Abstract
Developing children are particularly vulnerable to the effects of exposure to per- and polyfluoroalkyl substances (PFAS), a group of endocrine disrupting chemicals. We hypothesized that early life exposure to PFASs is associated with poor metabolic health in children.
We studied the association between prenatal and postnatal PFASs mixture exposure and metabolic syndrome (MetS) score in children, and the role of inflammatory proteins.
In 1,101 mothers-child pairs from the Human Early Life Exposome project, we measured the concentrations of PFAS in blood collected in pregnancy and at 8 years (range=6–12 years). We applied Bayesian Kernel Machine regression (BKMR) to estimate the associations between exposure to PFAS mixture and the cardiometabolic factors as age and sex- specific z-scores of waist circumference (WC), systolic and diastolic blood pressures (BP), and concentrations of triglycerides (TG), high-density lipoprotein (HDL-C) and low-density lipoprotein (LD-C) cholesterol. We measured thirty six inflammatory biomarkers in child plasma by protein panels and examined the underlying role of inflammatory status for the exposure-outcome association by integrating the three panels into a network.
Exposure to the PFAS mixture was positively associated with HDL-C and systolic BP, and negatively associated with WC, LDL-C and TG. When we examined the independent effects of the individual chemicals in the mixture, prenatal PFHxS was negatively associated with HDL-C and prenatal PFNA was positively associated with WC and these were opposing directions from the overall mixture. Further, the network consisted of five distinct communities connected with positive and negative correlations. The selected inflammatory biomarkers were positively, while the postnatal PFAS were negatively related with the included cardiometabolic factors, and only prenatal PFOA was positively related with the pro-inflammatory cytokine IL-1beta and WC.
Our study supports that prenatal, rather than postnatal, PFAS exposure might contribute to an unfavorable lipidemic profile and adiposity in childhood.
Keywords: PFAS, Cardiometabolic risk, HDL, inflammation, adiposity, BKMR
1. Introduction
Cardiovascular disease (CVD) is the most common cause of death in adults worldwide (Collaborators 2018a). At the same time, the main risk factors for CVD, abdominal adiposity, insulin resistance, hypertension and dyslipidemia are manifested at progressively younger ages (Collaborators 2018b). In Europe, 6% out of 19,000 2–11 year-olds had metabolic syndrome, a cluster of the CVD risk factors, and more than 30% of 3,500 13–18 year-olds had elevated blood cholesterol, blood glucose or blood pressure (Ahrens et al. 2014; Henriksson et al. 2017). Such unfavorable cardio-metabolic status in early life can track through adolescents and adulthood, posing as high as five times increased risk for hypertension, dyslipidemia, diabetes and CVD (Franks et al. 2010; Juonala et al. 2011). As a lifestyle characterized by unhealthy diet, inactivity and a sedentary behavior cannot completely explain the rising prevalence of CVD and its risk factors, emerging experimental and human evidence shows that endocrine disrupting chemicals may play a key role for this epidemic (Barouki et al. 2012; Bhatnagar 2017; Tang-Peronard et al. 2011).
Per- and polyfluoroalkyl substances (PFAS) are environmentally persistent chemicals, with toxicological properties and potential health concerns, as recently summarized (EFSA et al. 2020). Widespread human exposure through diet along with the persistence and mobility of PFAS led to measurable concentrations in blood and other tissues among general populations worldwide (EFSA et al. 2018; EFSA et al. 2020). Disturbances in lipid metabolism and increased total serum cholesterol levels was identified as the most critical PFAS-induced health effect in humans, while the evidence of effects on other cardiometabolic factors was neither sufficient nor consistent (EFSA et al. 2020). More specifically for early life exposures, prenatal low-dose PFAS exposure have not been consistently linked with obesogenic effects, with positive (Braun et al. 2016; Halldorsson et al. 2012; Hoyer et al. 2015; Mora et al. 2017) and null associations (Andersen et al. 2013; Barry et al. 2014). There are few studies exploring child insulin resistance (Fleisch et al. 2017), dyslipidemia (Manzano-Salgado et al. 2017), and the composite metabolic syndrome (Manzano-Salgado et al. 2017), and two studies considered postnatal PFAS exposures, in addition to prenatal (Fleisch et al. 2017; Li et al. 2021). After birth, postnatal PFAS exposure can substantially deviate from the prenatal exposure, with variations in PFAS concentrations between different congeners (Fromme et al. 2010; Kingsley et al. 2018; Papadopoulou et al. 2015; Papadopoulou et al. 2016), and postnatal PFAS exposure might substantially induce toxicity effects revealed later. The epidemiologic evidence of combined prenatal and postnatal PFAS exposure at environmentally relevant concentrations remains inconsistent and with large knowledge gaps (Rappazzo et al. 2017).
Chronic inflammation has been suggested as the underlying mechanism through which PFAS exposure can contribute to the disease exacerbation, especially due to their affinity to the peroxisome proliferator-activated receptors (PPARs), which are responsible for adipocyte differentiation and regulators of lipid and glucose metabolism and inflammation (Behr et al. 2020; Cheng et al. 2019; Mirza et al. 2019). Animal and human evidence supports this biological pathway, for asthma, impaired immune function and liver injury (Deng et al. 2020; Pennings et al. 2016; Tan et al. 2013; Yang et al. 2021). Chronic inflammation has a crucial role to play in the development of metabolic disease, but the extent to which PFAS exposure can contribute to such a process is unknown (Bussler et al. 2017; Rubin et al. 2011).
Our aim was to examine the association between prenatal and postnatal PFAS mixture exposure with cardiometabolic health by Bayesian Kernel Machine Regression, and explore the role of inflammatory biomarkers by constructing an integrated network, in a well-characterized study of 1,101 mother-child pairs.
2. Methods
2.1. Study population
This study is part of the HELIX project (Maitre et al. 2018), a collaboration across six ongoing longitudinal population-based birth cohort studies in Europe: the Born in Bradford (BiB) study in the UK (Wright et al. 2013), the Étude des Déterminants pré et postnatals du développement et de la santé de l’Enfant (EDEN) study in France (Heude et al. 2016), the INfancia y Medio Ambiente (INMA) cohort in Spain (Guxens et al. 2012), the Kaunas cohort (KANC) in Lithuania (Grazuleviciene et al. 2009), the Norwegian Mother, Father and Child Cohort Study (MoBa) (Magnus et al. 2016) and the RHEA Mother Child Cohort study in Crete, Greece (Chatzi et al. 2017). Within the larger HELIX (n=31,472 mother-child pairs), a subcohort of 1,301 children (approximately 200 children in each participating cohort) was selected for detailed characterization of a broad suite of environmental exposures, including several environmental chemicals (Maitre et al. 2018). During 2013–2015, a follow-up of the children was conducted with clinical examinations, computer assisted interviews and biological sample collection by trained personnel. Eligibility criteria for inclusion in the subcohort were: (a) age 6–11 years at the time of the visit, with a preference for ages 7–9 years if possible; (b) sufficient stored pregnancy blood and urine samples available for analysis of prenatal exposure biomarkers; (c) complete address history available from first to last follow-up point; (d) no serious health problems that may affect the performance of the clinical testing or impact the volunteer’s safety (e.g., acute respiratory infection). Each cohort selected participants at random from the eligible pool within the entire cohort and invited them to participate in this subcohort until the required number of participants was reached. Our study population consists of 1101 mother-child pairs from the HELIX subcohort, based on availability of information on pre- and postnatal PFAS exposure and complete data on childhood cardiometabolic factors and protein concentrations at follow-up (mean age 8 years; range=6.0 to 12 years).
All participating families provided written informed consent. Approval for the HELIX project was obtained from the local ethical committees at each site.
2.2. PFAS concentrations in pregnancy and childhood
PFAS concentrations were measured in maternal biological samples collected prenatally or at birth and in children’s biological samples collected during the HELIX follow-up (Tamayo-Uria et al. 2019). Maternal samples were collected at mean week of gestation (SD) 27 (1) in BiB, 26 (1) in EDEN, 14 (2) in INMA, 39 (1) in KANC, 19 (1) in MoBa and 14 (4) in Rhea. Child blood samples were collected at mean age (SD) 7 (0.2) years old in BiB, 11 (0.6) in EDEN, 9 (0.6) in INMA, 7 (0.5) in KANC, 9 (0.5) in MoBa and 7 (0.3) in RHEA. Four PFASs were analysed in maternal plasma for the BiB, INMA, MoBa and RHEA cohorts, in maternal serum for the BiB and EDEN cohorts and in maternal whole blood in the KANC cohort and in child plasma for all cohorts: perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorononanoate (PFNA). Perfluoroundecanoate (PFUnDA) was measured in all child samples. From the INMA cohort, only 15 (7%) women had available PFUnDA concentrations and maternal PFUnDA was not included in our analyses as it would result in the exclusion of a cohort.
All the chemical analyses were performed at the Section for Environmental Exposure and Epidemiology at the Norwegian Institute of Public Health (NIPH), Oslo, Norway, except for 208 maternal samples from the INMA cohort which were analyzed at the Institute for Occupational Medicine, RWTH Aachen University, Germany (Manzano-Salgado et al. 2015). PFUnDA concentrations were not available for these samples. Concentrations were determined using column switching liquid chromatography (LC) coupled to a triple quadrupole mass spectrometer in serum or plasma samples and online solid phase extraction with ultra-high performance LC coupled with tandem mass spectrometry in whole blood samples (Haug et al. 2009; Poothong et al. 2017). Maternal PFAS concentrations were measured in serum or plasma in 5 of the 6 participating cohorts and these results were assumed comparable, while for the one cohort with available PFAS concentrations in maternal whole blood, 1:2 ratios were assumed for whole blood:serum/plasma and whole blood concentrations were multiplied by a factor of two to be comparable with the other cohorts.
The limit of detection (LOD) was 0.02 μg/L and the limit of quantification (LOQ) was 0.05 μg/L for samples assessed at NIPH, while for samples assessed at RWTH Aachen University, LODs ranged from 0.05–0.1μg/L. Values below LOQ were replaced with the observed values, whenever available. For non-observed concentrations, singly imputed values were obtained using a quantile regression approach (Haug et al. 2018). A detailed description of the analytical methods, the quality assurance and quality control has been published elsewhere (Haug et al. 2018).
2.3. Child cardiometabolic factors
During the HELIX follow-up, specific training workshops were organized to standardize the clinical assessment between the cohorts (Maitre et al. 2018). In these workshops, all field workers participated and were trained to obtain measurements that were comparable to those measured by an experienced anthropometrist. Waist circumference (WC) was measured to the nearest 0.1 cm, midway between the lowest rib margin and the iliac crest, using a flexible tape and recorded in duplicate. The mean of the measurements was used. Height was measured to the nearest 0.1 cm with a stadiometer (Seca 213, California, USA) and weight to the nearest 0.1 kg with a digital scale, without shoes and with light clothing. Blood pressure was taken in sitting position after 5 min of rest using the OMRON 705-CPII automated oscillometric device. The mean of three consecutive measurements that were taken with 1 min intervals was used. Blood pressure was measured towards the end of the visit to ensure that children had not consumed anything that may affect the results (chocolate, cola drinks) in the previous hour. Systolic (SBP) and diastolic (DBP) blood pressures and pulse rate from each measurement were recorded. The concentrations (mg/dL) of HDL and LDL cholesterol (HDL-C and LDL-C) and triglycerides (TG) were measured in child non-fasting serum using homogenous enzymatic colorimetric methods on a Modular Analytics System from Roche Diagnostics GmbH Mannheim and according to the manufacturer’s instructions. We constructed age and sex- specific z-scores for child WC, TG, HDL-C, LDL-C. SBP and DBP z-scores were additionally standardized for child height and TG were log-transformed before standardization, following previous methodology (Ahrens et al. 2014).
2.4. Inflammatory status in children
Child blood samples were collected using standardized protocols during the HELIX follow-up. A set of 43 proteins were a priori selected based on the literature and on the commercially available kits from Luminex xMAP multiplex platform (Luminex Corp). We selected three kits for the subsequent analyses, to assess 50 measurements that represented 43 unique proteins: the human cytokines 30plex magnetic panel (Cat #. LHC6003M), the human apoliprotein 5-plex magnetic panel (LHP0001M) and the humam adipokine 15-plex magnetic panel (LHC0017M) (Supplementary Table S1). All the analyses were conducted at the University Pompeu Fabra Centre for Genomic Regulation Proteomics Unit in Barcelona, Spain.
All samples were randomized to ensure a representation of each cohort in each measurement plate (batch) and we made no distinctions by cohort or gender. For protein quantification, an 8-point calibration curve per plate was performed with protein standards provided in the Luminex kit and following the procedures described by the vendor. Commercial heat inactivated, sterile-filtered plasma from human male AB plasma (Sigma Cat #. H3667) was used as constant controls to control for intra- and inter-plate variability. Four control samples were added per plate. No duplicate measurements were done for the HELIX samples. All samples were diluted ½ for the 30-plex kit, ¼ for the 15-plex kit and 1/2500 for the 5-plex kit. The coefficients of variation for each protein estimated by plate and then averaged ranged from 3.42% to 36%. The derived raw intensities were converted to ng/ml (5-plex kit: adiponectine, CRP, APO-A1, APO-B, APO-E) and to pg/ml (15 and 30-plex kits) using the calculated standard curves of each plate and accounting for the dilutions that were made prior to measurement.
Further, we obtained the limit of detection (LOD) as well as the lower and upper quantification limits (LOQ1 and LOQ2, respectively) from the calibration curves for each protein. Seven proteins were excluded from further analysis due to low detection rates (detection frequency < 30%), namely IL7, VEGF, GMCSF, Lipocalin2, RANTES, Resistin and SAA. In addition, seven proteins were measured in two different plex (IL1beta, IL6, IL8, IL10, MCP1, HGF, TNFalfa) and the measure with lower quality was excluded from the analysis. For the included proteins (n=36), data was log transformed to reach normal distribution. Then, we subtracted the difference between the overall protein average minus the plate specific protein average to account for the plate batch effect. All values between LOQ2 and LOQ1 were imputed using a truncated normal distribution implemented in the truncdist R package (Nadarajah and Kotz 2006).. Thirty five proteins were included to describe the inflammatory status of the HELIX children: two adipokines (adiponectin and leptin), three apolipoproteins (apoA1, apoB, apoE), four CC chemokines (monocyte chemoattractant protein 1[MCP-1], Eotaxin, macrophage inflammatory protein 1-alpha [MIP-1α], macrophage inflammatory protein-1β [MIP-1β], three CXC chemokines (Interleukin 8 [IL-8], monokine induced by gamma interferon [MIG], interferon gamma-induced protein 10 [IP10]), two interferons (IFN-α and IFN-γ), ten interleukins (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-15, IL-17), the interleukin-1 receptor antagonist (IL-1RA), the interleukin 2 receptor (IL-2R), epidermal growth factor (EGF), basic fibroblast growth factor (FGF-2), granulocyte colony-stimulating factor (G-CSF), hepatocyte growth factor (HGF), plasminogen activator inhibitor-1 (PAI-1), connecting peptide (C-peptide), C-reactive protein (CRP), tumor necrosis factor-α (TNF-α) and the TNF cytokine B cell-activating factor (BAFF).
2.5. Statistical analysis
We examined the association of prenatal and postnatal exposure to the PFAS with the cardiometabolic factors by fitting Bayesian Kernel Machine Regression (BKMR) models. All our models were adjusted for the same set of confounders, identified based on previous knowledge and a directed acyclic graph (DAG) approach (Supplementary Figure S1): cohort, maternal age (in years), parity (nulliparous/multiparous), maternal education level (low, middle, high), maternal pre-pregnancy BMI (in kg/m2), child ethnicity (White European, Other), age at examination (in years) and sex (male/female).
BKMR is a non-parametric flexible modeling approach that can accommodate for correlation, non-linearity and interaction effects when estimating the exposure (PFAS mixture)-response associations (Bobb et al. 2015). As the correlations of PFASs within maternal or child samples are higher than between mother-child pairs (Papadopoulou et al. 2016; Tamayo-Uria et al. 2019; Verner et al. 2016), we conducted a hierarchical variable selection. In the first level, variable selection is done at the group level (i.e. choosing whether the group of all prenatal exposures and the group of all postnatal exposures are useful in predicting the outcome); and at the second level, variable selection is done on the exposures within each group.
The main models are described as:
where is each of the cardiometabolic factors for each participant , is the high-dimensional exposure-response function which can incorporate both non-linear relationships and interactions among exposures and is estimated using a Gaussian kernel machine representation. Further, is a vector of covariates and their associated regression coefficients and . PFAS were log-transformed for the BKMR analyses and BKMR was fitted using the Markov chain Monte Carlo algorithm with 10,000 iterations. Once fitted, BKMR provides a posterior inclusion probability (PIP) for each of the exposures, which constitutes a measure of the relative importance of each exposure within the function to the overall mixture effects. Since we used a hierarchical variable selection method two sets of PIPs were obtained, the groupPIP representing the probability of inclusion for each of two groups (pre and postnatal PFASs) and the conditional PIPs (condPIP) which represented the probability that a particular chemical within the group was included in the model. Note that group-level or individual PFASs-level PIPs are not constrained to sum to 1. Credible intervals obtained from the BKMR model incorporated the additional uncertainty due to estimation of a high-dimension set of exposures and accounting for multiple-testing penalty. We further estimated the overall joint effect of exposure to the PFAS mixture by providing an estimate of the change in the outcome when the PFAS mixture exposure is increasing up to 95th percentile, compared to holding all PFAS at their 25th percentile (reference level). We also explored the gender differences by stratification in the BKMR analyses, as metabolic effects in children of prenatal PFAS exposure have been previously suggested to differ by sex (Fleisch et al. 2017). The time of collection of the maternal samples varied by cohort, and the PFAS concentrations are expected to be lower by increasing trimester for reasons described by Fisher et al (Fisher et al. 2016), including the increased mother: fetus transfer ratio and maternal blood dilution due to increased weight gain as the gestation progresses (Supplementary Table S3). Therefore, we conducted stratified BKMR analysis, by trimester of maternal sample collection. As secondary analyses, we examined the association of prenatal and postnatal exposure to the PFAS with the cardiometabolic factors by linear regression models by mutually adjusting for all PFASs, among other confounders.
As a second step and in order to examine the role of child inflammatory status in the association between PFAS exposure and cardiometabolic health, we constructed and integrated network by applying the xMWAS method (Uppal et al. 2018). The input data were all the prenatal and postnatal PFAS concentrations (n=9), the measured concentrations of the proteins (n=36) and the z-scores of the cardiometabolic factors that had an association with the PFAS mixture as estimated by the previous step. Therefore, z-score of diastolic BP was excluded from the network analysis. The xMWAS provides an automated framework for integrative and differential network analysis through three steps: 1) pairwise data integration; 2) visualization of a multi-data integrative network; and 3) multilevel community detection. For step 1, we applied sparse Partial Least Squares regression (sPLS), a dimension reduction technique, for pairwise data integration and for generating the correlation matrices. sPLS performs simultaneous data integration and variable selection using a LASSO penalty for the loading vectors. For step 3, we used the betweenness centrality measure (BCM) to evaluate the importance of nodes and variables with BCM≥0.20 were considered important components of the identified network. Only associations that were significant at p<0.05 were included in network analysis and the correlation threshold was set to 0.6.
All significance levels were set to 0.05 in this study. We used STATA to calculate summary statistics and used R (version R 3.6.2, R Development Core Team) for all other analyses including the BKMR and the xMWAS.
3. Results
Regarding PFAS concentrations in maternal and child blood, PFOS, PFOA, PFNA and PFHxS were detected in more than 97% of the samples, with PFOS and PFOA being the most abundant substances (Table 1). We observed strong positive correlations within mothers and children, with the highest between PFOS and PFHxS in mothers (rho=0.71) and between PFOS and PFNA in children (rho=0.64). Moderate positive correlations were found between mother-child pairs, with the strongest between mother-child PFHxS and mother-child PFOS (rho=0.50 and 0.49, respectively).
Table 1.
PFAS concentration (in μg/L) in maternal samples collected in pregnancy and in child samples in the HELIX subcohort (n=1,101 mothers-child pairs).
| PFAS concentrations (in μg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Maternal samples a | Child samples b | ||||||||
|
| |||||||||
| PFOA | PFNA | PFHxS | PFOS | PFOA | PFNA | PFUnDA | PFHxS | PFOS | |
| Samples >LOD (%) | 99.6% | 97.8% | 97.1% | 100% | 100% | 99.5% | 66.2% | 99.7% | 99.7% |
| 10th | 0.80 | 0.23 | 0.19 | 2.36 | 0.95 | 0.18 | 0.02 | 0.10 | 0.73 |
| 25th | 1.34 | 0.42 | 0.30 | 3.99 | 1.17 | 0.29 | 0.03 | 0.18 | 1.22 |
| 50th | 2.22 | 0.69 | 0.53 | 6.15 | 1.53 | 0.47 | 0.06 | 0.34 | 1.93 |
| 75th | 3.29 | 1.10 | 0.88 | 9.16 | 1.96 | 0.73 | 0.10 | 0.56 | 3.11 |
| 90th | 4.37 | 1.58 | 1.39 | 14.41 | 2.43 | 1.14 | 0.17 | 0.82 | 4.63 |
| Spearman correlation coefficients | |||||||||
| Maternal samples | |||||||||
| PFNA | 0.61 | ||||||||
| PFHxS | 0.65 | 0.29 | |||||||
| PFOS | 0.64 | 0.46 | 0.71 | ||||||
| Child samples | |||||||||
| PFOA | 0.20 | −0.01 | 0.15 | 0.14 | |||||
| PFNA | 0.16 | 0.21 | 0.20 | 0.39 | 0.44 | ||||
| PFUnDA | 0.21 | 0.14 | 0.19 | 0.28 | 0.25 | 0.51 | |||
| PFHxS | 0.26 | −0.11 | 0.50 | 0.47 | 0.40 | 0.39 | 0.33 | ||
| PFOS | 0.25 | 0.20 | 0.26 | 0.49 | 0.43 | 0.64 | 0.50 | 0.58 | |
PFASs analyzed plasma samples for the BIB, INMA, MoBa and RHEA cohorts, in serum samples for the BIB and EDEN cohorts and in whole blood in the KANC cohort.
PFASs were analyzed in plasma samples collected at 6-12 years.
3.1. Prenatal and postnatal PFAS exposure and cardiometabolic factors in childhood
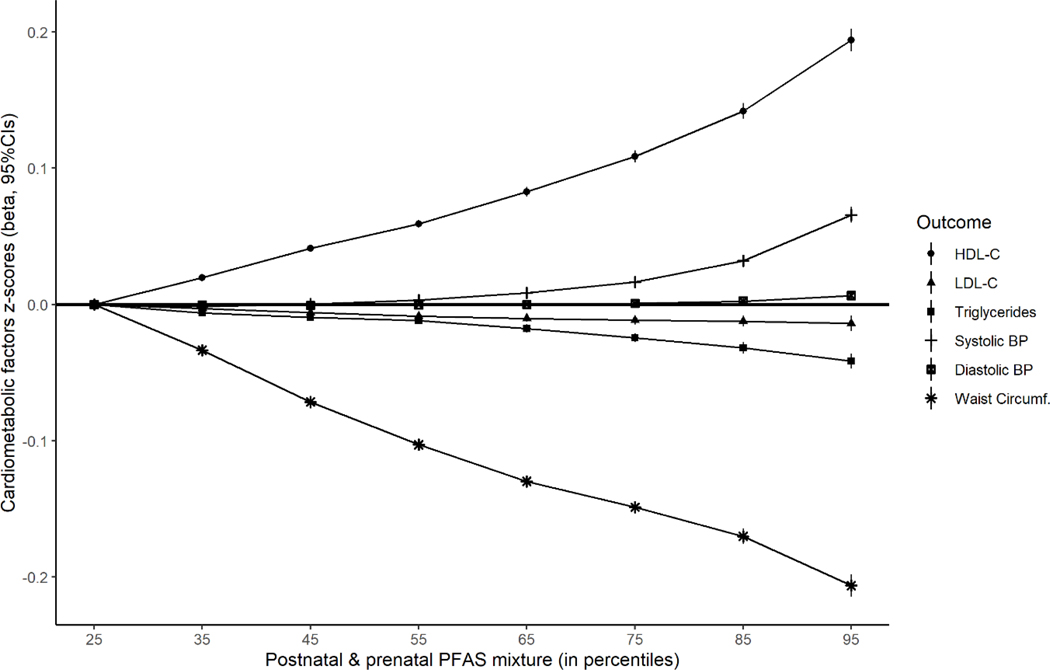
By applying BKMR analyses, we observed that pre- and post-natal PFAS mixture exposure was positively associated with HDL-C and Systolic BP, and negatively associated with WC, LDL-C, TG (Figure 1). Among those, the strongest dose-response associations were found for HDL-C (positive) and WC (negative), while the positive association with Systolic BP was seen in high PFAS mixture exposure levels (>50th percentile). More specifically, when exposure of the PFAS mixture was in the 95th percentile vs 25th percentile, the increase in HDL-C z-score was 0.19 (95% CI=0.19,0.20) and the decrease in WC z-score was −0.21 (95% CI=−0.21,−0.20) (Supplementary Table 2). For all cardiometabolic factors, postnatal PFASs were contributing more to the mixture than prenatal, according to the group posterior inclusion probabilities (PIPs), besides Systolic BP where PIPs were similar (Supplementary Table 2). Regarding the contribution of individual PFAS in the identified mixture, prenatal PFHxS and postnatal PFUnDA were the main contributors for HDL-C and TG, and pre- and post-natal PFOA for LDL-C. For both blood pressures, prenatal PFOS and postnatal PFNA, and for WC, prenatal PFNA and postnatal PFOA were identified as the main contributors to the BKMR mixture (Supplementary Table 2). As expected, the univariate exposure-response association for the PFAS that was contributing the most to the derived mixture, was in agreement with the observed association of the overall mixture. More specifically, postnatal PFOA was negatively associated with WC and LDL-C, and postnatal PFUnDA with TG and postnatal PFUnDA was positively associated with HDL-C. For systolic BP, this comparison was less straight-forward (Supplementary Figure S2). However, when all the PFAS in the mixture were held on the 50th percentile, prenatal PFHxS was negatively associated with HDL-C, prenatal PFNA was positively associated with WC, and postnatal PFOS was positively associated with LDL-C, and these were opposing directions from the overall mixture (Supplementary Figure S2).
Figure 1.
Joint effect (h(z), 95%CIs) of the pre- and post-natal PFASs mixture on the cardiometabolic factors by increasing PFAS mixture levels (from 25th to 95th percentile), compared to low PFASs mixture (reference: 25th percentile), using Bayesian kernel machine regression (BKMR) model, adjusted for maternal age and education, pre-pregnancy BMI, parity, cohort, child ethnicity, age and sex.
In stratified analyses, the positive association with HDL-C and the negative association with WC and the specific BKMR derived PFAS mixtures, persisted independently of gender, while for the other cardiometabolic factors associations were attenuated (Supplementary Table S2 & Supplementary Figure S3). After stratifying by the trimester of maternal blood sample collection, we obtained similar positive associations with the derived PFAS mixture and HDL-C for all subsamples as for the overall sample, while for the subsample with 2nd trimester measurements the associations were stronger (Supplementary Table S4 and Figure S4). Similarly for LDL-C, TG and Systolic BP, in the 2nd trimester sub-sample the effect estimates were similar to the overall sample, though with wider confidence intervals, while for WC the all subsamples were in line with the overall negative association with the derived PFAS mixture, but this association was significant only among the 3rd trimester sub-sample.
In addition, our findings from the multi-pollutant linear regression models are in agreement with the results from the BKMR analysis (Supplementary Table S3). More specifically, postnatal PFUnDA and PFOS was driving the positive association with HDL-C, while a negative, though weak association was found with prenatal PFHxS. A weak positive association was also found between postnatal PFOS and LDL-C. Postnatal PFOA and postnatal PFUnDA was driving the negative association with WC and TG, respectively, as observed also in the BKMR analyses.
3.2. Integrated network analysis
By applying the xMWAS network analysis we were able to identify and visualize the complex network between prenatal and postnatal PFAS concentrations, inflammatory proteins in child’s blood and cardiometabolic factors in childhood. Eight PFAS, 17 proteins and the five cardiometabolic factors were selected and arranged in five communities, one per child outcome, connected through positive and negative correlations (Figure 2). The derived correlation matrix is presented in Supplementary Table S4 and all the possible connections between the triad, PFAS, inflammatory biomarkers and health outcomes are presented in Supplementary Table S5.
Figure 2.
Graph of the integrative network analysis of prenatal and postnatal PFAS, inflammatory protein concentrations in child’s blood and cardiometabolic factors in childhood as derived by the xMWAS. Five communities were detected by the multilevel community detection algorithm, and are represented by different colors.
The smallest community (dark blue color) comprised of postnatal PFUnDA and HDL-C connected by a positive correlation. This is in line with our findings from the BKMR analysis, where postnatal PFUnDA was the main contributor of the derived PFAS mixture that was positively associated with HDL-C. No protein was linked with postnatal PFUnDA and HDL-C. Postnatal PFOA was linked to LDL-C, IL-8 and HGF and they were all assigned in one community, connected by negative correlations (orange color). Postnatal PFOA was an important contributor to the derived BKMR PFAS mixture and was negatively associated with LDL-C. TG was assigned together with prenatal PFOA, PFNA and PFOS and MIP1-β in one community, connected by negative correlations only (green color). The BKMR derived mixture was also negatively associated with TG. After examining all possible connections linking PFAS, and inflammatory biomarkers with TG, between and within communities, an increase in prenatal PFOA, PFOA, PFNA and postnatal PFOA, was linked with lower TG, IL-8, MIG and MIP1-β and higher IL-1β (Supplementary Table S5).
Further, the largest community in the network comprised of Systolic BP, postnatal PFNA and ten inflammatory proteins (yellow color). Postnatal PFNA was also a major contributor to the derived PFAS mixture and was positively associated with Systolic BP in the BKMR analysis. We identified six inflammatory proteins that were linking postnatal PFNA and systolic BP, namely, IL-4, IL-13, MIP1-α, MIP1-β, MIG and IFN-α. Of those biomarkers, MIG and MIP1-β were also negatively correlated with prenatal PFOS.
WC was assigned in one community with postnatal PFHxS and PFOS, together with IL-1β, IL-6, leptin and MCP1 (light blue color). After examining all possible connections linking PFAS and inflammatory biomarkers with WC, between and within communities, we observed that an increase in postnatal PFHxS, PFNA, PFOA and PFOS, was linked with lower WC, leptin, IL-6 and IL-1β, HGF, IL-8, MCP-1, IL-4, IL-13, MIP1-α, MIP1-β, MIG and IFN-α. Interestingly, we identified a positive correlation between prenatal PFOA and WC, through IL-1β.
4. Discussion
In a well-characterized mother-child study, we found that exposure to a mixture of prenatal and postnatal PFAS was associated with an increase in HDL-C and decrease WC in childhood, even in low exposure levels. We also found a positive association between the PFAS mixture and Systolic BP, but for higher exposure levels (>50th percentile). Through the integrative network analysis we identified several inflammatory biomarkers positively and negatively correlated with the PFAS and all the studied cardiometabolic factors, except for HDL-C.
There is limited evidence of the association between early life PFAS exposure and child cardiometabolic health, with examination of both prenatal and postnatal windows of exposure. We found that exposure to a PFAS mixture, mostly reflecting childhood exposures, was associated with higher HDL-C at 8 years. This is in line with a study among US mother-child pairs that reported a positive cross-sectional association between PFAS exposure and HDL-C at 8 years (Mora et al. 2018). In the same study, mutually adjusted models for pre- and post-natal exposures were not presented and null associations were found for prenatal exposures (Mora et al. 2018). Null associations between prenatal PFAS and HDL-C at 4 years were also reported in a Spanish cohort (Manzano-Salgado et al. 2017). Overall, the epidemiological evidence for a positive association between PFAS exposure and HDL-C is largely consistent, in cross-sectional studies of background and high exposed populations and for several age groups (Canova et al. 2020; Chateau-Degat et al. 2010; Dalla Zuanna et al. 2021; Frisbee et al. 2010; Geiger et al. 2014; Li et al. 2020; Lin et al. 2020; Liu et al. 2018; Starling et al. 2014), but the longitudinal association is inconsistent. When we examined every PFAS individually while other PFASs in the mixture were held at their median levels, we found that prenatal PFHxS was associated with a reduction in HDL-C; suggesting that when postnatal exposures are moderate, prenatal PFAS exposures can have a detrimental effect on child’s HDL-C profile. In approximately 200 mother-child pairs from the HOME study, in the Cincinnati, Ohio area, prenatal and cord blood PFAS were associated with a reduction in HDL-C at 12 years, but when exposure occurred in childhood the association changed direction, in models adjusting for longitudinal exposures (Li et al. 2021). In another recent study of 306 pregnant women, Tian et al., reported that all prenatal PFASs were associated with a reduction in HDL-C in cord blood, when postnatal exposure has not occurred yet (Tian et al. 2021). Through the network analysis, we were not able to report a connection between the “HDL-C─postnatal PFUnDA” component with one of the studied inflammatory proteins, suggesting that higher PFUnDA exposure in healthy children was associated with higher HDL-C, also confirmed by the BKMR analyses, but did not modify the levels of the inflammatory biomarkers under study. Most children in our study had normal high levels of HDL-C, by comparison to the references curves produced by the IDEFICS consortium for European children (De Henauw et al. 2014). Other components that have been found to promote or obstruct the anti-inflammatory properties of HDL-C include high fat diet, trans-fatty acid consumption, dietary flavonoids, and the history of metabolic disorders or cardiovascular disease, while evidence of such effects in health young populations are scarce (Desgagne et al. 2016; Millar et al. 2017; Sadana et al. 2020; Su et al. 2021).
Similarly to HDL-C, we found a negative association between the derived PFAS mixture, mostly driven by postnatal PFOA, and WC at 12 years; but when keeping all PFAS in their median, prenatal PFNA was positively associated with WC. Similar opposing directions of the association between PFAS and WC at 12 years, for different windows of PFAS exposure were reported in the mother-child pairs from the HOME study (Li et al. 2021). Thus, our study adds to the evidence that prenatal PFAS exposure, rather than childhood, are more strongly associated with an unfavorable cardiometabolic profile in childhood. Nevertheless, our reported associations were weaker than those reported previously, and this could be attributed to the lower exposure levels. More specifically, maternal blood concentrations of PFAS in our study were lower than those reported in the populations of the studies with similar findings as ours from USA and Shanghai, and the median PFOA, PFOS and PFHxS levels reported for female population and pregnant population (mainly PFOS and PFHxS) in the U.S. National Health and Nutrition Examination Survey (NHANES 1999–2010 and NHANES 2003–2008-Pregnant, respectively) were higher than the 75th percentile of our study population (CDC 2021a; Jain 2013; Li et al. 2021; Mora et al. 2018; Tian et al. 2021) (Supplementary Figure S5, panel A). Similarly, children PFAS levels were relatively low compared to American populations (CDC 2021b; Li et al. 2021; Mora et al. 2018) (Supplementary Figure S5, panel B).
In the same study population, using an exposome approach to study a wide range of prenatal and postnatal exposures and blood pressure (BP), authors reported a positive association between maternal fish consumption and childhood PFOA with systolic BP (Warembourg et al. 2019). Fish consumption is a determinant of PFAS levels in maternal blood along with other contaminants, as reported previously (Papadopoulou et al. 2019). Using the BKMR methodology, we also found a positive association between exposure to PFAS mixture and systolic BP, but for levels of exposure above the 50th percentile. This might be explained by the U-shaped association with postnatal PFNA, one of the main contributors to the derived mixture. Our results, are in line with the HOME study, in terms of the positive association with BP, while they identified PFHxS exposure as the driver of this association (Li et al. 2021). In our study, PFAS exposure in early life was not associated with diastolic BP. The association between PFAS exposure and BP is more complex and an area in need of further investigation.
Through our network analysis, we identified a cluster of positive relationships between WC and the upregulation of pro-inflammatory adipokine, leptin, and the pro-inflammatory cytokines, IL-6and IL-1β. This is in line with the known role of leptin in obesity-induced inflammation, characterized by elevated release of pro-inflammatory cytokines (Kwaifa et al. 2020). In our study, postnatal PFAS were negatively linked to WC and to additional inflammatory biomarkers including HGF, IL-8, MCP-1, IL-4, IL-13, MIP1-α, MIP1-β, MIG and IFN-α, comprising a phenotype of low postnatal PFAS exposure and obesity-induced inflammation. We cannot exclude the possibility that low PFAS exposure might be explained by dilution effects of larger body mass.
Postnatal PFNA and prenatal PFOS were negatively linked with systolic BP and IL-4, IL-13, MIP1-α, MIP1-β, MIG and IFN-α. Inflammation is a key component in the pathophysiology of hypertensive disorders, and a marker of disease development and progression (Tanase et al. 2019). Our results suggest a negative link between PFAS exposure and this inflammatory response accompanied by high BP.
Additionally, postnatal PFOA was negatively linked to LDL-C, TG, HGF and IL-8, and prenatal PFOA, PFNA and PFOS were negatively linked with TG, and through that with IL-8, MIG and MIP1-β. Increased levels of MIP-1beta and interleukin (IL)-8 have been observed in patients with familial hypercholesterolaemia and are suggested to promote an atherosclerotic inflammatory process that are involved in early atherosclerosis (Holven et al. 2003). The hepatocyte growth factor (HGF) is a marker of endothelial damage and an interaction with serum lipids has been reported, towards an unfavorable disease prognosis for patients with dyslipidemia and high HGF levels (Bell et al. 2016; Zhu et al. 2020).
Overall PFAS exposure in childhood were mostly negatively linked with the clusters of cardiometabolic factors-inflammatory proteins and this is in line with the previous evidence on their role on suppression of inflammatory response. More specifically, from previous epidemiological evidence, PFAS exposure has been associated with suppressed antibody response to vaccination and increased occurrence of asthma, suggesting reduced immunological response, as well as lower levels of proteomic markers of inflammation (Chang et al. 2016; Pennings et al. 2015; Rappazzo et al. 2017; Salihovic et al. 2020).
Nevertheless, we found a positive relationship between prenatal PFOA and IL-1beta concentrations and WC and negative relationship with TG. PFOA-induced pro-inflammatory cytokine production, including IL-1beta, has been demonstrated in human lung and liver cells, supporting the concern that PFAS exposure may increase the risk of acute lung toxicity and hepatotoxicity (Sorli et al. 2020; Zhang et al. 2021). Other experimental evidence supports the role of PFOA as potent immunotoxicant, acting by inducing the activation of NF-kB pathway and altering the IL-1β expression in zebrafish spleen (Zhang et al. 2014). Our findings are in line with the experimental evidence but there is a need of additional epidemiological evidence for further interpretation.
One strength of this study is the use of a mixture approach to explore the association between the prenatal and postnatal PFAS exposure with child metabolic health. The BKMR methodology allowed us to examine the overall mixture effect, the independent effects of the individual chemicals in the mixture as well as interactions between them. The BKMR does not require the effects of all mixture members to be in the same direction, as other methodologies (i.e. WQS). Given the inconsistent findings from cross- sectional and prospective studies examining the associations of PFAS exposure and cardiometabolic factors, we could not assume the direction of the association. In addition, given the large cross-sectional nature of our data (postnatal PFAS, cardiometabolic factors and inflammatory biomarkers) we considered the xMWAS network analysis as an appropriate methodology to describe some of the complex relationships between the three panels of information, even without being able to discuss causal inferences. This method did not allow adjustment for confounders and our results are prone to confounding by measured or unmeasured factors and should be interpreted with caution. Nevertheless, the molecular exacerbations underlying the association between early life PFAS exposure and cardiometabolic factors is under current investigation.
The PFAS exposure profile in childhood can substantially divert from the gestational exposure profile, due to the effect of key factors, such as transplacental transfer, early –life exposure through breastfeeding and later dietary exposures, while there are large variations by congener. Mutually adjusted models for pre- and post-natal exposures would most probably provide a less biased effect estimate. Especially for prenatal PFAS exposures, adjusting for postnatal levels is not expected to produce a large bias amplification due to co-exposure because of the relatively weak correlations between maternal and child PFAS concentrations (Weisskopf et al. 2018).
Longitudinal birth cohorts, from which our study draws resources, are exposed to the risk of loss of subjects to follow-up and possible selection bias. Maitre et al. reported that the distribution of family characteristics in the HELIX subcohort, is somewhat different than the entire HELIX cohort, which consist of 31,472 mother-child pairs from the six source birth cohorts (Maitre et al. 2018). More specifically, children of low educated mothers (entire cohort vs. subcohort: 23% vs. 7%), children of parents foreign to the country of the cohort (21% vs. 11%) and firstborns (nulliparous women: 51% vs. 46%) were less likely to participate in the HELIX follow-up examination. We previously found that low maternal education is associated with lower maternal and child PFAS levels, meaning that mother-child pairs with high prenatal and postnatal PFAS exposure are more likely included in our analysis (Montazeri et al. 2019). On the other hand, the inclusion of less firstborn children could mean lower prenatal PFAS exposure in our study, since parity is a strong determinant of maternal PFAS concentrations, but this can vary substantially by the inter-pregnancy interval (Brantsaeter et al. 2013; Papadopoulou et al. 2015). Regarding the outcome, overweight and obesity in the HELIX subcohort was similar to what has been reported in cohort or country-specific reports (de Bont et al. 2020; Harskamp-van Ginkel et al. 2020; Kadawathagedara et al. 2018). Overall selection and self-reporting bias is probable in birth cohorts based on voluntary enrolment and face-to-face interviews, while exposure assessment based on biomarkers and a thorough examination of the outcome by trained research assistants might reduce this bias in our study. Finally, non-fasting insulin levels were also assessed in children samples. However, given the moderate correlation between fasting and non-fasting insulin it was not that this will provide an adequate measure of glucose-insulin homeostasis (Hancox and Landhuis 2011) and we have decided not to include it in our analysis. Therefore, the absence of this measure is a limitation of our study.
5. Conclusion
In this large mother-child study, we found that exposure to a mixture of prenatal and postnatal PFAS was associated with higher HDL-C and lower adiposity at 8 years, and postnatal PFAS were the main contributors to the derived BKMR mixtures. Postnatal PFAS exposure was also linked to lower levels of inflammatory biomarkers in child’s blood. Nevertheless, when we examined individual PFAS while other PFASs in the mixture were held at their median levels, we found that prenatal PFAS exposures were associated with an unfavorable cardiometabolic profile of lower HDL-C and increased adiposity, but these associations were weak. We found a positive relationship between prenatal PFOA and concentrations of the pro-inflammatory cytokine, IL-1beta, which was itself linked with larger WC, suggesting increased inflammation but more studies are needed to confirm this association.
Supplementary Material
Acknowledgements
We acknowledge the input of the entire HELIX consortium. We are grateful to all the participating families in the six countries who took part in this cohort study (BiB, EDEN,INMA,KANC,MoBa,andRhea cohorts) and those families who came in for a clinical examination of their children and who, in addition, donated blood and urine to this specific study. The authors are equally grateful to all the fieldworkers for their dedication and efficiency in this study. A full roster of the INMA and Rhea projects investigators can be found at: https://www.proyectoinma.org/proyecto-inma/investigadores/ and http://www.rhea.gr/en/about-rhea/the-rhea-team/. BiB is possible only because of the enthusiasm and commitment of the children and parents in BiB. We are grateful to all the participants, health professionals and researchers who have made BiB happen.
Funding
This work was supported by the Norwegian Research Council (project No. 268465).
The HELIX project is funded by the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no 308333 – the HELIX project. The HELIX program is built on six existing cohorts that received previous funding, including the major ones listed below. The Born in Bradford (BiB) study presents independent research commissioned by the National Institute for Health Research Collaboration for Applied Health Research and Care (NIHR CLAHRC) and the Programme Grants for Applied Research funding scheme (RP-PG-0407–10044). INMA data collections were supported by grants from the Instituto de Salud Carlos III, CIBERESP, and the Generalitat de Catalunya-CIRIT. KANC was funded by the grant of the Lithuanian Agency for Science Innovation and Technology (6–04-2014_31V-66).
For a full list of funding that supported the EDEN cohort, refer to: Heude B et al. Cohort Profile: The EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int J Epidemiol. 2016 Apr;45(2):353–63. The Norwegian Mother, Father and Child Cohort Study (MoBa) is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. The Rhea project was financially supported by European projects, and the Greek Ministry of Health (Program of Prevention of Obesity and Neurodevelopmental Disorders in Preschool Children, in Heraklion district, Crete, Greece: 2011–2014; ‘Rhea Plus’: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012–2015).
Dr. Maribel Casas received funding from Instituto de Salud Carlos III (Ministry of Economy and Competitiveness) (MS16/00128).
Dr. Leda Chatzi was supported by NIH/NIHES R01 ES029944, R01ES030691, R01ES030364, R21 ES029681, R21 ES028903, and P30 ES007048–23.
Dr. David Conti was supported by P01CA196569, R01CA140561, R01 ES016813, R01 ES029944, R01ES030691, R01ES030364.
Dr. Nikos Stratakis was supported by NIH/NIHES R21 ES029681 and P30 ES007048–23, and NIH/NIDDK P30 DK048522–24.
The CRG/UPF Proteomics Unit is part of the Spanish Infrastructure for Omics Technologies (ICTS OmicsTech) and it is a member of the ProteoRed PRB3 consortium which is supported by grant PT17/0019 of the PE I+D+i 2013–2016 from the Instituto de Salud Carlos III (ISCIII) and ERDF.
ISGlobal affiliated researchers acknowledge support from the Spanish Ministry of Science, Innovation and Universities, “Centro de Excelencia Severo Ochoa 2013–2017”, SEV-2012–0208, and “Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya” (2017SGR595).
Footnotes
CRediT authorship contribution statement
Eleni Papadopoulou: Conceptualization, Methodology, Formal analysis, Writing - original draft, Visualization, Funding acquisition. Nikos Stratakis: Conceptualization, Methodology, Formal analysis, Writing - review & editing. Xavier Basagaña: Methodology, Formal analysis, Writing - review & editing. Anne Lise Brantsæeter: Methodology, Writing - review & editing, Funding acquisition. Maribel Casas: Methodology, Investigation, Writing - review & editing. Serena Fossati: Methodology, Investigation, Writing - review & editing. Regina Gražulevičienė: Methodology, Investigation, Writing - review & editing. Line Småstuen Haug: Methodology, Investigation, Writing - review & editing. Barbara Heude: Methodology, Investigation, Writing - review & editing. Léa Maitre: Methodology, Investigation, Writing - review & editing. Rosemary RC McEachan: Methodology, Investigation, Writing - review & editing. Oliver Robinson: Methodology, Investigation, Writing - review & editing. Theano Roumeliotaki: Methodology, Investigation, Writing - review & editing. Eduard Sabidó: Conceptualization, Methodology, Investigation, Writing - review & editing. Eva Borràs: Investigation, Sample analysis. Jose Urquiza: Methodology, Investigation, Writing - review & editing. Marina Vafeiadi: Methodology, Investigation, Writing - review & editing. Yinqi Zhao: Methodology, Investigation, Writing - review & editing, Formal analysis. Remy Slama: Conceptualization, Writing - review & editing. John Wright: Conceptualization, Writing - review & editing. David V Conti: Conceptualization, Methodology, Formal analysis, Writing - review & editing. Martine Vrijheid: Conceptualization, Methodology, Writing - review & editing Funding acquisition. Lida Chatzi: Conceptualization, Methodology, Investigation, Writing - review & editing, Funding acquisition, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data statement
The HELIX data warehouse has been established as an accessible resource for collaborative research involving researchers external to the project. Access to HELIX data is based on approval by the HELIX Project Executive Committee and by the individual cohorts. Further details on the content of the data warehouse (data catalog) and procedures for external access are described on the project website (http://www.projecthelix.eu/index.php/en/data-inventory).
6. References
- Ahrens W; Moreno LA; Marild S; Molnar D; Siani A; De Henauw S; Bohmann J; Gunther K; Hadjigeorgiou C; Iacoviello L; Lissner L; Veidebaum T; Pohlabeln H; Pigeot I; consortium I. Metabolic syndrome in young children: definitions and results of the IDEFICS study. International journal of obesity 2014;38 Suppl 2:S4–14 [DOI] [PubMed] [Google Scholar]
- Andersen CS; Fei C; Gamborg M; Nohr EA; Sorensen TI; Olsen J. Prenatal Exposures to Perfluorinated Chemicals and Anthropometry at 7 Years of Age. Am J Epidemiol 2013; [DOI] [PubMed] [Google Scholar]
- Barouki R; Gluckman PD; Grandjean P; Hanson M; Heindel JJ Developmental origins of non-communicable disease: implications for research and public health. EnvironHealth 2012;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry V; Darrow LA; Klein M; Winquist A; Steenland K. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environ Res 2014;132:62–69 [DOI] [PubMed] [Google Scholar]
- Behr AC; Plinsch C; Braeuning A; Buhrke T. Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicol In Vitro 2020;62:104700 [DOI] [PubMed] [Google Scholar]
- Bell EJ; Larson NB; Decker PA; Pankow JS; Tsai MY; Hanson NQ; Wassel CL; Longstreth WT Jr.; Bielinski SJ Hepatocyte Growth Factor Is Positively Associated With Risk of Stroke: The MESA (Multi-Ethnic Study of Atherosclerosis). Stroke 2016;47:2689–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A. Environmental Determinants of Cardiovascular Disease. Circ Res 2017;121:162–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF; Valeri L; Claus Henn B; Christiani DC; Wright RO; Mazumdar M; Godleski JJ; Coull BA Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015;16:493–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsaeter AL; Whitworth KW; Ydersbond TA; Haug LS; Haugen M; Knutsen HK; Thomsen C; Meltzer HM; Becher G; Sabaredzovic A; Hoppin JA; Eggesbo M; Longnecker MP Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int 2013;54:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM; Chen A; Romano ME; Calafat AM; Webster GM; Yolton K; Lanphear BP Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity 2016;24:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussler S; Penke M; Flemming G; Elhassan YS; Kratzsch J; Sergeyev E; Lipek T; Vogel M; Spielau U; Korner A; de Giorgis T; Kiess W. Novel Insights in the Metabolic Syndrome in Childhood and Adolescence. Horm Res Paediatr 2017;88:181–193 [DOI] [PubMed] [Google Scholar]
- Canova C; Barbieri G; Zare Jeddi M; Gion M; Fabricio A; Dapra F; Russo F; Fletcher T; Pitter G. Associations between perfluoroalkyl substances and lipid profile in a highly exposed young adult population in the Veneto Region. Environ Int 2020;145:106117. [DOI] [PubMed] [Google Scholar]
- Chang ET; Adami HO; Boffetta P; Wedner HJ; Mandel JS A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit Rev Toxicol 2016;46:279–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateau-Degat ML; Pereg D; Dallaire R; Ayotte P; Dery S; Dewailly E. Effects of perfluorooctanesulfonate exposure on plasma lipid levels in the Inuit population of Nunavik (Northern Quebec). Environ Res 2010;110:710–717 [DOI] [PubMed] [Google Scholar]
- Chatzi L; Leventakou V; Vafeiadi M; Koutra K; Roumeliotaki T; Chalkiadaki G; Karachaliou M; Daraki V; Kyriklaki A; Kampouri M; Fthenou E; Sarri K; Vassilaki M; Fasoulaki M; Bitsios P; Koutis A; Stephanou EG; Kogevinas M. Cohort Profile: The Mother-Child Cohort in Crete, Greece (Rhea Study). International journal of epidemiology 2017;46:1392–1393k [DOI] [PubMed] [Google Scholar]
- Cheng HS; Tan WR; Low ZS; Marvalim C; Lee JYH; Tan NS Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators, G.B.D.C.o.D. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018a;392:1736–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators, G.B.D.R.F. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018b;392:1923–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Zuanna T; Savitz DA; Barbieri G; Pitter G; Zare Jeddi M; Dapra F; Fabricio ASC; Russo F; Fletcher T; Canova C. The association between perfluoroalkyl substances and lipid profile in exposed pregnant women in the Veneto region, Italy. Ecotoxicol Environ Saf 2021;209:111805 [DOI] [PubMed] [Google Scholar]
- de Bont J; Diaz Y; Casas M; Garcia-Gil M; Vrijheid M; Duarte-Salles T. Time Trends and Sociodemographic Factors Associated With Overweight and Obesity in Children and Adolescents in Spain. JAMA Netw Open 2020;3:e201171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Henauw S; Michels N; Vyncke K; Hebestreit A; Russo P; Intemann T; Peplies J; Fraterman A; Eiben G; de Lorgeril M; Tornaritis M; Molnar D; Veidebaum T; Ahrens W; Moreno LA; consortium I. Blood lipids among young children in Europe: results from the European IDEFICS study. Int J Obes (Lond) 2014;38 Suppl 2:S67–75 [DOI] [PubMed] [Google Scholar]
- Deng P; Wang C; Wahlang B; Sexton T; Morris AJ; Hennig B. Co-exposure to PCB126 and PFOS increases biomarkers associated with cardiovascular disease risk and liver injury in mice. Toxicol Appl Pharmacol 2020;409:115301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgagne V; Guay SP; Guerin R; Corbin F; Couture P; Lamarche B; Bouchard L. Variations in HDL-carried miR-223 and miR-135a concentrations after consumption of dietary trans fat are associated with changes in blood lipid and inflammatory markers in healthy men - an exploratory study. Epigenetics 2016;11:438–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA; Chain P.o.C.i.t.F.; Knutsen HK; Alexander J; Barregard L; Bignami M; Bruschweiler B; Ceccatelli S; Cottrill B; Dinovi M; Edler L; Grasl-Kraupp B; Hogstrand C; Hoogenboom LR; Nebbia CS; Oswald IP; Petersen A; Rose M; Roudot AC; Vleminckx C; Vollmer G; Wallace H; Bodin L; Cravedi JP; Halldorsson TI; Haug LS; Johansson N; van Loveren H; Gergelova P; Mackay K; Levorato S; van Manen M; Schwerdtle T. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J 2018;16:e05194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA; Chain P.o.C.i.t.F.; Schrenk D; Bignami M; Bodin L; Chipman JK; Del Mazo J; Grasl-Kraupp B; Hogstrand C; Hoogenboom LR; Leblanc JC; Nebbia CS; Nielsen E; Ntzani E; Petersen A; Sand S; Vleminckx C; Wallace H; Barregard L; Ceccatelli S; Cravedi JP; Halldorsson TI; Haug LS; Johansson N; Knutsen HK; Rose M; Roudot AC; Van Loveren H; Vollmer G; Mackay K; Riolo F; Schwerdtle T. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 2020;18:e06223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M; Arbuckle TE; Liang CL; LeBlanc A; Gaudreau E; Foster WG; Haines D; Davis K; Fraser WD Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study. Environ Health 2016;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF; Rifas-Shiman SL; Mora AM; Calafat AM; Ye X; Luttmann-Gibson H; Gillman MW; Oken E; Sagiv SK Early-Life Exposure to Perfluoroalkyl Substances and Childhood Metabolic Function. Environmental health perspectives 2017;125:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PW; Hanson RL; Knowler WC; Sievers ML; Bennett PH; Looker HC Childhood obesity, other cardiovascular risk factors, and premature death. The New England journal of medicine 2010;362:485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee SJ; Shankar A; Knox SS; Steenland K; Savitz DA; Fletcher T; Ducatman AM Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med 2010;164:860–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H; Mosch C; Morovitz M; Alba-Alejandre I; Boehmer S; Kiranoglu M; Faber F; Hannibal I; Genzel-Boroviczeny O; Koletzko B; Volkel W. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environmental science & technology 2010;44:7123–7129 [DOI] [PubMed] [Google Scholar]
- Geiger SD; Xiao J; Ducatman A; Frisbee S; Innes K; Shankar A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere 2014;98:78–83 [DOI] [PubMed] [Google Scholar]
- Grazuleviciene R; Danileviciute A; Nadisauskiene R; Vencloviene J. Maternal smoking, GSTM1 and GSTT1 polymorphism and susceptibility to adverse pregnancy outcomes. Int J Environ Res Public Health 2009;6:1282–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M; Ballester F; Espada M; Fernandez MF; Grimalt JO; Ibarluzea J; Olea N; Rebagliato M; Tardon A; Torrent M; Vioque J; Vrijheid M; Sunyer J. Cohort Profile: The INMA--INfancia y Medio Ambiente--(Environment and Childhood) Project. IntJEpidemiol 2012;41:930–940 [DOI] [PubMed] [Google Scholar]
- Halldorsson TI; Rytter D; Haug LS; Bech BH; Danielsen I; Becher G; Henriksen TB; Olsen SF Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. EnvironHealth Perspect 2012;120:668–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancox RJ; Landhuis CE Correlation between measures of insulin resistance in fasting and non-fasting blood. Diabetol Metab Syndr 2011;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harskamp-van Ginkel MW; Ierodiakonou D; Margetaki K; Vafeiadi M; Karachaliou M; Kogevinas M; Vrijkotte TGM; Chatzi L. Gestational sleep deprivation is associated with higher offspring BMI and blood pressure. Sleep 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug LS; Sakhi AK; Cequier E; Casas M; Maitre L; Basagana X; Andrusaityte S; Chalkiadaki G; Chatzi L; Coen M; de Bont J; Dedele A; Ferrand J; Grazuleviciene R; Gonzalez JR; Gutzkow KB; Keun H; McEachan R; Meltzer HM; Petraviciene I; Robinson O; Saulnier PJ; Slama R; Sunyer J; Urquiza J; Vafeiadi M; Wright J; Vrijheid M; Thomsen C. In-utero and childhood chemical exposome in six European mother-child cohorts. Environment international 2018;121:751–763 [DOI] [PubMed] [Google Scholar]
- Haug LS; Thomsen C; Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. Journal of chromatography A 2009;1216:385–393 [DOI] [PubMed] [Google Scholar]
- Henriksson P; Henriksson H; Gracia-Marco L; Labayen I; Ortega FB; Huybrechts I; Espana-Romero V; Manios Y; Widhalm K; Dallongeville J; Gonzalez-Gross M; Marcos A; Moreno LA; Castillo MJ; Ruiz JR; group H.s. Prevalence of ideal cardiovascular health in European adolescents: The HELENA study. International journal of cardiology 2017;240:428–432 [DOI] [PubMed] [Google Scholar]
- Heude B; Forhan A; Slama R; Douhaud L; Bedel S; Saurel-Cubizolles MJ; Hankard R; Thiebaugeorges O; De Agostini M; Annesi-Maesano I; Kaminski M; Charles MA; Grp EM -c.C.S. Cohort Profile: The EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. International journal of epidemiology 2016;45:353–363 [DOI] [PubMed] [Google Scholar]
- Holven KB; Myhre AM; Aukrust P; Hagve TA; Ose L; Nenseter MS Patients with familial hypercholesterolaemia show enhanced spontaneous chemokine release from peripheral blood mononuclear cells ex vivo. Dependency of xanthomas/xanthelasms, smoking and gender. Eur Heart J 2003;24:1756–1762 [DOI] [PubMed] [Google Scholar]
- Hoyer BB; Ramlau-Hansen CH; Vrijheid M; Valvi D; Pedersen HS; Zviezdai V; Jonsson BA; Lindh CH; Bonde JP; Toft G. Anthropometry in 5- to 9-Year-Old Greenlandic and Ukrainian Children in Relation to Prenatal Exposure to Perfluorinated Alkyl Substances. Environmental health perspectives 2015;123:841–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17–39 years: data from National Health and Nutrition Examination Survey 2003–2008. J Toxicol Environ Health A 2013;76:409–421 [DOI] [PubMed] [Google Scholar]
- Juonala M; Magnussen CG; Berenson GS; Venn A; Burns TL; Sabin MA; Srinivasan SR; Daniels SR; Davis PH; Chen W; Sun C; Cheung M; Viikari JS; Dwyer T; Raitakari OT Childhood adiposity, adult adiposity, and cardiovascular risk factors. The New England journal of medicine 2011;365:1876–1885 [DOI] [PubMed] [Google Scholar]
- Kadawathagedara M; Botton J; de Lauzon-Guillain B; Meltzer HM; Alexander J; Brantsaeter AL; Haugen M; Papadopoulou E. Dietary acrylamide intake during pregnancy and postnatal growth and obesity: Results from the Norwegian Mother and Child Cohort Study (MoBa). Environ Int 2018; [DOI] [PubMed] [Google Scholar]
- Kingsley SL; Eliot MN; Kelsey KT; Calafat AM; Ehrlich S; Lanphear BP; Chen A; Braun JM Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environ Res 2018;165:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaifa IK; Bahari H; Yong YK; Noor SM Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N; Liu Y; Papandonatos GD; Calafat AM; Eaton CB; Kelsey KT; Cecil KM; Kalkwarf HJ; Yolton K; Lanphear BP; Chen A; Braun JM Gestational and childhood exposure to per- and polyfluoroalkyl substances and cardiometabolic risk at age 12 years. Environ Int 2021;147:106344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y; Barregard L; Xu Y; Scott K; Pineda D; Lindh CH; Jakobsson K; Fletcher T. Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water. Environ Health 2020;19:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY; Lee HL; Hwang YT; Su TC The association between total serum isomers of per- and polyfluoroalkyl substances, lipid profiles, and the DNA oxidative/nitrative stress biomarkers in middle-aged Taiwanese adults. Environ Res 2020;182:109064 [DOI] [PubMed] [Google Scholar]
- Liu HS; Wen LL; Chu PL; Lin CY Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013–2014. Environ Pollut 2018;232:73–79 [DOI] [PubMed] [Google Scholar]
- Magnus P; Birke C; Vejrup K; Haugan A; Alsaker E; Daltveit AK; Handal M; Haugen M; Høiseth G; Knudsen GP; Paltiel L; Schreuder P; Tambs K; Vold L; Stoltenberg C. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). International journal of epidemiology 2016; [DOI] [PubMed] [Google Scholar]
- Maitre L; de Bont J; Casas M; Robinson O; Aasvang GM; Agier L; Andrusaityte S; Ballester F; Basagana X; Borras E; Brochot C; Bustamante M; Carracedo A; de Castro M; Dedele A; Donaire-Gonzalez D; Estivill X; Evandt J; Fossati S; Giorgis-Allemand L; J RG; Granum B; Grazuleviciene R; Bjerve Gutzkow, K.; Smastuen Haug, L.; Hernandez-Ferrer C; Heude B; Ibarluzea J; Julvez J; Karachaliou M; Keun HC; Hjertager Krog N.; Lau CE; Leventakou V; Lyon-Caen S; Manzano C; Mason D; McEachan R; Meltzer HM; Petraviciene I; Quentin J; Roumeliotaki T; Sabido E; Saulnier PJ; Siskos AP; Siroux V; Sunyer J; Tamayo I; Urquiza J; Vafeiadi M; van Gent D; Vives-Usano M; Waiblinger D; Warembourg C; Chatzi L; Coen M; van den Hazel P; Nieuwenhuijsen MJ; Slama R; Thomsen C; Wright J; Vrijheid M. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open 2018;8:e021311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano-Salgado CB; Casas M; Lopez-Espinosa MJ; Ballester F; Basterrechea M; Grimalt JO; Jimenez AM; Kraus T; Schettgen T; Sunyer J; Vrijheid M. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res 2015;142:471–478 [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB; Casas M; Lopez-Espinosa MJ; Ballester F; Iniguez C; Martinez D; Romaguera D; Fernandez-Barres S; Santa-Marina L; Basterretxea M; Schettgen T; Valvi D; Vioque J; Sunyer J; Vrijheid M. Prenatal Exposure to Perfluoroalkyl Substances and Cardiometabolic Risk in Children from the Spanish INMA Birth Cohort Study. Environmental health perspectives 2017;125:097018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar CL; Duclos Q; Blesso CN Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv Nutr 2017;8:226–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza AZ; Althagafi II; Shamshad H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur J Med Chem 2019;166:502–513 [DOI] [PubMed] [Google Scholar]
- Montazeri P; Thomsen C; Casas M; de Bont J; Haug LS; Maitre L; Papadopoulou E; Sakhi AK; Slama R; Saulnier PJ; Urquiza J; Grazuleviciene R; Andrusaityte S; McEachan R; Wright J; Chatzi L; Basagana X; Vrijheid M. Socioeconomic position and exposure to multiple environmental chemical contaminants in six European mother-child cohorts. Int J Hyg Environ Health 2019;222:864–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM; Fleisch AF; Rifas-Shiman SL; Woo Baidal JA; Pardo L; Webster TF; Calafat AM; Ye X; Oken E; Sagiv SK Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environ Int 2018;111:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM; Oken E; Rifas-Shiman SL; Webster TF; Gillman MW; Calafat AM; Ye X; Sagiv SK Prenatal Exposure to Perfluoroalkyl Substances and Adiposity in Early and Mid-Childhood. Environmental health perspectives 2017;125:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah S; Kotz S. R Programs for Computing Truncated Distributions. Journal of Statistical Software 2006; [Google Scholar]
- Papadopoulou E; Haug LS; Sabaredzovic A; Eggesbo M; Longnecker MP Reliability of perfluoroalkyl substances in plasma of 100 women in two consecutive pregnancies. Environmental research 2015;140:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou E; Haug LS; Sakhi AK; Andrusaityte S; Basagana X; Brantsaeter AL; Casas M; Fernandez-Barres S; Grazuleviciene R; Knutsen HK; Maitre L; Meltzer HM; McEachan RRC; Roumeliotaki T; Slama R; Vafeiadi M; Wright J; Vrijheid M; Thomsen C; Chatzi L. Diet as a Source of Exposure to Environmental Contaminants for Pregnant Women and Children from Six European Countries. Environ Health Perspect 2019;127:107005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou E; Sabaredzovic A; Namork E; Nygaard UC; Granum B; Haug LS Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): The association with breastfeeding and maternal PFAS concentrations. Environ Int 2016;94:687–694 [DOI] [PubMed] [Google Scholar]
- Pennings JL; Jennen DG; Nygaard UC; Namork E; Haug LS; van Loveren H; Granum B. Cord blood gene expression supports that prenatal exposure to perfluoroalkyl substances causes depressed immune functionality in early childhood. Journal of Immunotoxicology 2015;27 [DOI] [PubMed] [Google Scholar]
- Pennings JL; Jennen DG; Nygaard UC; Namork E; Haug LS; van Loveren H; Granum B. Cord blood gene expression supports that prenatal exposure to perfluoroalkyl substances causes depressed immune functionality in early childhood. J Immunotoxicol 2016;13:173–180 [DOI] [PubMed] [Google Scholar]
- Poothong S; Thomsen C; Padilla-Sanchez JA; Papadopoulou E; Haug LS Distribution of Novel and Well-Known Poly- and Perfluoroalkyl Substances (PFASs) in Human Serum, Plasma, and Whole Blood. Environmental science & technology 2017;51:13388–13396 [DOI] [PubMed] [Google Scholar]
- Rappazzo KM; Coffman E; Hines EP Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int J Environ Res Public Health 2017;14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DA; McMurray RG; Hackney AC; Harrell JS Relationship between cardiovascular risk factors and adipokines in adolescents. Horm Res Paediatr 2011;76:123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadana P; Lin L; Aghayev M; Ilchenko S; Kasumov T. Early Pro-Inflammatory Remodeling of HDL Proteome in a Model of Diet-Induced Obesity: (2)H2O-Metabolic Labeling-Based Kinetic Approach. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihovic S; Lind L; Larsson A; Lind PM Plasma perfluoroalkyls are associated with decreased levels of proteomic inflammatory markers in a cross-sectional study of an elderly population. Environ Int 2020;145:106099 [DOI] [PubMed] [Google Scholar]
- Sorli JB; Lag M; Ekeren L; Perez-Gil J; Haug LS; Da Silva E; Matrod MN; Gutzkow KB; Lindeman B. Per- and polyfluoroalkyl substances (PFASs) modify lung surfactant function and pro-inflammatory responses in human bronchial epithelial cells. Toxicol In Vitro 2020;62:104656 [DOI] [PubMed] [Google Scholar]
- Starling AP; Engel SM; Richardson DB; Baird DD; Haug LS; Stuebe AM; Klungsoyr K; Harmon Q; Becher G; Thomsen C; Sabaredzovic A; Eggesbo M; Hoppin JA; Travlos GS; Wilson RE; Trogstad LI; Magnus P; Longnecker MP Perfluoroalkyl Substances During Pregnancy and Validated Preeclampsia Among Nulliparous Women in the Norwegian Mother and Child Cohort Study. Am J Epidemiol 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X; Zhang G; Cheng Y; Wang B. New insights into the emerging effects of inflammatory response on HDL particles structure and function. Mol Biol Rep 2021;48:5723–5733 [DOI] [PubMed] [Google Scholar]
- Tamayo-Uria I; Maitre L; Thomsen C; Nieuwenhuijsen MJ; Chatzi L; Siroux V; Aasvang GM; Agier L; Andrusaityte S; Casas M; de Castro M; Dedele A; Haug LS; Heude B; Grazuleviciene R; Gutzkow KB; Krog NH; Mason D; McEachan RRC; Meltzer HM; Petraviciene I; Robinson O; Roumeliotaki T; Sakhi AK; Urquiza J; Vafeiadi M; Waiblinger D; Warembourg C; Wright J; Slama R; Vrijheid M; Basagana X. The early-life exposome: Description and patterns in six European countries. Environ Int 2019;123:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X; Xie G; Sun X; Li Q; Zhong W; Qiao P; Sun X; Jia W; Zhou Z. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One 2013;8:e61409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase DM; Gosav EM; Radu S; Ouatu A; Rezus C; Ciocoiu M; Costea CF; Floria M. Arterial Hypertension and Interleukins: Potential Therapeutic Target or Future Diagnostic Marker? Int J Hypertens 2019;2019:3159283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Peronard JL; Andersen HR; Jensen TK; Heitmann BL Endocrine-disrupting chemicals and obesity development in humans: a review. Obesity reviews : an official journal of the International Association for the Study of Obesity 2011;12:622–636 [DOI] [PubMed] [Google Scholar]
- Tian Y; Miao M; Ji H; Zhang X; Chen A; Wang Z; Yuan W; Liang H. Prenatal exposure to perfluoroalkyl substances and cord plasma lipid concentrations. Environ Pollut 2021;268:115426 [DOI] [PubMed] [Google Scholar]
- Uppal K; Ma C; Go YM; Jones DP; Wren J. xMWAS: a data-driven integration and differential network analysis tool. Bioinformatics 2018;34:701–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner MA; Ngueta G; Jensen ET; Fromme H; Volkel W; Nygaard UC; Granum B; Longnecker MP A Simple Pharmacokinetic Model of Prenatal and Postnatal Exposure to Perfluoroalkyl Substances (PFASs). Environ Sci Technol 2016;50:978–986 [DOI] [PubMed] [Google Scholar]
- Warembourg C; Maitre L; Tamayo-Uria I; Fossati S; Roumeliotaki T; Aasvang GM; Andrusaityte S; Casas M; Cequier E; Chatzi L; Dedele A; Gonzalez JR; Grazuleviciene R; Haug LS; Hernandez-Ferrer C; Heude B; Karachaliou M; Krog NH; McEachan R; Nieuwenhuijsen M; Petraviciene I; Quentin J; Robinson O; Sakhi AK; Slama R; Thomsen C; Urquiza J; Vafeiadi M; West J; Wright J; Vrijheid M; Basagana X. Early-Life Environmental Exposures and Blood Pressure in Children. J Am Coll Cardiol 2019;74:1317–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG; Seals RM; Webster TF Bias Amplification in Epidemiologic Analysis of Exposure to Mixtures. Environ Health Perspect 2018;126:047003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J; Small N; Raynor P; Tuffnell D; Bhopal R; Cameron N; Fairley L; Lawlor DA; Parslow R; Petherick ES; Pickett KE; Waiblinger D; West J; Born in Bradford Scientific Collaborators, G. Cohort Profile: the Born in Bradford multi-ethnic family cohort study. International journal of epidemiology 2013;42:978–991 [DOI] [PubMed] [Google Scholar]
- Yang M; Li LY; Qin XD; Ye XY; Yu S; Bao Q; Sun L; Wang ZB; Bloom MS; Jalava P; Hu LW; Yu HY; Zeng XW; Yang BY; Dong GH; Li CW Perfluorooctanesulfonate and perfluorooctanoate exacerbate airway inflammation in asthmatic mice and in vitro. Sci Total Environ 2021;766:142365 [DOI] [PubMed] [Google Scholar]
- Zhang H; Fang W; Wang D; Gao N; Ding Y; Chen C. The role of interleukin family in perfluorooctanoic acid (PFOA)-induced immunotoxicity. J Hazard Mater 2014;280:552–560 [DOI] [PubMed] [Google Scholar]
- Zhang R; Yao Y; Tu L; Luan T; Chen B. Non-targeted metabolomics of multiple human cells revealing differential toxic effects of perfluorooctanoic acid. J Hazard Mater 2021;409:125017. [DOI] [PubMed] [Google Scholar]
- Zhu Z; Wang A; Guo D; Bu X; Xu T; Zhong C; Peng Y; Xu T; Peng H; Chen J; Ju Z; Geng D; He J; Zhang Y. Association between serum hepatocyte growth factor and prognosis of ischemic stroke: The role of blood lipid status. Nutr Metab Cardiovasc Dis 2020;30:492–499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.