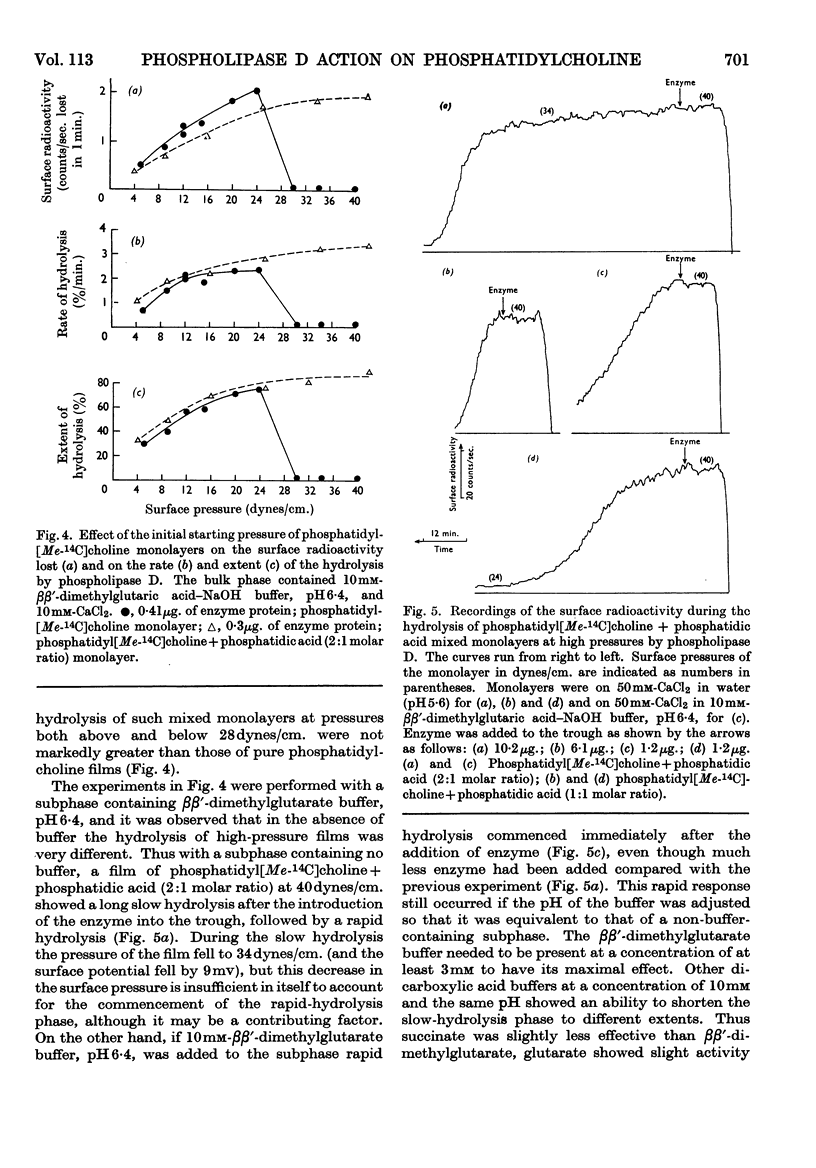
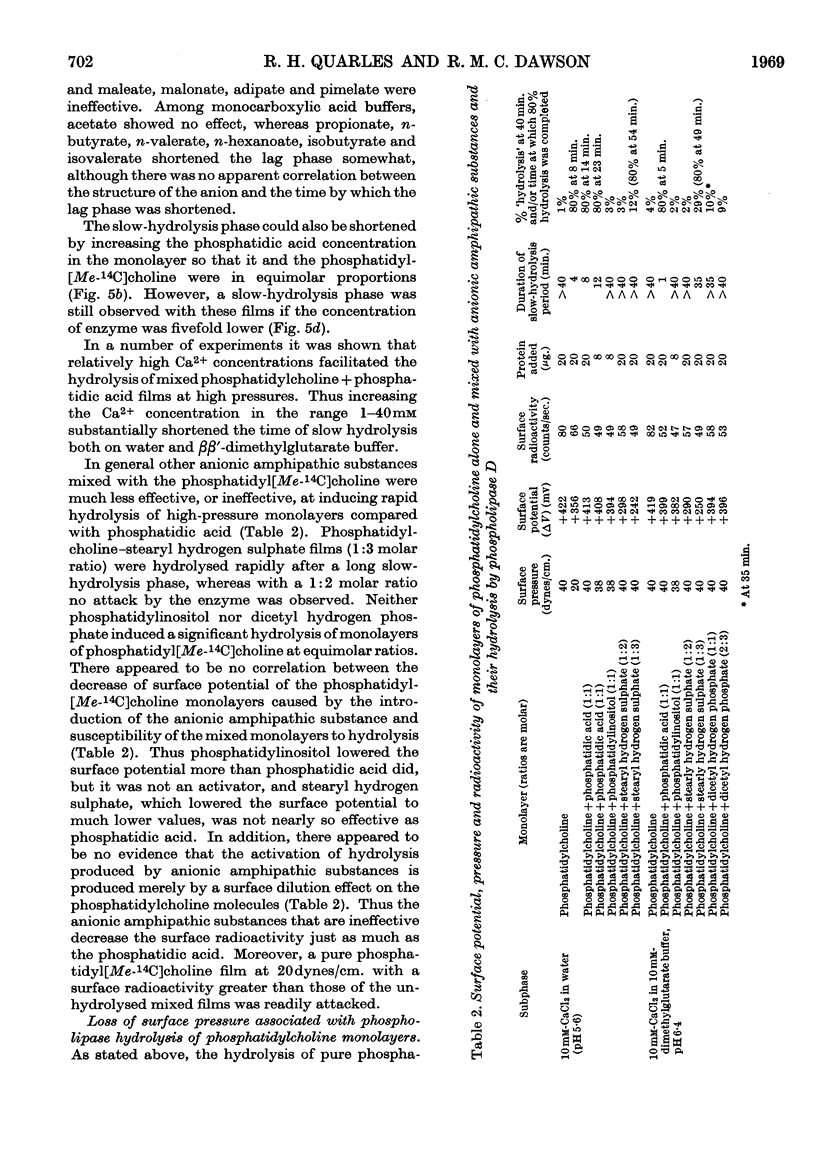
1. The hydrolysis of monolayers of phosphatidyl[Me-14C]choline at the air/water interface by phospholipase D (phosphatidylcholine phosphatidohydrolase) was investigated by a surface-radioactivity technique by using a flow counter. 2. Phosphatidylcholine of high specific radioactivity was prepared biosynthetically in good yield from [Me-14C]choline by using Saccharomyces cerevisiae. 3. At initial monolayer pressures between 12 and 25 dynes/cm. the hydrolysis occurred in two stages, an initial slow hydrolysis followed by a rapid hydrolysis. Below 3dynes/cm. and above 28dynes/cm. no enzymic hydrolysis of pure phosphatidylcholine monolayers could be detected. 4. The rapid hydrolysis was proportional to the enzyme concentration in the subphase, its pH optimum was 6·6, and 0·2mm-Ca2+ was required for maximal activity. 5. Hydrolysis of the film was accompanied by a pronounced fall in the surface pressure even though the phosphatidic acid formed did not leave the film. When the pressure fell to low values the hydrolysis ceased even if the film was only partially hydrolysed. 6. Above monolayer pressures of 28dynes/cm. enzymic hydrolysis could be initiated by inclusion of phosphatidic acid (and less effectively stearyl hydrogen sulphate) in the film, although the rates were not appreciably higher than those observed at 25dynes/cm. with a pure phosphatidylcholine film. 7. The initiation of the hydrolysis by phosphatidic acid was facilitated by the inclusion of high Ca2+ concentrations and certain carboxylic acid buffer anions in the subphase, although these did not activate by themselves. 8. The initiation of the hydrolysis at high pressures could not be related to any change in the surface potential brought about by the addition of the long-chain anions to the film, nor could it be ascribed to a surface dilution effect. 9. The results are discussed in relation to previous studies on the hydrolysis of phosphatidylcholine particles by the enzyme and also similar investigations on phosphatidylcholine monolayers with other phospholipases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D., DAWSON R. M. Electrokinetic requirements for the reaction between Cl. perfringens alpha-toxin (phospholipase C) and phospholipid substrates. Biochim Biophys Acta. 1962 May 7;59:103–115. doi: 10.1016/0006-3002(62)90701-1. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., DAWSON R. M. The physicochemical requirements for the action of Penicillium notatum phospholipase B on unimolecular films of lecithin. Biochem J. 1960 Apr;75:133–138. doi: 10.1042/bj0750133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colacicco G., Rapport M. M. Lipid monolayers: action of phospholipase A of Crotalus atrox and Naja naja venoms on phosphatidyl choline and phosphatidal choline. J Lipid Res. 1966 Mar;7(2):258–263. [PubMed] [Google Scholar]
- Condrea E., Fabian I., De Vries A. Action of plastid phospholipase D on free and lipoprotein-bound phospholipids. Experientia. 1964 Oct 15;20(10):557–558. doi: 10.1007/BF02150288. [DOI] [PubMed] [Google Scholar]
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., BANGHAM A. D. The activation of surface films of lecithin by amphipathic molecules. Biochem J. 1959 Jul;72:493–496. doi: 10.1042/bj0720493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Hauser H. On the mechanism of the stimulation by anionic amphipaths of lecithin hydrolysis by phospholipase B of penicillium notatum. Biochim Biophys Acta. 1967 Jun 6;137(3):518–524. doi: 10.1016/0005-2760(67)90132-4. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. Some properties of purified phospholipase D and especially the effect of amphipathic substances. Biochem J. 1967 Jan;102(1):76–86. doi: 10.1042/bj1020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Dawson R. M. Stability of unimolecular films of 32P-labeled lecithin. Biochem J. 1967 Oct;105(1):401–407. doi: 10.1042/bj1050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Dawson R. M. The binding of calcium at lipid-water interfaces. Eur J Biochem. 1967 Mar;1(1):61–69. doi: 10.1007/978-3-662-25813-2_11. [DOI] [PubMed] [Google Scholar]
- Hughes A. The action of snake venoms on surface films. Biochem J. 1935 Feb;29(2):437–444. doi: 10.1042/bj0290437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATES M. Effects of solvents and surface-active agents on plastid phosphatidase C activity. Can J Biochem Physiol. 1957 Feb;35(2):127–142. [PubMed] [Google Scholar]
- Palmer F. B., Dawson R. M. Complex-formation between triphosphoinositide and experimental allergic encephalitogenic protein. Biochem J. 1969 Mar;111(5):637–645. [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. A shift in the optimum pH of pospholipase D produced by activating long-chain anions. Biochem J. 1969 May;112(5):795–799. doi: 10.1042/bj1120795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. O., Schulman J. H. Influence of calcium, cholesterol, and unsaturation on lecithin monolayers. J Lipid Res. 1967 May;8(3):215–226. [PubMed] [Google Scholar]


